Introduction
An esthetic smile is characterized by providing facial esthetic correlation, lip dynamics, and harmonizing the red and white aesthetics of the oral cavity (1). On the other hand, a gummy smile is defined as a non-pathological esthetic condition that results in disharmony, where an excessive amount of gum tissue is visible when smiling (2). The etiology of a gummy smile involves factors such as abnormal tooth eruption, lip hyperactivity, excessive vertical maxillary growth, tooth extrusion, overbite, as well as a short and prominent upper lip (3).
There are different treatments for a gummy smile, which are defined based on the etiology of the problem in various clinical cases (4). These treatments may include periodontal surgeries (5), laser surgeries (6), the application of botulinum toxin (BTX) (7), orthodontics (8), orthognathic surgeries (9), lip repositioning (10), polyester threads (11), or even a combination of the treatments mentioned above.
After proper clinical diagnosis and establishing the etiology, treatment with BTX can be a therapeutic alternative when a gummy smile is caused by labial hypercontraction, as suggested in cases of muscular hyperfunction. BTX treatment is considered an effective alternative for reducing a gummy smile due to the ease and safety of the applications (12). The advantages of this treatment include ease and safety during application, the use of a reduced amount of the product, rapid action, low risk, and a reversible effect, which is not the case with invasive surgical treatments (13).
In light of the foregoing, the aim of this study is to report a clinical case describing the use of BTX in cases of gummy smile associated with muscular hyperfunction, as well as to present the results obtained.
Case report
Following the patient's signature on an Informed Consent Form (ICF), this case was submitted and approved by the Research Ethics Committee of UPF (CEP/UPF) (Opinion number 5.785.215).
A 21-year-old female patient, of fair complexion, sought dental care for the evaluation and possible treatment of a gummy smile. She had aesthetic concerns regarding gum exposure when smiling and wished to improve this aspect.
During the anamnesis, the patient reported not having any drug allergies or significant health problems. She had previously undergone local anesthesia without any complications (ASA Classification I).
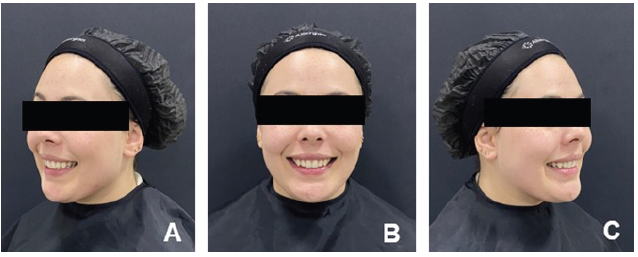
In the same session, photographs were taken, and facial analysis was conducted (Figure 1). The patient exhibited a facial pattern type I, with no apparent scars on the facial region and a Fitzpatrick skin phototype type II. Facial propor- tions were measured, with the upper third measuring 6.5cm, the middle third 6.5cm, and the lower third 7cm.
The gummy smile was determined to be mixed, and three points for the application of BTX Type A (Botox-Allergan Aesthetics Brazil-Guarul- hos, SP) were established (Figure 2).
After proper dilution of the product, 2 IU of toxin were injected at each point, with half a needle introduced and applied slowly, totaling 6 IU. During the first session, complete resolution of the patient's gummy smile was observed, with the dose used and treatment being effective immediately.
With this in mind, a post-operative follow-up of 90 days was proposed to assess the effectiveness and stability of the therapeutic effects achie- ved. Figure 3 shows the appearance of the smile 30 days after the first session, with noticeable positive results and no evident changes. Figure 4 shows the clinical appearance after 60 days, still without significant alterations.
Figure 5 shows the clinical appearance of the smile 90 days after the first session, with no significant changes observed in the measure- ments. Therefore, the results obtained in the first session remained stable.
It should be noted that the patient had a 5 mm gummy smile initially, and in 90 days, there was no return to gingival exposure at those levels or clinically visible alterations.
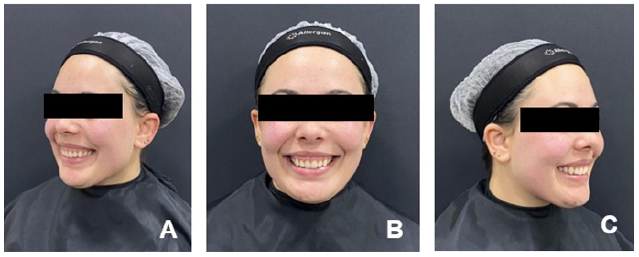
Figure 1 Initial clinical appearance of the smile during the anamnesis A. Left profile B. Frontal C. Right profile.
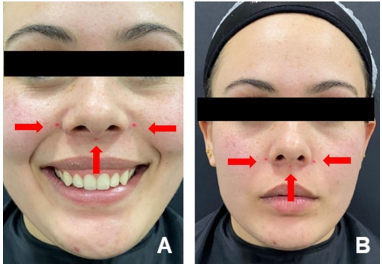
Figure 2 Three injection points for the toxin (red spheres): central region, at the point of greatest contraction of the nasolabial sulcus, and two other points lateral to the wing of the nose.
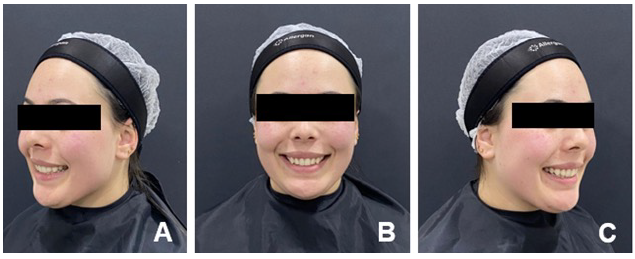
Figure 3 Clinical appearance of the smile 30 days after the procedure: A. Left profile; B. Frontal; C. Right profile.
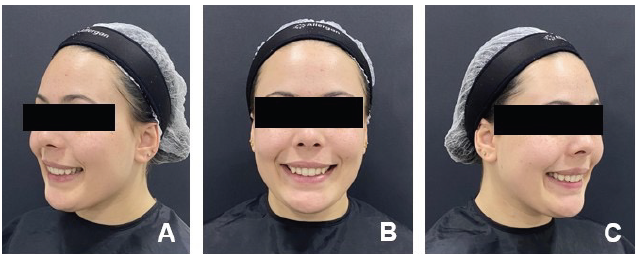
Figure 4 Clinical appearance of the smile 60 days after the procedure: A. Left profile; B. Frontal; C. Right profile.
Discussion
The excessive exposure of gingival tissue when smiling is a common concern among dental patients and is often seen as an aesthetic issue
(14). The patient in this case had an initial gingival exposure of 5mm, with the etiological factor diagnosed clinically, attributed to the hyperfunction of the upper lip.
BTX has been widely used in the control and treatment of muscle hyperfunction, not only in the aesthetic field but also in cases of temporomandibular disorders (15). Application points lateral to the nasal wing can be used in the conventional technique for treating anterior gummy smile. In cases of posterior gummy smile, the product should be applied at the point of greatest contraction of the nasolabial sulcus during smiling and 2cm laterally to the first point. In cases of mixed gummy smile, application should be performed at all points (13).
The use of BTX following the Yonsei point technique involves three muscles: the levator labii superioris and ala of the nose, the levator labii superioris, and the zygomatic minor. This technique represents a safe and effective point for the application of BTX type A, with no complications observed (16). The patient in this case had a mixed gummy smile, with extensive gum exposure when smiling, greater than 3mm. Therefore, the proposed treatment was to perform BTX type A injections at three points: two lateral to the nose wing and one midpoint in the nasolabial sulcus.
Razmaite and Trakiniene (2021) reviewed the main points of BTX application in the treatment of gummy smiles, highlighting these points as they pass through the major target muscles of the techniques described in the literature: the levator labii superioris, the levator labii superioris and ala of the nose, and the zygomatic minor (17). Similarly to what was done in this study, Mazzuco and Hexsel (2010) also recommend the application of BTX at a central point in the nasolabial sulcus in cases of posterior or mixed gummy smiles (13).
The average units for bilateral application to the nose wings range from 4 to 6 IU, with the severity of the gummy smile being the main factor in determining the required quantity for treatment, and in extreme cases justifying a slight increase in dosage (14). In the case discussed in this study, an average of 2 IU of BTX type A was used at each of the three points, totaling 6 IU required to resolve the case.
The patient in the described case remained under postoperative follow-up, with visible stability of the results up to 90 days after the procedure. The reversibility of this technique remains a disadvantage, with a duration of 4 to 6 months being the observed average for the effects of type A BTX treatments. The stability of BTX results in cases of gummy smile is up to 8 weeks, with no complete return to initial measurements within 12 weeks (18), which aligns with the results observed in the clinical case presented in this study (90 days).
It is of utmost importance to emphasize that the treatment in question has temporary results, and the final outcome achieved may vary from patient to patient. However, its safety, reversibility, and the fact that it is a less invasive procedure are still the main advantages of this technique. Therefore, it is suggested that further clinical studies with longer follow-up periods be conducted to progressively gain a better understanding of the longterm effects of BTX application in the treatment of gummy smile.
Conclusion
In light of the case presented, it can be concluded that the application of BTX in the treatment of gummy smile is a safe and easily executable technique for the patient. The obtained results were satisfactory both from a professional perspective and from the patient's personal perspective, restoring aesthetics and well-being.
Right to privacy and informed consent
The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is is in possession of this document.
Protection of human and animal subjects
The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data
The authors declare that they have followed their work center protocols on access to patient data and for its publication.
Author contribution statement
Conceptualization and design: A.K.D., F.G.D., and J.C.M.
Literature review: A.K.D., and J.C.M.
Methodology and Validation: P.N., and F.G.D.
Formal analysis: A.K.D., and J.P.D.C.
Investigation and data collection: A.K.D., P.R.M.S., and M.C.S.F.
Resources: A.K.D.
Data analysis and interpretation: P.N., P.R.M.S., and M.C.S.F.
Writing-original draft preparation: M.S.T., and J.P.D.C.
Writing-review & editing: F.G.D
Supervision: M.S.T., E.D., and J.P.D.C.
Project administration: A.K.D., M.S.T., E.D., and J.P.D.C.
Funding acquisition: A.K.D.