Introduction
Cancer is a leading cause of death in the world. Despite synthetic drugs used in current therapies have improved patient prognosis, the toxicity and development of secondary resistance remain a serious concern for researchers (Qazi et al., 2018). It is generally accepted that plant-derived products provide health-related benefits, specifically for the prevention and treatment of several diseases. Crude herbal or botanical preparations have shown promising utility for chronic illnesses such as oncologic disorders, diabetes, heart diseases, and neurodegenerative processes, among others. The herbal bioactive compounds can provoke changes in plasma nutrient availability, therefore, in the cell microenvironment. By this mode of action, botanicals became a valuable source of anticancer compounds, which can affect tumor growth by modifications induced in antitumor immune response, cancer cell proliferation, its survival, and spread (Kanarek et al., 2020). The mentioned properties are supported by numerous preclinical studies, which provide ample evidence that botanicals regulate multiple cancer hallmark pathways, including cell cycle, apoptosis, angiogenesis, invasion, and metastasis (Puccinelli & Stan, 2017).
In addition, these natural compounds can also interfere with the early stages of tumor development, acting as chemopreventive agents (Butt et al., 2013). Carcinogenesis is a biological process hallmarked by its diffuse and multifocal presence, with high statistical chances to progress to malignancy, alterated DNA synthesis, and changes in cell protection mechanisms (Ryan & Faupel-Badger, 2016). This process, in which the normal cell is transformed into a cancer cell, is commonly divided into 3 different stages: initiation, promotion, and progression (Weinstein et al., 1984). Among an increased list of chemical and environmental carcinogens, inflammation is a factor widely related to neoplastic transformation. In the interplay between inflammation and cancer, reactive oxygen/nitrogen species, cytokines and prostaglandins act as promotors of carcinogenesis by induction of DNA damage. The interference with the inflammatory process and its intermediaries will reduce the tumor transformation, neoplastic progression, and the development of metastases and recurrences (Piotrowski et al., 2020). For this reason, anti-inflammatory and anti-oxidant properties of botanic extracts could represent a crucial aid for the prevention of carcinogenesis mediated by the inflammatory processes (Serrano et al., 2018).
The plant kingdom represents an endless supply of bioactive compounds with potential activity to control diseases. In particular, the Asteraceae family includes more than 20 thousand species around the world and is considered the most evolved botanical family due to the floral structure and its chemical composition. In America, it is within the most important families of plants in relation to the number of species reported with medicinal properties (Thomas et al., 2009). Tessaria (Ruiz & Pavon, 1753) is a genus of the Asteraceae family, sometimes considered as Pluchea, composed of up to 17 proposed species. It is distributed from the southwest of the United States to Argentina; including Brazil, Bolivia, Chile, Colombia, Costa Rica, Ecuador, Panamá, Paraguay, Perú, Uruguay, and Venezuela (Tropicos.org, 1982). Currently, there are only 5 accepted and confirmed species: T. absinthioides (Hook. & Arn.) DC.; T. ambigua DC.; T. dodoneifolia (Hook. & Arn.) Cabrera; T. fastigiata (Griseb.) Cabrera and T. integrifolia Ruiz & Pav (The Plant List, 2013). All these species are reported in the bibliography by their health care implications and, often, scientific studies demonstrate novel valuable biological properties. Because of the mentioned antecedents, considering the Tessaria species as undervalued within the oncologic field, the goal of the current systematic review is to summarize the available genus information relevant to cancer research and treatment.
Material and Methods
A literature search on the Tessaria genus was performed to identify texts describing species with ethnopharmacological reports of use and other papers related to its scientific studies. The current review includes 77 references selected from websites (The plant list and Tropicos.org) and scientific databases as PubMed, Science Direct, SciELO, Google Scholar, LILACS, and Library Genesis. The main descriptors used were Tessaria and the constitutive genus species (especially, T. absinthioides, T. ambigua, T. dodoneifolia, T. fastigiata, and T. integrifolia), cancer, cytotoxicity, antitumoral, carcinogenesis, tumor growth, metastasis, angiogenesis, and, finally, other words related to the chemical characterization as phytochemicals, phenolic compounds, and all the specific compounds mentioned in Table 3. Altogether, more than 4500 articles and documents were reviewed.
Distribution and Ethnopharmacological Uses of Tessaria spp.
Cancer is a group of diseases that represent a worldwide problem and, often, conventional therapy is limited by the cost and side effects of used drugs. With few exceptions, folk medicine has not reported information about cancer diagnosis and treatment. Because of this, exploring plants with registered ethnobotanical properties resulted an important strategy to find effective natural products for oncologic purposes. For this reason, during the last decades, many plants with reports of ethnomedicinal use were studied to develop anticancer plant-based drugs with improved potency and better tolerance by the patients (Tariq et al., 2017).
Because the natural distribution of Tessaria spp. includes regions and cultures of Mesoamerica and South America, the ethnopharmacological properties of the species are widely registered (Table 1). Among others, there are reports of biological actions related to inflammation, cell proliferation, immune system response, and liver and kidney protection. Because of the relation between these properties and the tumor growth or treatment toxicity, the mentioned attributes make the genus a valuable candidate for plant-based cancer research.
Table 1 Ethnopharmacological Information of Tessaria Tessaria spp.
| Species | Popular names | Ethnopharmacological uses | Product | Geographical distribution | Ref. |
| T.absinthioides | Pájaro bobo, chilca, suncho | Hypocholesterolemiant, | Dried leaves infusion | Argentina | Barboza et al., 2009 |
| rosado, suncho negro, brea | balsamic, expectorant | ||||
| Sorona, brea | Diabetes | Leaves infusion | Chile | Madaleno & Delatorre Herrera, 2013- | |
| Pájaro bobo, suncho negro | Empacho (digestive disorder) | Leaves infusion | Argentina | Campos-Navarro & Scarpa2013, | |
| Pájaro bobo, Sorona, hierba | Rheumatism, prostate | Leaves infusion | Perú, Bolivia, Chile, | Torres-Carro et al., 2017 | |
| de zorra | illness, cancer, | Argentina | |||
| T.ambigua | Pájaro bovo | Antitussive, hepatic, tonic, | Part not specified | Argentina | Barboza et al., 2009 |
| depurative, laxative | |||||
| T.dodonefolia | hierba dulce, ka´a he´ê | sweetening | Young shoots | Paraguay | Nanayakkara et al., 1988 |
| Chilca dulce,suncho, chilca, | abortifacient, vaginal | Leaf | Argentina | Barboza et al., 2009 | |
| chilca negra, suncho negro | mycosis, anuria, urin with | ||||
| blood, emmenagogue | |||||
| T.fastigiata | Uri uri | antiinfflammatory | Part not specified | Bolivia | Parejo et al., 2003 |
| T.integrifolia | Pájaro bobo | hepatic and renal insufficiency, hepatitis. | Leaves | Perú | Feo et al., 1990 |
| Aliso del río, aliso, aliso | Diuretic, asthma, febrifuge, | Aereal parts infusion | Argentina | Barboza et al., 2009 | |
| bobo, bobo, buibé, pájaro | astringent, cicatrizant | ||||
| bobo, palo bobo | Antigonorrheal, | Leaf and flowers | Argentina, Peru | Barboza et al., 2009, Peluso, et al., 1995 | |
| antiallergic,antiasthmatic, | |||||
| antiinflammatory, diuretic | |||||
| --- | Malnutrición | All plant | Bolivia | Feo et al., 1990 | |
| pájaro bobo, huapariu, tseco | asthma, antipiretic, | Part not specified | Perú | Silva-Correa et al., 2018 | |
| antiinflammatory, diuretic |
Note: derived from research.
Reported Biomedical Properties Of Tessaria Species Different From Cancer
Considering that cancer is a very heterogeneous group of diseases, it is not possible to predict when future discoveries will report other activities of Tessaria spp. relevant for oncology. For this reason, Table 2 summarizes the available information about other biomedical, scientifically tested properties of the genus.
Antioncologic Effects Of Tessaria spp.
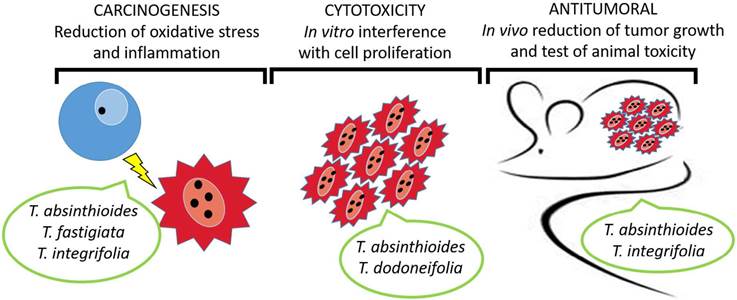
Cancer is caused by a multistep process that results in uncontrolled cell proliferation. The available information about Tessaria species effects concerning cancer establishment and progression is schematized in Figure 1. To the present, only 4 species have been scientifically studied for biological activities with oncological relevance; in these cases, the natural compounds demonstrated anticarcinogenic, cytotoxic, and antitumoral properties. According to our knowledge, T. ambigua has no previous reports of biological properties related to cancer.
The Role of the Tessaria Genus in Carcinogenesis
In some opportunities, the carcinogenesis process is driven by oxidative stress and inflammation, which determines the aberrant gene expression of tumor cells and those sites surrounding the lesion (Lechner & Stoner, 2019). As with other plants, Tessaria species are a source of phenolic compounds reported as natural antioxidants with clear inhibitory effects of carcinogenesis.
T. absinthioides is the most studied specimen because of its anti-inflammatory and antioxidant properties. Torres Carro et al. (2015; 2017) evidenced the capability of the hydromethanolic extracts obtained from the plant aerial parts to interfere with the inflammation process. Extracts act by a reduction in
Table 2 Biomedical properties of Tessaria spp. not cancer related.
| Specie | Activity | Plant source | Cite |
|---|---|---|---|
| T.abs. | Insecticidal and repelent | Sesquiterpenes from aerial parts. | García et al., 2003; García et al. 2017 |
| Virucidal | Essential oils from leaves. | García et al., 2003; | |
| Organic extract (dichloromethane: methanol) | Visintini Jaime et al., 2013 | ||
| Gastric cytoprotection | Sesquiterpenes from aerial parts. | Donadel et al., 2005 | |
| Antibacterial | Methanolic extract from leaves | Romero et al., 2016 | |
| Hypoglycemic and antiatherogenic | Aqueous extract from leaves | Quesada et al., 2021 | |
| T.amb. | Insecticidal | Penduletin from aerial parts | Sosa et al., 2000 |
| T.dod. | Antifungal | Flavonones from aerial parts | Soberón et al., 2020 |
| T.fas. | Antiasthmatic, analgesic, immunomodulatory | Casticin from leaves | Chan et al., 2018 |
| T.int. | Antispasmodic | Aqueous extract from aerial parts | Silva-Correa, 2011 |
| Gastric cytoprotection | Ethanolic extract from leaves | Correa et al., 2014 | |
| Leishmanicidal | Sesquiterpenes from leaves | Silva-Correa et al., 2018 |
T. abs.: T. absinthioides; T. amb.: T. ambigua; T. dod.: T. dodoneifolia; T. fas.: T. fastigiata; T. int.: T. integrifolia.
Note: derived from research.
the activity of the pro-inflammatory enzymes lipoxygenase (LOX), cyclooxygenase (COX2), secretory phospholipase A2 (PLA2), and hyaluronidase. Moreover, diminished production of nitric oxide (NO) by a reduction in the activity of nitric oxide synthase enzyme (iNOS) and the stabilization of human red blood cells membrane was also demonstrated. In the same studies, the antioxidant capability of hydromethanolic extract was established by determining the iron-chelating capacity and the 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging effect. Later, Gómez et al. (2019) confirmed the antioxidant power of Argentinean and Chilean T. absinthioides decoctions by 2,2 diphenyl picrylhydrazyl (DPPH) scavenging activity, ferric reducing antioxidant power assay (FRAP), Trolox Equivalent Antioxidant Activity (TEAC), and reduction of lipid peroxidation in human erythrocytes. The above-mentioned biological properties were attributed to the presence of caffeoylquinic acid derivatives, as well as vanillic acid, protocatechuic, taxifolin, chlorogenic acid, quercetin, and rutin, among other phenolic compounds evidenced in the extracts.
In T. integrifolia, the antioxidant and anti-inflammatory properties were demonstrated by studying changes in the migration and the superoxide anion secretion of activated human macrophages. In this work, the biological properties were also related to the caffeoylquinic acid present in the aerial parts of the plant (Peluso et al., 1995). Later, Ono et al. (2000) described the antioxidants effects of eudesmane derivatives present in the methanolic extract obtained from leaves, evidenced by the ferric thiocyanate method and DPPH scavenging assay.
T. fastigiata is the other species in the genus reported as an antioxidant. The unique available study used DPPH scavenging activity, nitroblue tetrazolium (NBT)/hypoxanthine superoxide assay, and the hydroxyl radical scavenging activity to evidence the biological activity attributable to the phenolic compounds present in the leaves hydromethanolic extract.
Altogether, the presented evidence makes Tessaria plants a very important source of natural compounds to prevent biological oxidations and inflammation, reducing the impact of both key factors of carcinogenesis.
Cytotoxicity of Tessaria Genus Against in Vitro Cells
In recent times, an important scientific effort was focused on discovering novel, effective, and affordable anticancer agents from natural sources. Because of this, a large number of botanicals have been explored for their cytotoxic potential against in vitro models of cancer cells (Dehelean et al., 2021). In this section, the available evidence of cytotoxicity is analyzed in relation to the capability of make interference in cancer cell metabolism, the inhibition of cell proliferation, and the cell death induction.
Between the reviewed Tessaria species, the evidence clearly indicates that botanicals exert a selective cytotoxic effect against cancer cells. By these results it was demonstrated that the treatment with natural compounds of normal, non-tumoral cells induces lower or null toxicity; in contrast, when cancer cell lines were assayed, the cytotoxic effects were potent, similar to those evidenced by the conventional chemotherapeutic drugs used as a positive control.
Persia et al. (2017) demonstrated the selective cytotoxic effects of T. absinthioides leaves aqueous extract. The study reported a dose-response effect on HeLa (cervix cancer), Gli-36 (glioblastoma), HCT-116 (colorectal cancer) and MCF-7 (breast cancer) human cancer cell lines. The extract potency, measured as CV50 (50% of cell viability) in all cases, was similar to 5-fluoracile, a chemotherapic agent used as a positive control. Interestingly, in the same study and conditions, the cytotoxicity determined by the extract on non-tumoral HBL100 cell line was significantly lower in relation to the other cancer cell lines tested; also, in these cell lines, the measured toxicity was notably diminished in relation to the effect induced by 5-fluoracile.
In another study, eudesmane semi-synthetic derivatives from T. absinthioides affected the proliferation of A2780 (ovarian), HeLa (cervix), SW1573 (non-small cell lung), T47D (breast) and WiDr (colorectal) human solid tumor cell lines. In a dose-response experimental design, the treatment determined metabolic cytotoxicity (measured by SRB assay) and cell cycle arrest in G2/M phase. In the study, 5-fluoracil was used as positive control, and its potency, measured as GI50 (50% of growth inhibition), was always lower than the plant-derived compound (León et al., 2009).
There are, at least, other 3 works of in vitro studies related to the cytotoxicity of Tessaria´s compounds. In the course of these studies, not directly related to cancer research, were tested caffeoylquinic derivatives and flavonoids from T. absinthioides (Torres Carro et al., 2015), caffeoylquinic derivatives from T. integrifolia (Peluso et al., 1995) and the ethanolic extract of T. dodoneifolia with content of naringenin and pinocembrin (Soberón et al., 2020). In all of these studies was reported none or slight toxicity induced by treatment on cultured non-tumoral murine macrophages and human peripheral blood lymphocytes (PBL).
The presented evidence in this section for the Tessaria derived compounds coincides with the observation of the selective cytotoxicity against cancer cells, originally proposed by Persia et al. (2017).
Preclinical Evidence AboutTessaria sp. Antitumoral Effects
Often, the therapeutic potential of some natural products is limited by the presence of xenobiotics’ effects. In other cases, the in vitro evidenced cytotoxic properties cannot be reproduced in vivo because of bioavailability limitations of the bioactive phytoconstituents (Piroozmand et al., 2020). In the case of T. absinthioides, both limitations were analyzed on the aqueous extract by the study of oral toxicity and by the determination of its antitumoral effects against colorectal-induced cancer. The T. absinthoides aqueous extract oral toxicity was tested and discarded in males and females of Sprague Dawley rats. At doses up to 2000 mg kg-1, a single administration of T. absinthioides did not determine acute toxic effects. No animals died immediately or within 14 days after administration and were not evidenced changes of body weight nor other clinical signs of toxicity. After euthanasia, necropsy did not evidence changes on tissues or organs. On the other hand, the study of 28 oral repeated doses up to 1000 mg kg-1 d-1 did not show toxic evidence either. After administration, no significant changes were registered in body weight, organs weight or organs histological appearance. Neither changes were present in blood cell counting nor blood serum biochemistry (Persia et al., 2017).
T. integrifolia inflorescences infusion was also tested by its oral toxicity in the 28 days repeated doses experimental design. By the use of Rattus norvegicus var. albina, the study concluded that no significant toxic effects were observed in the males or females analyzed. After administration of 500 mg kg-1 day-1 doses, the histopathologic analysis showed neither cell damage nor necrosis in the liver, lungs, stomach, brain, ovary or testis. Only a mild to moderate glomerular congestion was evidenced and was attributable to the sesquiterpene lactones present in the plant sample. In conclusion, the study demonstrated that there was not significant toxicity determined by oral administration of T. integrifolia during 28 days and for the long term administration of the infusion, specific studies need to be performed to discard potential kidney damages (Julián Dávalos & Vásquez Muñoz, 2016).
About the in vivo antitumoral effects, T. absinthioides aqueous extract was tested in a colorectal cancer model induced by dimethylhydracine (DMH). In BALC/c mice, the oral administration of 300 mg animal-1 day-1 significantly increased the median survival of animals. While the median survival in Tessaria treated animals was 30 weeks, in the untreated group survival was significantly lower (24 weeks). It is important to note that 5-fluoracil was used as a positive control drug; in this group, the median survival was 27 weeks. In spite of the fact that no statistical differences were observed between the survival of Tessaria and 5-fluoracil groups, the in vivo results confirm the similar potency evidenced by both compounds in vitro. To finish, animals treated with Tessaria did not evidence toxic symptoms related to the oral administration of the extract during the entire assay (up to 38 weeks) (Persia et al., 2017).
Only preliminary evidence exists about T. absinthioides efficiency against in situ and metastatic murine syngeneic melanomas (personal observations); until now, no other Tessaria species were reported in the bibliography by its antitumoral properties.
Tessaria Genus as the Origin of Anticancer Phytochemicals
The phytochemicals are bioactive non-nutrient vegetal compounds that have health-related effects. More than 5,000 phytochemicals have been identified; if well, their health benefits are still to be fully understood. Several studies have strongly demonstrated that phytochemicals have many different mechanisms of action related to cancer (Liu, 2004).
Table 3 Tessaria spp. Phytochemicals and Its Anticancer Properties.
| Phytochemical | Source | Anticancer effect by target… | Ref | |||
| carcinogenesis | tumor growth | metastasis | angiogenesis | |||
| Amyrin | T. amb. | yes | Wen et al., 2018 | |||
| Artemisinin | T. abs. | yes | yes | yes | Slezakova & Ruda-Kucerova, 2017 | |
| Caffeoylquinic acid and derivatives | T. abs. | yes | yes | In et al., 2016 | ||
| Caryophyllene oxide | T. abs. | yes | yes | Fidyt et al., 2016 | ||
| Casticin | T. abs. | yes | yes | yes | yes | Ramchandani et al., 2020 |
| Chrysosphanol | T. int. | yes | yes | yes | Hsu et al., 2020 | |
| Citric acid | T. abs. | yes | Ying et al., 2013 | |||
| Enoic acid derivatives | T. abs. | yes | yes | Oliveira et al., 2018 | ||
| Eriodictyol | T. dod. | yes | yes | yes | Li et al., 2020 | |
| Eudesmane derivatives | T. abs. | yes | yes | yes | yes | Liang et al., 2017 |
| Eudesmol (gamma) | T. abs. | yes | Furtado et al., 2018 | |||
| Eugenol | T. int. | yes | Fathy et al., 2019 | |||
| Eupatorin | T. abs. | yes | yes | yes | Razak et al., 2019 | |
| Gallic acid | T. int. | yes | Rezaei-Seresht et al., 2019 | |||
| Ginnalin A | T. abs. | yes | yes | Bi et al., 2018 | ||
| Gurjunene (alfa) | T. abs. | yes | yes | Yongram et al., 2019 | ||
| Hyperyn | T. int. | yes | Li et al., 2012 | |||
| Ilicic acid | T. abs. | yes | León et al., 2009 | |||
| Linalool | T. int. | yes | Pan & Zhang, 2019 | |||
| Mannoheptulose | T. abs. | yes | Board, et al., 1995 | |||
| Naringenin | T. dod. | yes | yes | yes | yes | Joshi, et al., 2018 |
| Pinoresinol | T. int. | yes | yes | Ning et al., 2019 | ||
| Protocathechuic | T. abs. | yes | yes | Deng et al., 2020 | ||
| Quercetin | T. dod. | yes | yes | yes | yes | Tang et al., 2020 |
| Rhamnetin | T. abs. | yes | yes | Lan et al., 2019 | ||
| Sakuranetin | T. dod. | yes | yes | Stompor, 2020 | ||
| Taxifolin | T. abs. | yes | yes | yes | Wang et al., 2020 | |
| Terpinen-4-ol | T. abs. | yes | Shapira et al., 2016 | |||
| Tessaric acid and derivatives | T. abs. | yes | León et al., 2009 | |||
| Trifolin | T. int. | yes | Kim et al., 2016 | |||
| Vanillic acid | T. abs. | yes | yes | yes | Gong et al., 2019 | |
T. abs.: T. absinthioides; T. amb.: T. ambigua; T. dod.: T. dodoneifolia; T. fas.: T. fastigiata; T. int.: T. integrifolia
Note: derived from research.
Table 3 presents the phytochemicals derived from Tessaria spp. with reported anticancer actions. Until now, the 5 confirmed species of the genus (T. absinthioides, T. ambigua, T. dodonaeifolia, T. fastigiata, and T. integrifolia), were chemically analyzed and their phytochemical constituents described (Torres-Carro et al., 2017; García et al., 2003a; Gómez et al., 2019; Ono et al., 2007; Guerreiro et al., 1990; Bailac et al., 1998; Caballero Palacios, 2014). By these studies, more than 30 phenolic compounds were identified in Tessaria with reported anticancer efficacy, including sesquiterpenes, flavonoids, phenolic acids, and lignans. All of these chemicals affect tumor growth, modifying proliferation or viability; while 15 compounds interfere with carcinogenesis, 10 reduce the metastasis process, and 8 decrease angiogenesis.
The above-mentioned phytochemicals and their scientifically proven effects make the genus Tessaria a valuable source of natural compounds for future cancer research and treatment.
Conclusions
Botanicals, nutraceuticals, and herbals are plant-derived materials with medical benefits that aim for disease prevention or treatment. They represent a particular promise for cancer prevention due to their efficacy and safety profile. The wide chemical diversity features together with available epidemiological, preclinical, and clinical studies suggest an undeniable role of natural products in various approaches related to cancer prevention and treatment. Many of these natural compounds are responsible for antioxidant, anti-inflammatory, chemopreventive, and anticancer activities.
Some botanical constituents as polyphenols, phytoalexins, carotenoids, and flavonoids are specifically related to the expression and activity of multiple proteins such as epidermal growth factor receptor (EGFR), nuclear factor-kappa B (NF-κB), tumor necrosis factor-alpha (TNF-α), protein kinase B (PKB/AKT), mitogen-activated protein kinase (MAPK) and p53, between others targets. By the modification of these molecular targets, botanicals play a critical role in signal transduction pathways related to carcinogenesis, cell proliferation inhibition, invasion, and angiogenesis (Rahman et al., 2010).
Due to these important properties, it is urgent to perform a scientific validation of regional medicinal plants to precise their toxicological and pharmacological profiles with the goal of ensuring both the effectiveness and the safety in the use of ethnobotanicals. This information is highly valuable and necessary to improve the therapeutic approach of pathologies with unsatisfactory or toxic treatments, mainly cancer. In this field, the botanical complementary treatments rise as a promissory area to improve the potency of available therapies and/or reduce their toxic collateral effects.
The present review systematically summarizes the information available for the Tessaria genus related to cancer research and treatment. Then, it is imperative to move forward to complete the preclinical evidence related to its molecular mechanistic mode of action, pharmaceutical presentation and standardization, and the study of pharmacological interactions with current chemotherapeutics. In accordance with the presented evidence, based on its ethnopharmacological reports, biomedical explored properties, and phytochemical composition, it is possible to affirm that Tessaria spp represent a promissory source of botanicals for oncologic purposes, especially in the complementary treatment approach. To conclude, based on the folkloric reports of uses and the recently validated scientific information, the present revision intends to encourage new and deep research destined to promote the Tessaria derived botanicals as anticancer compounds.