Introduction
Non-invasive teeth whitening is well-known procedure, used to enhance tooth appearance and color (1). Tooth color is influenced by its intrinsic color, stains deposited within its bulk, and extrinsic stains formed on its surface (2). Enamel tissue is translucent and doesn’t completely obscure the underlying dentin color, determining the final tooth color (3).
Tooth whitening agents assist in improving the overall tooth whiteness by two approaches. First, changing teeth intrinsic color by using hydrogen or carbamide peroxides. These agents diffuse into the teeth and interact with stain molecules, leading to alteration in teeth structure surface, reflecting the light differently and giving whiter teeth (1,4). Meanwhile, the use of bleaching components remains highly controversial according to safety. Several studies documented alterations in enamel surface morphology, mineral content, roughness and microhardness in teeth subjected to bleaching (5,6,7).
The second approach is removing the extrinsic stains formed on teeth surface completely by the abrasive action of the toothpastes. Whitening toothpastes are designed with enhanced chemical and physical cleaning potentials. They include ingredients as variation of abrasive components, enzymes and phosphate salts, which are designed to remove the extrinsic stains and control their formation (8). Stain removal effectiveness is significantly influenced by size, hardness and quantity of the abrasive agents, beside to the amount of toothpaste used (9,10,11). Antitartar agents that are usually added to toothpastes, can bind with dental pellicles, preventing stains attached to salivary proteins from adhering to the enamel surface (10). Additionally, charcoal has high surface area and porosity that absorbs the extrinsic stains formed on the teeth in its pores then brushed away, resulting in tooth color change (12).
Lately, there is an increasing attention among the individuals to have bright smile. This results in higher use of the easily accessible whitening toothpastes. However, certain whitening toothpastes may exert negative influence on dental hard tissues (13). Thus, the aim of this study was to have updated knowledge concerning the effect of different whitening toothpastes on the enamel surface morphology, chemical profile and their whitening efficiency.
Materials and methods
Ethical aspect
This study was validated by “Research Ethics Committee” Faculty of Dentistry, Ain Shams University, Cairo, Egypt with number: (FDASU-Rec IR032312).
Sample sizw calculation
The reference values were obtained from earlier study (14), using G*Power software 3.1.9.7. The input parameters were 0.4881 effect size, 0.8 power, and 0.05 (α err prob) according to one-way ANOVA power analysis. The calculated total sample size was 60 teeth.
Teeth collection
Sixty human permanent maxillary incisors were collected from Oral surgery Department, Faculty of Dentistry, Ain-Shams University with informed consents obtained from donors. The teeth used in the study were inspected under the stereomicroscope (Olympus SZ40, Japan) at 10x magnification to ensure that the teeth were free of crack lines, restorations, cavities, hypoplasia, white spot or abnormal enamel surfaces (14).
Experimental desing
The 60 teeth were randomly divided into 5 groups (12 teeth/group) as following:
Gp I: Control teeth were brushed only with no toothpaste application.
Gp II: Teeth were brushed with Pearl-based whitening toothpaste. “Crest 3D White Brilliance with Pearl Glow”.
Gp III: Teeth were brushed with Charcoal-based whitening toothpaste “Crest 3D White with Charcoal”.
Gp IV: Teeth were brushed with Alumina-based whitening toothpaste “ZACT Stain fighter”.
Gp V: Teeth were brushed with Sodium bicarbo- nate salt and lemon-based whitening toothpaste. “Dabur Herb’l Salt & Lemon, fluoride free”.
The ingredients of the tested whitening toothpastes are presented in (Table 1).
Brushing procedure
Each tooth was brushed with bean sized whitening toothpaste wetted with distilled water twice daily (1 min each) by using standard electro- nic soft hair toothbrush for four weeks, except Group I which brushed with no toothpaste. During the entire experiment, all teeth were kept in 37°C artificial saliva, that was renewed daily (15,16).
The morphology, chemical profile and color of each tooth were investigated at the middle of the middle third of its labial enamel surface.
Enamel surface morphology examination
Teeth were mounted on the metal holder of FEI/Quanta FEG 250 scanning electron microscope (SEM), attached with energy dispersive X-ray analyzer (EDXA) (Holland), (SEM-EDXA unit at Desert Research Center, Egypt), where all teeth were investigated by using circular back scattered detector (CBS) at 20 KV with spot size 3.5 nm. Scanning electron micrographs were acquired at magnifications x1000 and x5000 (17).
Chemical profile analysis
For chemical profile analysis of all teeth, labial enamel surface Calcium (Ca) and Phosphorous (P) weight % (wt%) as well as Ca/P ratio were determined using EDXA with S-UTW detector, count rate (1800-2000 counts/second) at resolution 135.8 eV. Five points were randomly selected and measured in each tooth at magnification x1000 (18).
Colorimetric evaluation
Evaluation of tooth color was implemented by using double-beam Agilent Cary 5000 UV-Vis-NIR spectrophotometer (Agilent Technologies, USA) at National Institute of Standards, Egypt, with spectral relative irradiance of CIE D65 standard illuminant and 10° standard observer. The obtained spectral reflectance curves data of the visual spectrum (380-780 nm) were converted to L*, a*, b*, which are units of the uniform CIE L* a* b* color space. Three measurements for each parameter/tooth were done and the mean value was used. L*, a* and b* initial values (I) of each specimen were taken from the middle of the middle third as baseline data. Then, after four weeks of tooth brushing, final values (F) were recorded from the same location of each specimen. Tooth color changes (ΔE) were calculated by using the following equations: ΔL*= L*F - L*I, Δa* = a*F - a*I, Δb* = b*F - b*I , ΔΕ = (ΔL*2 + Δa*2 + Δb*2)1/2 (19).
Moreover, whitening index (WID) was calculated before and after tooth brushing as following: WID(I)= 0.511L*I − 2.324a*I − 1.100b*I, WID(F)=0.511L*F − 2.324a*F − 1.100b*F, Whiteness change (ΔWID) = WID(F) - WID(I) (20).
Perception of tooth color and whiteness change was assessed by two thresholds: perceptibility threshold (PT) of ΔΕ was set at 1.2 and ΔWID at 0.72, and acceptability threshold (AT) of ΔΕ was set at 2.7 and ΔWID at 2.60 (12,19,20,21,22).
Statiscal analysis
EDXA chemical profile results and color measurements data were explored for normality using Kolmogorov-Smirnov and Shapiro-Wilk Test. One‐way ANOVA was performed, followed by Tukey's post hoc test. Confidence interval: 95%, margin of error accepted: 5%, and P-value <0.05 was considered significant.
Table 1 Brand names and ingredients of the tested whitening toothpastes.
| Brand Name | Manufacturer | Ingredients |
|---|---|---|
| Crest 3D White Brilliance with Pearl Glow | Procter & Gamble Manufacturing, Germany | Pearl powder, Hydrated Silica, Glycerin, Sodium Hexametaphosphate, Aqua, PEG-6, Aroma, Mica, Trisodium phosphate, Sodium Lauryl Sulfate, citric acid, Xanthan Gum, Sucralose, CI 77891, Sodium Benzoate, Sodium Hydroxide, Sodium citrate, Potassium Sorbate, Sodium Fluoride (1450 ppm). |
| Crest 3D White with Charcoal | Procter & Gamble Manufacturing, Germany | Charcoal powder, Hydrated Silica, Aqua, Mica, Sorbitol, Disodium Pyrophosphate, Sodium Lauryl Sulfate, Aroma, Sodium Hydroxide, Cellulose Gum, CI 77891, Limonene, Sodium Fluoride (1450 ppm). |
| ZACT Stain fighter | LION, Indonesia | Alumina, Silica, Calcium Carbonate, Sorbitol, Water, PEG-8, Sodium Lauryl Sulfate, Flavor, Xanthan Gum, Butylparaben, Zinc Oxide, Sodium Benzoate and Caprylic/ Capric Triglyceride and Sodium Monofluorophosphate (1100 ppm). |
| Dabur Herb’l Salt & Lemon, fluoride free | Dabur Ltd, Egypt | Sodium bicarbonate (Salt), Silica, Lemon Extract, Enzyme, Sorbitol, aqua, Glycerin, Polyethylene glycol, Sodium Lauryl Sulfate, Titanium dioxide, Tetra sodium pyrophosphate, Persica extract, Citric acid, Flavor, Sodium Carboxy Methyl Cellulose, Carrageenan, Trisodium Orthophosphate, Sodium Saccharin, Methyl paraben, Propyl paraben. |
Results
Surface morphological results
Control group (Gp I)
Scanning electron micrographs demonstrated relatively uniform prismatic enamel surface with areas of rodless enamel in some regions. Perikymata grooves and ridges were well-defined. Shallow concavities of enamel rod ends could be seen especially at perikymata grooves, in addition to some scratches of different depths (Figure 1.a & Figure 2.a).
Pearl based-toothpaste (Gp II)
Some regions of enamel surface appeared aprismatic, while others revealed numerous enamel rod ends. Nearly well-defined perikymata grooves and ridges were observed with few scratches and pit-like depressions (Figure 1.b & Figure 2.b).
Charcoal based-toothpaste (Gp III)
Smooth enamel surface exhibited areas of aprismatic enamel, others with plenty of enamel rod ends and areas with less demarcated fish-scale appearance. Furthermore, the surface revealed shallow perikymata grooves and ridges with few shallow scratches (Figure 1.c & Figure 2.c).
Alumina based-toothpaste (Gp IV)
Prismatic enamel surface appeared with fish-scale appearance and less pronounced periky- mata grooves and ridges. The surface showed few less accentuated scratches, some pits, depressions of different sizes, crater-shaped depressions and accentuated prismatic sheath in few areas (Figure 1.d & Figure 2.d).
Sodium bicarbonate salt and lemon extract based-toothpaste (Gp V)
This group demonstrated irregular prismatic enamel surface with fish-scale appearance, regions of aprismatic enamel, ill-defined perikymata grooves and ridges, and numerous scratches of different depths. Nearly the entire surface showed widening of prism sheath, crater-like depressions and large number of pits, pores, depressions of different sizes and several neighboring pits fused forming linear depressions (Figure 1.e & Figure 2.e, f).
Chemical profile results
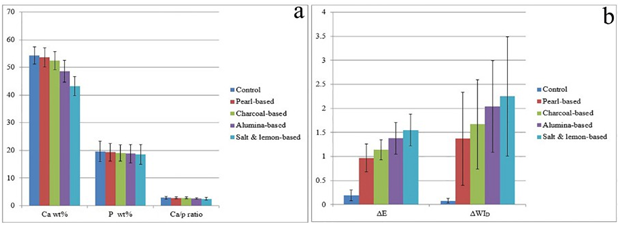
There was statistically significant difference among the groups concerning Ca wt% and Ca/p ratio with insignificant difference regarding P wt%. The significantly highest mean values of Ca wt% and Ca/p ratio were shown in Control, Pearl-based and Charcoal-based groups. Alumina-based group presented a significantly lower value, followed by Salt & lemon-based with the significantly lowest value (Table 2 & Figure 3.a).
Tooth color change results
The positive mean values of ΔL and negative values of Δa and Δb indicated lighter, less red and less yellow tooth color after four weeks of tooth brushing respectively. Concerning perception of tooth color change and whiteness change, only Alumina-based and Salt & lemon-based groups surpassed PT of color change (1.2 <ΔE <2.7), while all groups, other than the control, exceed PT of whiteness change (0.72 < ΔWID < 2.60) (Table 3).
There was highly statistically significant difference between the groups according to ΔL, Δb, ΔE and ΔWID, and insignificant difference concerning Δa. According to ΔE and ΔWID, Salt & lemon-based, then Alumina-based groups presented the significantly highest values, followed by Charcoal-based one. Pearl-based revealed significantly lower value, while the significantly lowest value was shown in the control (Table 3 & Figure 3.b).
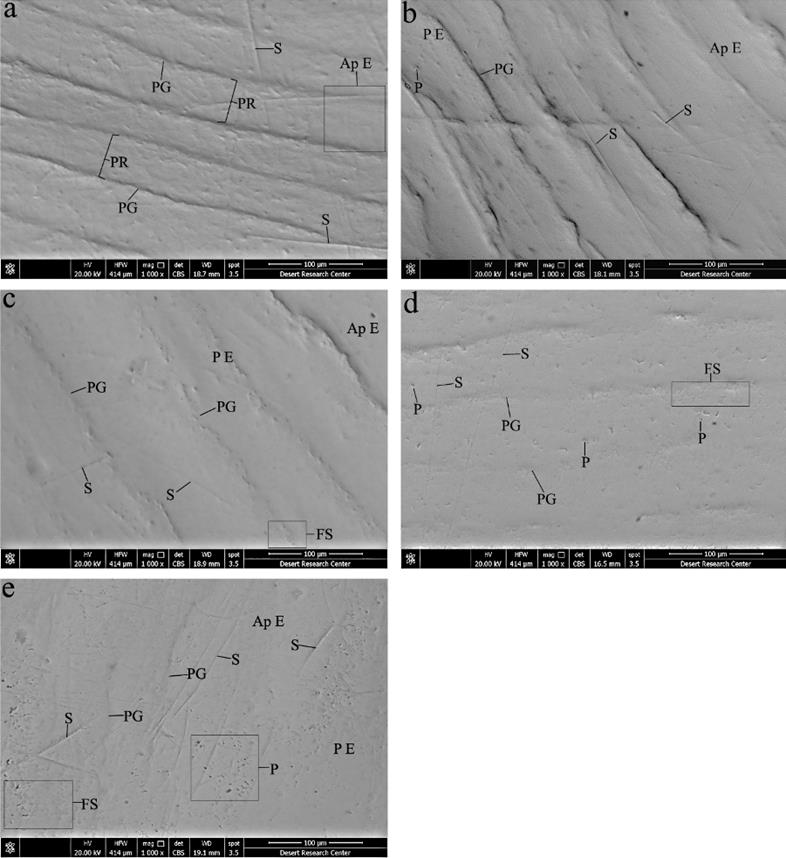
Figure 1 Scanning electron micrographs of enamel surface (x1000). (a)- Gp I; A relatively uniform prismatic enamel surface, aprismatic enamel (Ap E) in some regions, well-defined perikymata grooves (PG) and ridges (PR), and some scratches of different depths (S). (b)- Gp II; Some regions of aprismatic enamel (Ap E), other areas of prismatic enamel (P E), more or less well-defined perikymata grooves (PG), few scratches (S) and pit-like depressions (P). (c)- Gp III; Smooth enamel surface with areas of aprismatic enamel (Ap E), other prismatic enamel areas (P E) and areas with less demarcated fish-scale appearance (FS), shallow perikymata grooves (PG) with few shallow scratches (S). (d)- Gp IV; Prismatic enamel surface with fish-scale appearance (FS), less pronounced perikymata grooves (PG), few less accentuated scratches (S), some pits and depressions (P). (e)- Gp V; Irregular prismatic enamel surface (P E) with fish scale appearance (FS), areas of aprismatic enamel (Ap E), ill-defined perikymata grooves (PG), numerous scratches of different depths (S), large number of pits and pores of different sizes (P).
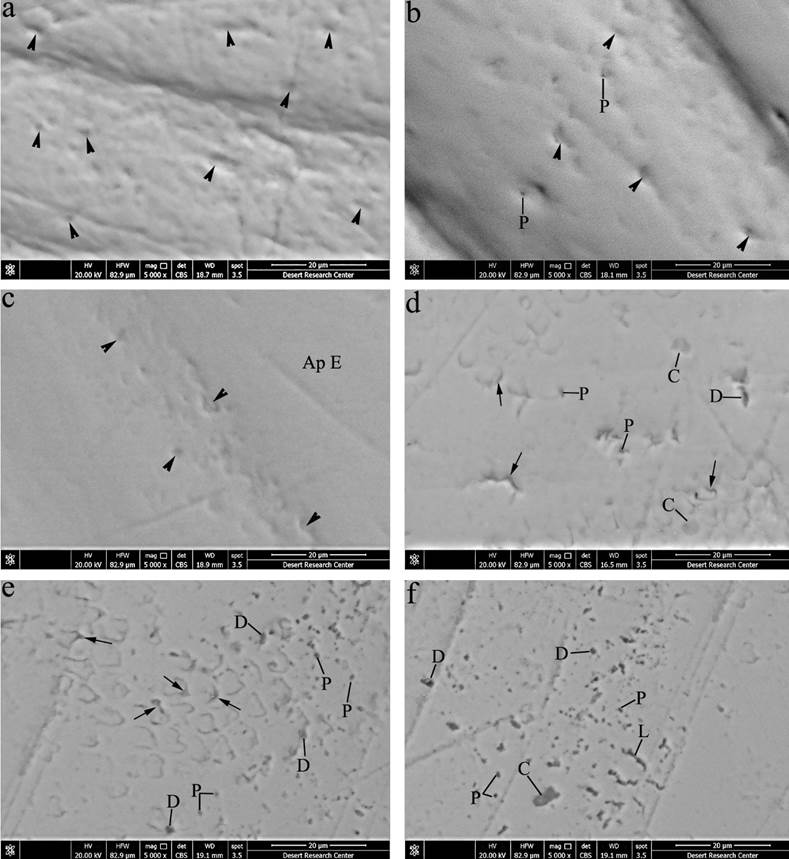
Figure 2 Scanning electron micrographs at higher magnification of enamel surface (x5000). (a)- Gp I; Prismatic enamel surface with shallow concavities of enamel rod ends (arrows heads). (b)- Gp II; Prismatic enamel with numerous enamel rod ends (arrows heads) and few pits (P). (c)- Gp III; Plenty of enamel rod ends (arrows heads) over areas of prismatic enamel and areas with aprismatic enamel (Ap E). (d)- Gp IV; Fish-scale appearance of prismatic enamel surface with some pits (P), depressions (D), crater-shaped depression (C) and accentuated prismatic sheath in few areas (arrows). (e, f)- Gp V; Prismatic enamel surface with fish-scale appearance showed widening of prism sheath (arrows), crater-like depressions (C), large number of pits and pores (P), depressions of different sizes (D) and several neighboring pits fused forming linear depressions (L).
Table 2 Comparison between groups according to Ca wt%, P wt% and Ca/p ratio.
| - | - | Control (n=12) | Pearl-based (n=12) | Charcoal- based (n=12) | Alumina- based (n=12) | Salt & lemon- based (n=12) | Test value | p-value |
|---|---|---|---|---|---|---|---|---|
| Ca wt% | Mean ± SD | 54.28 ± 3.10A | 53.60 ± 3.43A | 52.41 ± 3.25A | 48.60 ± 3.90B | 43.23 ± 3.50C | 21.206 | <0.001** |
| - | Min/Max | 48.88/59.27 | 46.86/58.19 | 47.42/57.72 | 40.45/53.58 | 38.90/51.03 | - | - |
| P wt% | Mean ± SD | 19.62 ± 3.70 | 19.30 ± 3.20 | 19.00 ± 2.90 | 18.80 ± 3.30 | 18.50 ± 3.60 | 0.202 | 0.936 |
| - | Min/Max | 11.84/24.25 | 15.50/26.31 | 12.66/23.65 | 13.83/24.75 | 9.98/22.07 | - | - |
| Ca/p ratio | Mean ± SD | 2.85 ± 0.51A | 2.82 ± 0.27A | 2.80 ± 0.33AB | 2.63 ± 0.26BC | 2.42 ± 0.50C | 2.587 | 0.047* |
| - | Min/Max | 2.44/4.13 | 2.21/3.13 | 2.44/3.75 | 2.17/2.97 | 2.10/3.90 | - | - |
Using: One-way Analysis of Variance test & multiple comparison between groups through Post Hoc test: Tukey's test. Different capital letters indicate significant difference at (p<0.05) among means in the same row, p-value >0.05 is insignificant; *p-value <0.05 is signifi- cant; **p-value <0.001 is highly significant.
Table 3 Comparison between groups according to ΔL, Δa, Δb, ΔE & ΔWID.
| - | - | Control (n=12) | Pearl-based (n=12) | Charcoal-based (n=12) | Alumina-based (n=12) | Salt & lemon- based (n=12) | Test value | p-value |
|---|---|---|---|---|---|---|---|---|
| ΔL | Mean ± SD | 0.02 ± 0.15D | 0.12 ± 0.32D | 0.34 ± 0.25C | 0.62 ± 0.30B | 0.84 ± 0.27A | 19.668 | <0.001** |
| - | Min/Max | -0.24/0.21 | -0.36/0.54 | -0.01/0.71 | 0.16/1.03 | 0.55/1.36 | - | - |
| Δa | Mean ± SD | -0.02 ± 0.14 | -0.15 ± 0.23 | -0.16 ± 0.30 | -0.18 ± 0.25 | -0.20 ± 0.40 | 0.792 | 0.536 |
| - | Range | -0.22/0.24 | -0.49/0.38 | -0.54/0.49 | -0.74/0.17 | -0.91/0.49 | - | - |
| Δb | Mean ± SD | -0.03 ± 0.08A | -0.87 ± 0.27B | -1.02 ± 0.12BC | -1.19 ± 0.20CD | -1.23 ± 0.17D | 87.005 | <0.001** |
| - | Min/Max | -0.11/0.10 | -1.44/-0.37 | -1.30/-0.92 | -1.49/-0.85 | -1.49/-0.99 | - | - |
| ΔE | Mean ± SD | 0.19 ± 0.11D | 0.97 ± 0.29C | 1.14 ± 0.21BC | 1.38 ± 0.33AB | 1.55 ± 0.33A | 46.905 | <0.001** |
| - | Min/Max | 0.04/0.35 | 0.64/1.61 | 0.94/1.58 | 0.88/1.96 | 1.15/2.21 | - | - |
| ΔWID | Mean ± SD | 0.08 ± 0.06D | 1.37 ± 0.97C | 1.67 ± 0.93BC | 2.04 ± 0.95AB | 2.25 ± 1.24A | 9.695 | <0.001** |
| - | Min/Max | -0.79/0.73 | -0.66/3.00 | -0.12/3.05 | 0.62/3.89 | 0.24/4.45 | - | - |
Using: One-way Analysis of Variance test & multiple comparison between groups through Post Hoc test: Tukey's test. Different capital letters indicate significant difference at (p<0.05) among means in the same row, p-value >0.05 is insignificant; *p-value <0.05 is significant;
**p-value <0.001 is highly significant.
Discussion
Many studies have reported that tooth surface can be abraded after prolonged use of whitening toothpastes (23-24). It has been thought that enamel surface abrasion by toothpastes was depended not only on the abrasive constituents of the toothpastes, but also on the hardness of toothbrushes hair (16). In this study, electronic soft hair toothbrush was preferred and used by one operator to minimize its abrasiveness and to apply uniform brushing. Herein, the toothbrush abrasiveness alone to enamel surface was almost negligible, as control teeth revealed normal morphological features and chemical profile of the enamel surface. According to Berkovitz et al. (25), enamel surface, if unabraded, is aprismatic in most areas, thus more highly mineralized, and exhibited features as perikymata ridges and grooves with prism ends marking. Additionally, Ca and P wt% of control group in this study were comparable to those of intact enamel presented in previous research (26).
Toothpastes abrasives vary greatly in composition, where some formulations are being much more abrasive than others. In commercially available toothpastes, there is wide variety in the abrasive systems with different particle size. These agents may abrade the tooth structure with stain removal to unacceptable degree for obtaining good cleaning quality (27,28). Moreover, it has been previously reported that the daily use of whitening toothpaste may lead to decreased enamel microhardness, giving indirect evidence of hard dental tissues demineralization (29).
The present work could assess the extent of enamel surface morphological and chemical profile changes in different whitening toothpastes groups according to Mohs scale of hardness for different abrasive particles used (9,13,14,30). Herein, Pearl- based and Charcoal-based toothpastes (have lower Mohs scale of hardness than enamel) exerted little influence on the enamel surface morphology, being nearly comparable to the control with insignificant reduction in mineral content. In spite of the low abrasive action of charcoal containing toothpastes, prior studies illustrated few topographic alterations and some scratches on enamel surface with increased surface roughness of tooth enamel after charcoal toothpaste brushing (16,31). Moreover, the results after Crest 3D White with Charcoal brushing for 30 days in previous work, presented less defined perikymata and less numerous enamel rod ends (32).
Besides, it has been previously reported that the larger and more irregular shape of the particles, the more they abrade the dental tissue surfaces (9). Previous study examined the particles of different whitening toothpastes as charcoal, hydrated silica, calcium carbonate and alumina with SEM, and revealed that Alumina particles were the largest with sharp edges, showing the highest change in enamel surface morphology and roughness (14). The high Mohs scale value of Alumina in addition to its particles size and shape could be attributed to the obvious morphological enamel surface changes detected in Alumina-based group in the current study, alongside to the significant reduction in the mineral content.
Despite sodium bicarbonate has lower Mohs scale of hardness than enamel, the enamel surface of sodium bicarbonate Salt & lemon-based group in this study presented the most considerable alterations. Previous investigation showed comparable enamel surface pattern after and before brushing with sodium bicarbonate toothpastes with insignificant increase in surface roughness (33). Another study revealed that sea salt (equivalent hardness as sodium bicarbonate) and lemon toothpaste resulted in greater tooth surface roughness than charcoal toothpaste (34, 35).
Thus, the changes in the current study could be due to the acidic content present in Salt & lemon toothpaste, resulting in demineralization of the outer enamel surface and reduction of the surface hardness. Herein, this could be confirmed by the highest significant reduction in mineral content in Salt & lemon group. This is in accordance with former study, showing that Dabur Herb’l Salt & lemon toothpaste contains lemon extract with acidic pH. These produced more abrasion than those with neutral or basic pH, and showed significant reduction in the microhardness (36). Moreover, lemon extract acts as strong oxidizer as hydrogen peroxide, due to the presence of citric and malic acids, making the tooth color brighter (37,38). byproducts generated from the oxidizing reaction to the tooth enamel surface could lead to enamel erosion and porosities with alteration in its mineral content as priorly reported, after enamel surface hydrogen peroxide bleaching (18).
Proper color and whiteness measurements are important for the clinical practice to monitor the effectiveness of different agents in tooth whitening (20). There are two thresholds for tooth color differences assessment. PT indicates the least color difference which can be detected by the observer, meanwhile, AT refers to the color difference which is acceptable by the observer when compared to reference color (19). Regarding tooth whitening, the patients demand is to reach high color change exceeding AT (12).
In the current study, only Salt & lemon-based and Alumina-based groups exceeded PT of color change, and all groups, other than the control, exceed PT of whiteness change. Previous study demonstrated that toothpaste with alumina (ZACT) had the highest capability to remove stains compared to those containing hydrated silica, calcium carbonate or charcoal, where color alteration by the former toothpastes didn’t significantly differ (14). Other investigation reported that sea salt- lemon-based toothpaste exhibited better white- ning action than the charcoal one (34).
Although Salt & lemon-based, followed by Alumina-based group showed the major surface morphological changes, they demonstrated the highest color and whiteness changes. These results are consistent with prior study, stated that the increase in surface roughness improves ΔE and ΔL values, as the enamel surface roughness can diffuse reflection, making brightening effect (14). However, the increased enamel surface porosities and roughness in the oral cavity could increase plaque formation and retention, resulting in caries and periodontal disease risk, and could attribute to teeth discoloration (29).
Conclusions
After four weeks of brushing, Pearl-based and Charcoal-based toothpastes exerted little influence on enamel surface morphology and chemical profile. Meanwhile, Salt & lemon-based toothpaste group presented the most considerable alterations in the morphology and mineral content of the enamel surface, followed by Alumina-based one. Further, Salt & lemon-based then Alumina-based toothpastes showed higher whitening effects on teeth than the other groups.