INTRODUCTION
The balance of demineralization and remineralization in dental caries is fundamental to understand the presence and progress of initial or subclinical lesions (1,2,3). These cycles are a complex series of events on the tooth surface, which take place in ecological changes within the dental biofilm (4,5). At the beginning they are observed clinically as a small demineralized area. Remineralization is a net gain of ions towards the dental tissues, which replaces the loss due to the demineralization process. This occurs through a physical-chemical process that includes the supersaturation of ions in the solution in relation to enamel, the formation of nucleus and crystals (6,7). Since the control of dental caries remains a challenge (8) as one of the main public health problems in the world, its negative impacts should not be underestimated. Among other effects, dental pain, tooth loss, effects on general well- being and quality of life must be mentioned (9,10). It is important to look for effective treatments to prevent the disease in its incipient stages (1,2,3). The importance of fluoride is indisputable as a viable and economical method; most likely, it will continue to lead the prevention of dental caries. However, there are compounds that serve as adjuvants in the remineralization process (11). Currently there is a growing interest in options that do not contain fluoride. These compounds are biologically active, mainly derived from natural products such as tea (eg, Camellia Sinensis or green tea), coffee, grapes, propolis, as well as some Asian herbs and mushrooms (12), synthetics products such as amorphous calcium phosphate, casein and xylitol (13,14,15). Xylitol ((CHOH) 3(CH2OH) 2) is a non-acidic sweetener; it is associated with Ca in aqueous solution to inhibit the dissolution of enamel’s calcium or phosphate ions, thus acting as a calcium transporter required for enamel remineralization (16).
The objective of this article was to evaluate the remineralizing effect of toothpastes based on Xylitol, and of two biologically active natural botanical agents, Camellia sinensis and Juniperus communis. This exploration of its remineralizing potential was compared against a fluoridated toothpaste.
MATERIALS AND METHODS
An experimental in vitro study was carried out. The protocol was reviewed and approved by the Research and Ethics Committee of the Autonomous University State of Mexico.
We included 18 human premolars extracted for orthodontic reasons. All the patients signed an informed consent.
Bicuspids had no restorations, fractures or decay on the vestibular face. The teeth were stored in 0.2% thymol after extraction. Teeth were randomly assigned to one of six groups. Control group, which was not exposed to any procedure; Demineralization only group, exposed to an artificial demineralization process; Xilitol group, exposed to 100% Natural Dentiste toothpaste (Bangkok, Thailand); Camelia sinensis group, exposed to Fluocaril toothpaste (Bangkok, Thailand); Juniperus communis group, exposed to Splat Special Blackwood toothpaste (Moscow, Russia); and Toothpaste Control Group, exposed to Colgate Triple Action toothpaste (San Jose Iturbide, Guanajuato, Mexico).
ALL THE TEETH WERE SUBJECTED TO THE FOLLOWING PROCESSES FOR FURTHER ANALYSIS
ARTIFICIAL DEMINERALIZATION PROCESS
An artificial demineralization process was performed, for which the specimens were immersed in a demineralizing solution (2.2 Mm CaCl2, 2.2 Mm NaH2PO4, 0.05 Mm CH3COOH and 1M KOH) adjusting the pH to 4.4 during 96 hours at 37 ° C in an incubator (17).
CYCLIC PH MODEL
The specimens were exposed to a cyclic pH model for 15 days at 37°C in order to simulate the conditions of the oral environment.
Each specimen was immersed in a demineralizing solution (2.2Mm CaCl2, 2.2Mm NaH2PO4, 0.05 Mm CH3COOH, 1M KOH, pH 4.4, 10 mL/specimen) twice a day for 3 hours and for 2 hours in the remineralizing solution (1.5Mm CaCl2, 0.9Mm NaH2PO4, 0.15Mm KCl, pH 7.0, 10mL / specimen) between the demineralization cycles.
The specimens treated with the toothpastes were embedded in suspension of toothpaste according to their group (5 mL per specimen) for 60s before the onset of the first demineralization cycle and before and after the second demineralization cycle. Then, they were stored in remineralizing solution in an incubator at 37ºC for 16 hours to complete 24 hours of a cycle (17).
TEETH PREPARATION FOR ANALYSIS BY SEM AND EDS
We obtained 3x3 mm blocks of enamel from the vestibular surface of the teeth. They were cut with a carbide disc, then we cleaned of specimens for 5 minutes in separate containers, filled with tri-distilled water in an ultrasonic bath (Quantrex Q140, L & R Ultrasonics, NJ, USA). Next, the blocks were fixed to an aluminum specimen holder with carbon adhesive tape (SPI Supplies, USA).
The atomic percentages of Ca and P were evaluated, which were analyzed with EDS. In addition, the enamel surface was visualized by SEM.
The SEM analysis was performed using a scanning electron microscope (JEOL, JSM-6510LV, Japan) in the low vacuum mode at 10 Pa of pressure in the chamber, with an electron acceleration voltage of 15 kV and detecting backscattered electrons.
The morphology of the enamel surface was observed at a magnification of 500x at a working distance of 50 mm. We determined the atomic percentages (% at) of Ca and P using an X-ray detector system (Oxford Instruments, 7582, United Kingdom) coupled to the scanning electron microscope.
STATISTICAL ANALYSIS
Data were analyzed using the statistical package SPSS 23.0 (SPSS Inc., Chicago, USA). The one-way ANOVA test was performed with Bonferroni’s correction for multiple comparisons of atomic percentages of Ca and P; the value of p was adjusted to 0.001667. We also used the Pearson Correlation test between Ca and P, with a significance of p≤0.05.
RESULTS
QUANTITATIVE EVALUATION OF CHEMICAL COMPOSITION WITH EDS
The chemical composition of the enamel surface was determined before treatment, after a demineralization process and after the treatment with each of the remineralizing toothpastes, which were presented in at%.
Table 1 shows the results of the atomic percentage analysis of the Ca and P elements of each study group. In the Pearson correlation test between Ca and P, statistically significant correlations were observed in all groups (p <0.01), ranging from r=0.7413 (Xylitol Group) to r= 0.9510 (Control Group).
When performing the one-way ANOVA test for the comparison of P, statistically significant differences were found between the Control Toothpaste Group vs the Xylitol Group (p<0.001), the Control Toothpaste Group vs the Camelia Sinensis Group (p=0.003) and between the Control Toothpaste Group vs Juniperus Communis Group (p<0.001). With respect to Ca, statistically significant differences were obtained between the Xylitol Group vs. the Demineralization Group (p=0.001) and between the Control Toothpaste Group vs the Xylitol Group (p <0.001) (Table 2 and 3)
QUALITATIVE ANALYSIS WITH SEM OF THE SPECIMENS’ SURFACES
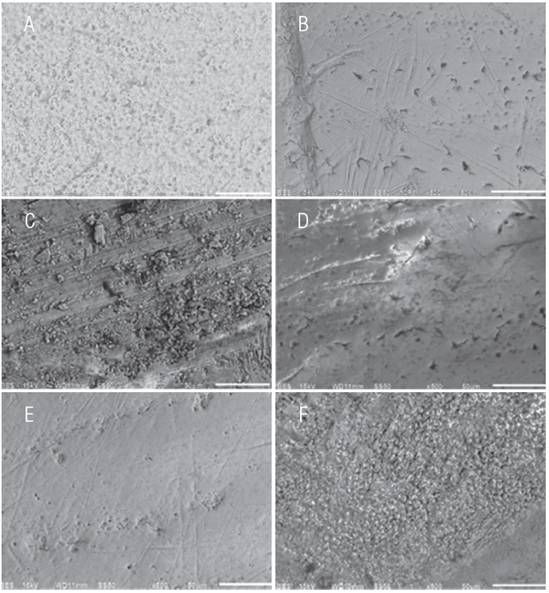
In Fig. 1 we observe the microphotographs representative of the SEM enamel surfaces analysis for the groups. In the pretreatment analysis of the enamel surface morphology, shown in Fig.1a), we observe the prisms exposed in a “key hole” shape, as well as in a circular form. On the enamel surface after artificial demineralization we can observe a smooth appearance without perikymata pattern, with irregular fissures, as well as small defects in its periphery and presence of numerous irregular holes (Fig.1b). In the enamel surfaces treated with the toothpastes studied, smooth surfaces with various porosities and grooves were found in the goups treated with Camellia Sinensis and Juniperus Communis toothpastes (Fig.1c-f).
Table 1 Analysis of the Ca and P element atomic percentage.
| Chemical element at% | Control Group | Demineralization Group | Xylitol Group | Camelia sinensis Group | Juniperus communis Group | Control Toothpaste Group | F | p value* |
| Ca | 16.96±3.56 | 15.22±1.57 | 21.01±3.05 | 17.19±4.48 | 17.77±1.67 | 14.66±4.70 | 6.8 | 0.000 |
| P | 11.38±2.33 | 11.15±1.10 | 12.93±1.56 | 12.26±2.02 | 13.03±1.00 | 9.31±2.51 | ||
| Correlation† | r= 0.9510 p<0.0010 | r= 0.9161 p<0.0010 | r=0.7413 p=0.0058 | r=0.9371 p<0.0010 | r=0.9161 p<0.0010 | 0.7626 p<0.0012 |
* Analysis of variance (ANOVA)
† Pearson correlation of Ca y P
Table 2 Multiple comparisons of Bonferroni for P.
| - | Control Group | Demineralization Group | Xylitol Group | Camelia sinensis Group | Juniperus communis Group |
| Demineralization Group | -0.2325 1.000 | - | - | - | - |
| Xylitol Group | 1.5525 0.662 | 1.785 0.319 | - | - | - |
| Camelia sinensis Group | .8775 1.000 | 1.11 1.000 | -0.675 1.000 | - | - |
| Juniperus communis Group | 1.65 0.491 | 1.8825 0.231 | 0.0975 1.000 | 0.7725 1.000 | - |
| Control Toothpaste Group | -2.0725 0.119 | -1.84 0.266 | -3.625 0.000 | -2.95 0.003 | -3.7225 0.000 |
*The p value was adjusted to a significance of 0.001667.
Table 3 Multiple Bonferroni comparisons for Ca.
| - | Control Group | Demineralization Group | Xylitol Group | Camelia sinensis Group | Juniperus communis Group |
| Demineralization Group | -1.74 1.000 | - | - | - | - |
| Xylitol Group | 4.04583 0.074 | 5.78583 0.001 | - | - | - |
| Camelia sinensis Group | .224167 1.000 | 1.96417 1.000 | -3.82167 0.116 | - | - |
| Juniperus communis Group | .811666 1.000 | 2.55167 1.000 | -3.23417 0.347 | .5875 1.000 | - |
| Control Toothpaste Group | -2.29833 1.000 | -.558333 1.000 | -6.34417 0.000 | -2.5225 1.000 | -3.11 0.431 |
*The p value was adjusted to a significance of 0.001667.
DISCUSSION
In the present study, the remineralizing effect of Xylitol, Camellia sinensis and Juniperus communis added to dentifrices was evaluated, using a fluoride toothpaste as a control. We studied the morphological and chemical changes of human premolars enamel surface after their treatment with the toothpastes previously mentioned. It was found that specimens treated with the Juniperus communis toothpaste had a higher atomic percentage of P; however, it cannot be definitively concluded if this result is due to the active component of Juniperus communis, since pyrophosphates are found within the ingredients of this toothpaste. On the other hand, when analyzing the atomic percentages of Ca, statistically significant differences were observed between the group treated with the Xylitol toothpaste and the control group. This ion gain benefits the remineralization process by the net addition into the enamel structure, which replaces what was previously lost by demineralization. It should be mentioned that this occurs through a physical-chemical process that includes the ions supersaturation of the solution respecting to enamel, the formation of nucleus and crystal growth (18,19).
It is well known that Xylitol in high concentrations has the ability to form compounds with calcium ions and penetrate the demineralized enamel, where it can contribute to transporting dissolved ions by decreasing the calcium and phosphate diffusion coefficient (15,20). Studies have shown greater remineralizing effect in both varnishes (15) and in toothpastes (21) when xylitol is added to fluoride. In our study we found a positive correlation between the presence of calcium and phosphorus; however, this was the weakest correlation, even compared with the control toothpaste group. In vivo studies have shown that the remineralization process can be accelerated because xylitol reduces the acidity of the bacterial biofilm, allowing fluoride to act under more favorable conditions (22).
Recent studies have shown the remineralizing effect of Camellia sinensis (23,24) Likewise, it has been shown that its addition to carbonated drinks reduces the demineralization they cause (25). In this study we found that the addition of this compound to toothpastes helps to increase calcium and phosphate ions, obtaining a strong correlation. These results are not definitive: in a dynamic environment such as the mouth, antibacterial effects should be evaluated (26). It should be determined whether remineralization is influenced by the variable fluoride levels in the Camellia sinensis leaves (27).
Reviewing the literature, no evidence was found of the Juniperus communis remineralizing effect (28) However, its antioxidant effect has been proven, which is directly related to its application to prevent oxidative damage to biological systems by reactive forms of oxygen H2, O2, O2- and OH) that are produced during cellular metabolism in organisms (29,30). It is worth mentioning that, in the present study, an almost perfect correlation was found between the amount of calcium and phosphate ions when applying the Juniperus communis toothpaste (28). For these reasons it is advisable to perform in vivo studies to evaluate these effects - both the effects of the different types of Juniperus by themselves, and in conjunction with pyrophosphates in the toothpaste.
Although the use of fluorides is essential in the prevention of dental caries, it is important to explore the value of available alternatives. The present research aims to expand the knowledge about remineralizing substances that are not based on fluoride. These have become attractive for patients interested in using products that are perceived to be biologically natural alternatives, or with fluorides excluded in their formulation. The cultural tendency to exclude fluorides from water- manifested in multiple votes in developed countries in which local populations reject the addition of fluorides to drinking water-makes it necessary to evaluate the different options available in the market for prevention of dental caries.
This study has the limitation that it was undertaken in a small sample. Despite its exploratory nature, our results confirm some past literature reports, and provide new evidence. Xylitol toothpaste showed the highest remineralizing property, both in the EDS analysis and in the SEM images. Although the Camelia sinensis toothpaste showed positive results by EDS, the SEM images showed a smooth surface, similar to the demineralized surface. The Juniperus communis toothpaste was associated with a high percentage of phosphate; this may be due to the fact that its composition includes pyrophosphates, which can form synthetic phosphates.