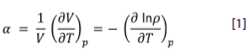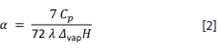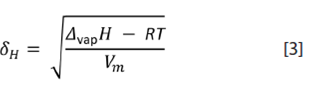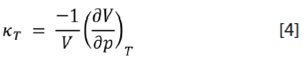Low oil prices are certainly good news for non-petroleum-producing economies and for high energy consumers but, an undesirable consequence is the growth in carbon emissions associated to its use. Low fossil-fuel prices could discourage further innovation and adoption of cleaner energy technologies, but nevertheless it is tenable the need to look for competitive green alternatives, where biodiesel stands out for its great versatility as technical material (Knothe, Dunn & Bagby, 1997). There is a manifested interest in Costa Rica to develop the production of vegetable oils for non-food purposes, initiatives that come from government as well as private sector.
Physic nut (Jatropha curcas), castor-oil (Ricinus communis), oil palm (Elaeis guineensis), wine palm (Attalea butyracea) and macaw palm (Acrocomia aculeata) were chosen for study in our laboratory, because they are oleaginous plants with convenient oil yields to produce biodiesel. These species are considered in the agro-industrial initiatives indicated above.
The plants are present around the world and can grow under adverse environmental conditions and need no optimal soils. Soybean (Glycine max) is included as comparison, because its oil is widely used to produce biodiesel. The periods of initiation of oil production suggest that good agronomical techniques must yet be developed, based on simultaneous multiple-species cultivation that could start yielding economical revenue from earlier times.
Table 1: Lipid production of some oil-producing plants
| Species | Lipid yield / t y-1 ha-1 | Onset of oil production |
| Elaeis guineensis | 5,5 | 4 years |
| Attalea butyraceae | 3,0 | 5 years |
| Acrocomia aculeata | 3,0 | 5 years |
| Jatropha curcas | 1,6 | 2 years |
| Ricinus communis | 1,5 - 2,5 | 6 months |
| Glycine max | 1,5 | 3 months |
Source: Bártoli (2008), Cristancho, Hanafi, Omar y Rafii (2011), Jachmanian, Pérez Gomar, Villamil y Villamil (2009), MAG (1991), Molina, Nouel-Borges, Sánchez-Blanco, Rojas-Castellanos y Espejo (2013), Smith (2015).
Based on previous results of biodiesel properties (Castellón-Elizondo, Lutz & Mata-Segreda, 2006), we should like to discuss the unimportance of fatty-acid chain composition on the thermodynamic properties of different biodiesel materials derived from the six vegetable oils in Table 1.
Biodiesel can be used not only as liquid fuel but also as alternative industrial solvent, lubricant and as fluid for transport of mechanical or thermal energies. These potential applications imply no foreseeable consequences on occupational or environmental safety, mainly because it has proved to be biodegradable at a minimum of 80% in the open environment within a month or two (Demirbaş, 2008; Lutz, Chavarría, Arias & Mata-Segreda, 2006; Sendzikiene, Makareviciene, Janulis & Makareviciute, 2007).
The generalisations derived from this discussion are useful to achieve acceptable prediction of physical properties of biodiesel materials that have not been synthesised yet.
Analysis
All methyl fatty monoesters were obtained by alkaline transesterification of the feedstock oils, as has been widely reported in the literature(see for example: Mittelbach & Remschmidt, 2004; Alvarado-Montero, 2012; Monge-Montero, 2014,). Amongst these agro-industrial possibilities, the fatty-acid chain composition differs, as shown in Table 2.
The cubic-expansion thermal coefficient (α) was the experimental quantity from which the isothermal compressibility coefficient (κT), the enthalpy of vaporization (Δ vap H°) and the Hildebrand solubility parameter (δH) were obtained, according to the soft-solid model for liquids (Castellón-Elizondo et al., 2006). Traditional methods were used for the evaluation of parameters such as kinematic viscosity and viscous-flow activation energy, and the enthalpy of combustion (Shoemaker & Garland, 1968), and need not be detailed in this paper.
Cubic expansion thermal coefficient (α):
Knowledge of the cubic expansion coefficient and isothermal compressibility of fluids is needed for the design of hydraulic equipment. The observable thermodynamic properties of materials can be understood in terms of the forces between the constituent molecules. The molecular interpretation of α provides a way to assess the system cohesivity, because stronger attractive intermolecular forces allow only small inter-particle spacing gained by thermal energy increase (temperature). This thermodynamic coefficient is easily obtained from the temperature effect on density (ρ) as indicated by equation [1], in the temperature range from 18°C up to 33°C.
α values are similar for all the cases in the set of biodiesel samples studied with average value (8,5±0,4) × 10-4 K-1. Thus, no significant volumetric differences should be expected when different types of biodiesel are subjected to identical temperature increase under constant pressure.
Biodiesel materials are mixtures of fatty esters, and as it is the case with their parent hydrocarbons, the physical properties chiefly depend on molecular size. Note that Ricinus biodiesel also conforms to this expectancy, despite the presence of a hydroxyl group attached to carbon atom 12 in the ricinoleic-acid chains. Thus, acyl chains mainly define the degree of intermolecular interactions in biodiesel materials.
Table 2: Fatty-acid chain profile of the six vegetable oils studied. Data are given as gas-chromatographic mole percentage.
| Oil | C8:0 | C10:0 | C12:0 | C12:1 | C14:0 | C14:1 | C16:0 | C16:1 | C18:0 | C18:1 (9c, 12-OH) | C18:1 (9c) | C18:2 (9c, 12c) | C18:3 (9c, 12c, 15c) |
| Elaeis guineensis | - | - | - | - | - | - | 44 | - | 5,0 | - | 40 | 10 | - |
| Jatropha curcas | - | - | - | - | - | - | 13 | - | 7,0 | - | 42 | 37 | - |
| Ricinus comunis | - | - | - | - | - | - | 3,0 | - | 3,0 | 80 | 5,0 | 5,0 | - |
| Attalea butyracea | - | - | - | - | - | - | 22 | 1,0 | 1,5 | - | 67 | 0,8 | 5,0 |
| Acrocomia aculeate | 6,0 | 3,0 | 37 | 1,5 | 12,5 | 0,7 | 7,0 | 0,8 | 2,5 | - | 19 | 2,0 | - |
Table 3: Thermodynamic and rheological properties of six types of methyl biodiesel.
| Methyl biodiesel | 104 α/K-1 | Δvap H/kJ mol-1 | δH / (J cm-3)½ | μ40°C/mm2 s-1 | E μ/kJ mol-1 | 1010 κT/Pa-1 | ρ/g cm-3 (T °C) | Δc H/MJ kg-1 |
| Elaeis guineensis | 8,2±0,1 | 106 ± 6 | 18,0 ± 0,5 | 6,97 ± 0,05 | 6,5 ± 0,4 | 7,2 ± 0,2 | 0,8878 (24 °C) | 36 ± 1 |
| Attalea butyraceae | 9,1±0,1 | 95 ± 1 | 17,3 ± 0,5 | 4,8 ± 0,2 | 15,0 ± 0,7 | 9,1 ± 0,3 | 0,8881 (22 °C) | 39 ± 1 |
| Acrocomia aculeata | 9,1±0,1 | 96 ± 1 | 17,3 ± 0,6 | 4,7 ± 0,4 | 14 ± 1 | 9,0 ± 0,5 | 0,88 (22 °C) | 38,1 ± 0,8 |
| Jatropha curcas | 8,3±0,2 | 104 ± 3 | 17,4 ± 0,5 | 4,77 ± 0,02 | 16,1 ± 0,5 | 8,17 ± 0,03 | 0,8741 (25 °C) | 38,8 ± 0,2 |
| Ricinus communis | 8,2±0,1 | 106 ± 3 | 17,2 ± 0,5 | 14,9 ± 0,9 | 31,6 ± 0,6 | 8,2 ± 0,2 | 0,9157 (22 °C) | 38 ± 1 |
| Glycine max | 8,3±0,2 | 105 ± 6 | 17,5 ± 0,3 | 4,6 ± 0,8 | 17 ± 1 | 7,7 ± 0,2 | 0,871 (24 °C) | 38,5 ± 0,7 |
Enthalpy of vaporisation (Δ vap H): The soft-solid model for liquids proposed by our laboratory (Castellón-Elizondo et al., 2006), gives an inverse relation between α and Δvap H:
where λ is a measure of the molecular packing density of the constituting particles according to their geometry (e. g., λ = 0,30 for spherical molecules, λ = 0,62 for rod-shaped molecules, λ = 1,2 for very long hydrocarbon molecules, and λ = 1,4 for triacylglycerols, as given by Lezcano-González and Mata-Segreda (2011). Readily comparison of the cohesiveness of liquids (Δvap H) can also be achieved through equation [2].
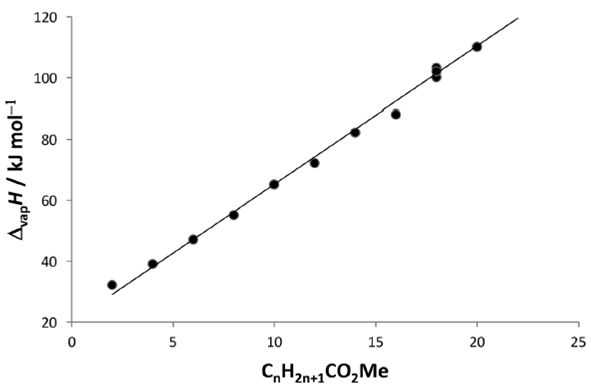
The Δvap H values are similar within experimental uncertainty (average 102 ± 5 kJ/mol), and as expected, are found of the same magnitude of those of pure fatty esters of similar chain length (e. g. methyl palmitate 88 kJ/mol, methyl stearate 100 kJ/mol, methyl oleate 103 kJ/mol, methyl linoleate 102 kJ/mol, methyl linolenate 102 kJ/mol). These values were obtained from the data collection of Chickos and Acree, Jr (2003). Again, the acyl chains are mainly responsible for the degree of cohesion of these systems, as is observed in Figure 1 for linear chain carboxylic esters.
Hildebrand solubility parameter (δ H ):
It is the square root of the so-called molar cohesive-energy density ((vap U = (vap H - RT). Again, δH does not vary significantly amongst the biodiesels considered (average 17,4 ± 0,3 (J/cm3)½). This property implies that biodiesel could replace some uses of industrial solvents such as benzene, toluene or a mixture of xylenes (δH ( 18 (J/cm3)½), fossil-derived solvents of great safety concern due to their volatility, toxicity and low flash point.
The traditional meaning of industrial solvent is that of organic liquids used on an industrial scale for the purpose of dissolving, suspending or changing the physical properties of other components of a final product. The selection of an appropriate solvent for a particular formulation is based on the knowledge of the chemical interactions amongst the mixture constituents. Besides thermodynamic considerations such as mutual miscibility, environmental and safety issues orient the substitution of old solvents for newer ones.
Isothermal compressibility coefficient (κ T ):
From the soft-solid model for liquids, one obtains:
This quantity is a measure of the resistance to compression. Again, κT values are similar for all samples, with an average value of (8,2±0,7) × 10-10 Pa-1.
The amount of work needed in compressing a substance isothermally and reversibly is determined by κT. A molecular interpretation of the result obtained is based on the condition that the average distance between molecules in liquids is greater than the corresponding to the potential-energy minimum, and it is similar for molecules of a particular geometry and degree of molecular packing (δ value). Then, it takes similar amounts of work to get the biodiesel systems past the minimum average intermolecular distance.
All biodiesel materials showed κT values similar to those of mechanical-power transmission fluids in the range from 6 ( 10-10 Pa-1 to 8 ( 10-10 Pa-1 (Štěpina & Veselý, 1992). This finding suggests an alternative use of the fatty esters as fluids for transmission of mechanical energy.
Kinematic viscosity (μ): Viscosity measures resistance to flow. In mechanical terms, it is considered as the resisting capability of a fluid to gradual deformation by an applied external stress.
Kinematic viscosity (viscosity/density is also called momentum diffusivity) measures transmission of momentum resulting from an applied shear stress.
All biodiesel μ values vary within a narrow range from 4,6 mm2 s-1 to 6,97 mm2 s-1 at 40 °C, except that for Ricinus biodiesel. It is tenable to think that the hydrogen-bonding interaction between sliding Ricinus biodiesel molecular layers provides a less efficient momentum transport mechanism (greater inertia) via hydrogen bonding, thus increasing the specific resistance to flow (Glasstone et al., 1941). This proposed mechanism for momentum diffusion agrees with the high de Guzmán activation energy for viscous flow, E μ . This last rheological parameter gives the effect of temperature on the viscosity of liquids (Atkins & de Paula, 2006):
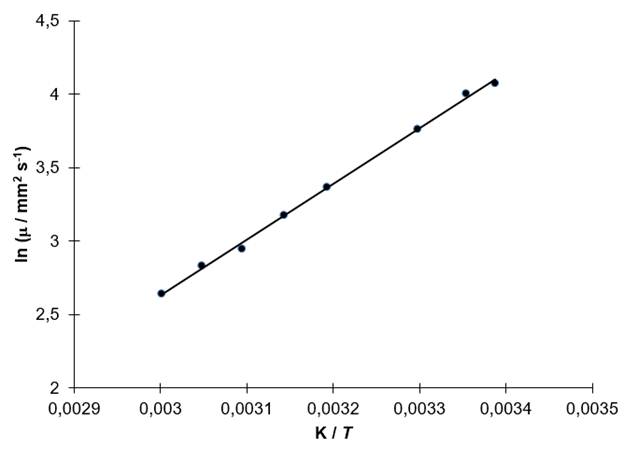
Equation [6] indicates that E μ is the relative change of μ per unit temperature change, ∂ 𝜇 𝜇 𝜕𝑇 . These values are obtained as the slope of graphs of ln μ vs. 1/T, in an Arrhenius-like fashion.
Figure 2 shows the results for Ricinnus biodiesel.
Eμ allows the estimation of viscosity values at different temperatures other than that of a standard testing procedure (40°C). An interesting result is the correlation between the Eμ values of biodiesels and the corresponding values for their oil feedstocks. Figure 3 shows the result.
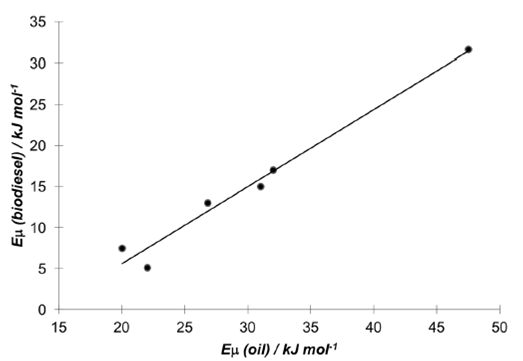
Figure 3 Correlation between the activation energy for viscous flow of different types de biodiesel and their source vegetable oil.
The regression equation is:
Eμ (biodiesel) / kJ mol-1 = [(0,94 ± 0,08) Eμ (oil) / kJ mol-1] - (13 ± 2) p < 0,0002 [7]
Equation [7] allows an estimation of the Eμ of a specific type of biodiesel from the viscosity of the parent vegetable oil, before the synthesis an evaluation of the methyl monoester is done.
Biodiesel is a mixture of fatty-acid esters. The polar ester moiety gives a greater degree of attractive intermolecular forces relative to their parent hydrocarbon molecules, as evidenced by their higher average ΔvapH (102 kJ/mol) vs. 40-50 kJ/mol currently accepted for gasoline or petrodiesel (Demirbaş, 2008). Amongst the different fatty-ester mixtures, the carbon chains level out the bulk thermodynamic physical properties of the different biomaterials. Thus, no significant differences are found in properties that arise from the intermolecular forces in biodiesel materials.
A salient difference is found in the rheological behaviour of Ricinus biodiesel, due to its mechanism for diffusion of momentum via hydrogen bonding associated to the hydroxyl group attached to carbon 12 of the ricinoleic-acid chains.
Though it might be taken as a lapalissade, it can be concluded that biodiesel materials are useful either as the ester mixture from a single feedstock, or as mixtures from different sources.












 uBio
uBio