Introduction
More than half of the world’s population has insufficient food consumption, even though food production has tripled World War II. There is a shared responsibility to change this situation and ensure that all people have access to healthy, quality food (Food and Agriculture Organization of the United Nations (FAO) et al., 2020; FAO, 2017). Humankind has developed agriculture as one of its main sources of food. Faced with this dilemma, high-yield agricultural production models have been implemented to supply the world market (Muller et al., 2017).
In order to achieve the aforementioned purpose, different strategies aimed at increasing food production have been developed, including new agricultural techniques, the use of improved seeds, and the use of plant protection products, such as pesticides. The application of pesticides on crops is becoming increasingly important due to the expansion of intensive agriculture around the outskirts of cities, destined for the domestic market (Rodríguez-Rojas & Peraza-Padilla, 2022; Sarkar et al., 2021).
It has been estimated that without the use of plant protection products (PPPs), a large amount of the world’s food would be lost (FAO et al., 2020; FAO, 2017). It is necessary to state that the use of pesticides worldwide has become essential for different crops (García-Hernández et al., 2018; Leyva-Morales et al., 2014). The tendency to increase is driven by the need to control pests and diseases that can interfere with crop production (Sarkar et al., 2021).
While the use of these chemical products benefits production processes, their misuse, application at inappropriate times, and use on crops for which they have not been registered make these substances a potential risk for human health and the environment. These products are generally toxic and can leave residues in the final product, thus diminishing its quality (Lagos-Alvarez et al., 2022; Leyva-Morales et al., 2017; Leyva Morales et al., 2014; López-Dávila et al., 2022).
Although pesticides are indispensable in today’s agriculture, their effect on humans and the environment can be considerable (López-Dávila et al., 2022; Sarkar et al., 2021). Pesticides are one of the main phytosanitary barriers and perhaps the biggest agricultural challenge of globalization. International regulations are becoming more stringent regarding the maximum permissible limits of pesticides in different products for human consumption. The massification of food production has triggered excessive pesticides use, with serious implications not only for food residues but also for the contamination of water sources, soil, air, and other living beings (Sarkar et al., 2021; Singh et al., 2019).
The presence of chemical residues in food of agricultural origin includes various residues commonly known as environmental contaminants, which come from agrochemicals used in agriculture and livestock farming (Bastidas-Bastidas et al., 2014; Lagos-Alvarez et al., 2022). While pesticides have been instrumental in combating many human diseases and hunger, their increased use has led to the development of certain toxic diseases, making them a double-edged sword. They can produce acute or chronic poisoning, depending on the speed of absorption or repeated exposure to the pesticide (Blanco-Valdes et al., 2022; López-Dávila et al., 2022).
It is important to note that organophosphates, dithiocarbamates, carbamates, and pyrethroids have a marked action on the central nervous system. Some of these a.i. act reversibly, but others act irreversibly. Organophosphates bind irreversibly to acetylcholinesterase, preventing the degradation of acetylcholine, which produces nervous hyperactivity that can end with death; they have high acute toxicity and are cause neurotoxic effects in wildlife (Han & Wang, 2019; López-Dávila et al., 2020). Carbamates pose a potential risk to human health because they can be absorbed by inhalation, ingestion, and sometimes through the skin. Pyrethroids act quickly against insect pests but can lead to adverse events affecting bacteria and even humans (Oliveira Jardim et al., 2018).
The main adverse events caused by the aforementioned a.i. in humans include vomiting, headache, poor coordination, tremor, salivation, diarrhea, irritability to sound and touch, burning, itching, and tingling progressing to numbness and moderate nervous manifestations. These compounds are also highly toxic to bees, fish, and aquatic arthropods. Therefore, it is important to use appropriate protective measures to avoid contact with the liquid and its vapors (López-Dávila et al., 2020; Tsakirakis et al., 2022), and to respect the recommended dosage and regulations concerning irrigation and the distance between selected crop and non-selected ones, as well as open water tanks (Vryzas, 2018).
In Cuba, to increase the productivity of agricultural systems, technological packages have been introduced where the use of pesticides is the main component. The province of Sancti Spíritus is composed of eight municipalities, six of which produce tobacco (Nicotiana tabacum). The province has varied agriculture, but the main crops harvested, according to their importance, are rice, tobacco, beans, roots and tubers, sugar cane, vegetables, onions and garlic, maize, and fruits (Oficina Nacional de Estadística e Información (ONEI), 2019).
In the province of Sancti Spíritus, agriculture is one of the main sectors of the economy. To substitute imports, significant quantities of pesticides are being used on priority crops to increase yields. So far, no studies have been found on pesticide in our environment. The risk they pose to human health and the environment has motivated the search for the main difficulties that may be associated with their use. Toxicity and ecotoxicological studies are variables to be monitored in environmental quality (Claus et al., 2021).
Several developed countries have created different models capable of assessing the pressure, effect, and impact of pesticides on man and the environment. In the pesticide reduction program in Flanders, Belgium, a pesticide risk indicator was developed to assess pesticide risk reduction. The Pesticide Occupational and Environmental Risk Indicator (POCER), based on Annex VI of the 91/414/EC European Directive, consists of a module assessing the risk to humans from occupational and non-dietary exposure and the risk to the environment. Each module is evaluated using risk indices (Vercruysse & Steurbaut, 2002).
The POCER indicator has already proven its usefulness in Belgium and other countries (Bozdogan et al., 2015; Bueno & Da Cunha, 2020; Claeys et al., 2005; Cunha et al., 2012; Houbraken et al., 2016; Yarpuz-Bozdogan & Bozdogan, 2016) as a tool for reducing toxic synthetic pesticides. POCER can be used as a decision-making tool to choose alternative pesticides concerning their pressure on humans and the environment. It can also assess the impact of all pesticide applications related to a crop within a year and evaluate alternative cropping systems. (Vercruysse & Steurbaut, 2002; Wustenberghs et al., 2018).
Due to the lack of studies evaluating the pollutant pressure caused by synthetic pesticides of high (eco)toxicity used in tobacco (N. tabacum) cultivation in the province of Sancti Spíritus on human health and the environment, it is hypothesized that the use of an (eco)toxicological indicator like POCER will allow for evaluating this pollutant pressure, filling the gap caused by the use of these pesticides.
This study aimed to evaluate the toxicological the (eco)toxicological pressure exerted by synthetic pesticides on tobacco crops (Nicotiana tabacum) in the province of Sancti Spíritus, Cuba.
Materials and methods
The conception of the study
A descriptive cross-sectional study was designed in the province of Sancti Spíritus, Cuba, between 2016 and 2019, to identify potencial risks to the environment and human health from the use of pesticides in tobacco (Nicotiana tabacum) cultivation.
Operation of the variables
From a database that collects all pesticides assigned by the Provincial Plant Protection Department of Plant Health for tobacco cultivation, pesticides were classified by biological function, chemical nature, formulation, and municipality. Data were also compiled for toxicological reference values, including no observed adverse effect level (NOAEL), acceptable operator exposure level (AOEL), acceptable daily intake (ADI), acute reference doses (ARfD), persistence in soil (half-life, DT50), effect concentration (EC50), no observed effect concentration (NOEC), lethal doses (LD50), and lethal concentration (LC50) in humans, terrestrial and aquatic organisms. The hazard classification criteria of the World Health Organization (World Health Organization (WHO), 2020) were used.
Data processing procedures
Data for all variables were summarized and tabulated. Absolute and relative frequencies (descriptive statistics) were calculated using the Statistical Package for the Social Sciences (SPSS) (v. 20) and expressed as percentages for each of the variable categories described. These analyses help achieve a better perception and connotation of the results, leading to the conclusions of the research.
Toxicity and ecotoxicity assessment
In the Pesticide Occupational and Environmental Risk (POCER) indicator, risk indices (RIs) for human health and the environment are calculated as the ratio between the predicted environmental concentration (PAC) and a toxicological reference value, such as an acceptable operator exposure level (AOEL). After assessing the relevant risk parameters, POCER calculations can be performed by inserting the parameters (equations 1-10) into the model, which results in ten values, one for each of the human and environmental compartments (Vercruysse & Steurbaut, 2002). The calculated RI values are logarithmically transformed, and benchmarks are set between a lower limit and an upper limit, resulting in a dimensionless value between 0 and 1 for each compartment, where 0 indicates a low risk and 1 indicates a high risk of exposure (Vercruysse & Steurbaut, 2002; Wustenberghs et al., 2018).
The total risk for human and environmental exposure is calculated in POCER by summing the values of the different components, assuming that all components are equally important. The risk to humans is therefore the sum of the risk to the applicator, the worker, the resident, and the bystander. The risk to the environment is calculated as the sum of the risk of persistence, leaching to groundwater, aquatic organisms, birds, earthworms, and bees. The calculation formula for each module is described below:
Operator
Pesticide operators are the people who mix, load, and apply pesticides (Tsakirakis et al., 2022). The exposure risk of pesticide operators in the POCER indicator is assessed using the European Prophetic Operator Exposure Model (EUROPOEM). This model has been produced as a draft, resulting in a Concrete Equation (CE), and is based on a database of relevant studies representing iEuropean practices (Vercruysse & Steurbaut, 2002). Subsets of this database have been formed for generic exposure scenarios. The data are segregated into the categories of mixing, loading, and application, with further designation according to determinants such as type of formulation and type of application equipment. The pesticide operator risk index (Operator RI) is calculated using EUROPOEM in equation (1) as the quotient of the internal exposure and the acceptable operator exposure level (AOEL).

Where, IE is the internal exposure during mixing/loading and application (mg/kg/day) and AOEL is the acceptable operator exposure level (mg/kg/day)
Worker
Workers interacting with the crop are exposed to contamination from pesticides that remain on the crop after application, such as during re-entry tasks like harvesting and folding. Dermal exposure through contact with crop foliage is considered the most significant route of exposure during re-entry activities. There is a direct relationship between exposure, the application rate, and the degree of contact between crop and the worker (Sarkar et al., 2021). During re-entry activities, inhalation exposure is very low compared to dermal exposure (Bozdogan et al., 2015; Sarkar et al., 2021). Therefore, only the dermal exposure of the worker is calculated. For risk assessment, the internal exposure, calculated as the dermal exposure multiplied by the dermal absorption factor, is compared to the systemic AOEL. The internal exposure should be divided by the worker’s body weight (default = 70 kg) since the AOEL is expressed in (mg/kg/day). The risk index for workers is calculated using equation (2).

Where, DE is dermal exposure (mg/kg/day) and Abde is dermal absorption (-)
Bystander
In most cases, bystander exposure occurs through airborne contact during the application process (Cunha et al., 2012; Tsakirakis et al., 2022). Currently, none of the internationally accepted models is available for bystander exposure assessment. In the POCER indicator, a bystander can experience both dermal and inhalation exposure. If it is assumed that bystanders are located at a distance of 8 m downwind of the treated field, then there could be a dermal or inhalation uptake. The risk index for the bystander is calculated using equation (3).

Where, DE is dermal exposure (mg/kg/day), I is inhalation exposure (mg/kg/day), AbI is absorption by inhalation (-), and BW is body weight
Resident
The exposure of residents is practically negligible if it is assumed that they are not involved in any activity related to the use of pesticides on crops. Very specific climatic conditions (sucha as wind) and proximity of the dwelling to the treated field would be required to consider any uptake by residents. The risk index for the resident is calculated using equation (4).

Where, DE is dermal exposure (mg/kg/day), I is inhalation exposure (mg/kg/day), AbI is absorption by inhalation (-), and Abde is dermal absorption (-).
Aquatic organisms
For agricultural conditions in some countries, exposure to aquatic organisms is primarily caused by pesticide runoff (Bueno & Da Cunha, 2020). Another route of exposure, leaching, is considered negligible. The Predicted Initial Environmental Concentration for aquatic organisms is calculated for a trench with a depth of 0.3 m and a width of 1 m. Since values are derivative (Vercruysse & Steurbaut, 2002), the factor 1000 is a conversion factor for the units. Equation (5) calculates the ratio of the Predicted Environmental Concentration (PEC) to the minimum standard for three groups of organisms (fish, daphnia and crustaceans). The safety factors used are defined in the Uniform Principles (Vercruysse & Steurbaut, 2002).

Where PEC superficial aquatic organisms is prediction of surface water concentration (g/L) and minimum standard (aquatic organisms) is low toxicity values for three groups of organisms (fish, daphnids and crustaceans) (g/L).
Birds
Birds can be exposed to pesticides when collecting food in a treated field. There are three different worst-case scenarios for bird exposure. The first scenario is the daily feed intake taken by the birds assumed to be in treated crops. Small birds have a daily feed intake of 30 % of their body weight (Tassin de Montaigu & Goulson, 2020; Vercruysse & Steurbaut, 2002). The default weight of the bird is assumed to be 10 g. The average concentration in the crop immediately after spraying is estimated by multiplying the application rate (kg a.i./ha). The Risk Index (RI) for birds is calculated in equation (6). The factor 10 is set by the Commodity Exchange Main Report.

Where PECbird is estimated total daily pesticide intake by birds (mg/day), LD50 is lethal dose for 50 % of the population (mg/kg/day), and BW is body weight (default = 0.01 kg).
Bees
The Risk Index for bees is only applicable when pesticides are actively used. In equation (7), the RI for bees is calculated. Factor 50 is established by the Uniform Principles (Vercruysse & Steurbaut, 2002).

Where, AD is application dose (g/ha) and LD50 is lethal dose for 50 % of the population (μg/bee).
Earthworms
During pesticide applications, some of the substance reaches the soil and may present a hazard to organisms such as earthworms. The risk index for earthworms is calculated using equation (8). Factor 10 is established by the main report of the Commodity Clearing House. For the initial estimation of the Estimated Pesticide Concentration in Soil (EPC in soil), it is assumed that the pesticide is concentrated in the top 5 cm of the soil. When pesticides are sprayed on crops, only a fraction reaches the soil beneath the plants, and for the other types of treatments, it is assumed that the total pesticide dose reaches the soil (Gentil-Sergent et al., 2021).

Where, PECsoil is estimated concentration in the soil (mg/kg) and LC50 is lethal concentration for 50 % of the population (mg/kg).
Soil persistence
The persistence time of a pesticide indicates its during in the soil. Acording to Annex VI of 91/414/EC (European Commission, 2022), no authorization of a plant protection product is granted if the DT50 of the pesticide in the soil is more than 90 days. In the Netherlands, no authorization is granted if the DT50 of the pesticide in the field is more than 180 days (Vercruysse & Steurbaut, 2002). These two principles are incorporated into the risk index for persistence in soil, represented by equation (9).

Where, DT50 is time in which 50 % of the pesticides disappear (days).
Groundwater
According to the EU Uniform Principles, the concentration (a.i.) of a pesticide in groundwater should be less than 0.1 mg/l (Vercruysse & Steurbaut, 2002). The Pesticide Leaching and Accumulation Model (PESTLA) is used to estimate the predicted Ambient Groundwater Concentration (AGW), which is the maximum concentration of active ingredient (a.i.) in groundwater, for all types of applications. The risk indices for groundwater are calculated with equation (10).

Where, PECgroundwater is estimated pesticide concentration in groundwater (μg/L), and 0.1 is the European drinking water limit (μg/L) reference (Vercruysse & Steurbaut, 2002).
For spray applications, only a fraction of the sprayed pesticide reaches the soil due to interception by the crop. This fraction depends on the growth stage of the crop and its surface plant area. For the other types of application such as seed and grain treatment, dipping, leaching, and powder applications directly on the field, 100 % of the a.i. reaches the soil (Cunha et al., 2012; Vercruysse & Steurbaut, 2002)
Integration of risk indices into total risk indicator values
For the integration of the risk indicators (RIs), the general method was developed by Beinat Van den Berg (1996). This method describes the extent to which a chosen index is exceeded as a dimensionless numerical value. First, a lower limit (LL) and an upper limit (UL) have to be established for the ten risk equations. Pesticides with a risk index value below the lower limit indicate a low risk (0), while when the upper limit is exceeded, a high-risk value of 1 is expected. Pesticides with a risk index below 1 fulfil the criteria formulated in the Uniform Principle of Directive 91/414/EC (European Commission, 2022). Secondly, the relative values of RI+, LL+, and UL+ are calculated by dividing respectively in equation 11 (Beinat & van den Berg, 1996).

Where, X is RI+, LL+, and UL+.
Thirdly, the risk of a pesticide to the different components is related to the extent to which the lower limit of pesticide components has been exceeded (Vercruysse & Steurbaut, 2002). The exceedance factor is calculated in equation (12).

Exceedance factor (EF) values less than or equal to 0 are set to 0 and indicate low risk, while EF values greater than or equal to 1 are set to 1 and indicate a high risk. Intermediate risk is found for values between 0 and 1. With the general method mentioned above, the pesticide risk to a particular component is expressed as a dimensionless value between 0 and 1.
The total risk of a pesticide to humans and the environment is calculated by summing the values of the ten components if it is assumed that all components are equally important. Thus, using the POCER indicator to calculate the total risk of a pesticide to humans and the environment will provide a value from 0 to 10 (Vercruysse & Steurbaut, 2002).
Tobacco samples analysis
Ten tobacco crop samples from farmers in the municipality of Cabaiguán underwent analysis using gas and liquid chromatography to detect the possible presence of trace pesticides. This approach enabled the traces to be correlated with the products applied. The municipality of Cabaiguán was selected due to its status as the highest tobacco producer and consumer of pesticides in this crop. Additionally, data from the interviews conducted with 50 farmers in the municipality of Cabaiguán, as part of the doctoral study by López-Dávila, Torres et al. (2020), were utilized to determine the pesticides used in the tobacco cultivation and to correlate them with those identified during the study period.
QuEChERS
For the analysis of the tobacco leaves, the QuEChERS method was utilized. QuEChERS stands for Quick, Easy, Cheap, Effective, Rugged & Safe, providing a highly beneficial analytical approach that simplifies the analysis of multiple pesticide residues in fruits, vegetables, cereals, and processed products. The method consist of several straightforward analytical steps, making it quick and easy to implement while minimizing susceptibility to errors. QUEChERS offers high recoveries for a wide range of pesticides belonging to different chemical classes (Huang et al., 2019; Musarurwa et al., 2019).
Procedure to analyze tobacco crop samples
Two grams of the homogeneous sample are added in 50 ml Teflon centrifuge capped tubes. Milli-Q water is added until the sample weight reaches 10 g. Then, 15 mL of acetonitrile (ACN) from VWR Prolabo, Belgium, is added to the samples. To extract the contaminating co-extracts, the following extraction salts from Sigma Aldrich, Belgium, are added to the samples: 1.5 g NaCl, 1.5 g Na3HCitrate dihydrate, 0.75 g Na2HCitrate sesquihydrate, and 6.0 g MgSO4. The addition of these salts reduces the noise in the chromatograms. The samples are mixed for 5 minutes at 300 rpm and then centrifuged for 5 min at 10,000 rpm in the centrifuge (Eppendorf, Belgium).
The solvent change differs in the samples for liquid chromatography with mass spectrometry detector (LC-MS/MS) compared to gas chromatography with electron capture detector (GC-ECD) (Claus & Spanoghe, 2020). In the case of LC-MS/MS, 1 mL is taken from the top layer of the tube and added to a 10 mL volumetric vial; 9 mL of Milli-Q water is added to the 10 mL vial. This dilutes the sample 10-fold. The mixture is thoroughly diluted to obtain a homogeneous solution. A subsample of approximately 1.5 mL is pipetted into an LC-MS/MS vial. For GC-ECD, 5 mL is taken from the top layer of the tube and added to an evaporation balloon. The solvent (ACN) is evaporated using a rotary evaporator (Buchi SL 200, Germany). Then, 5 mL of hexane is added to the balloons. A subsample of approximately 1.5 mL is pipetted into a GC-ECD vial (López Dávila, Houbkaren, et al., 2020).
Analytical procedure
Samples are analyzed using LC-MS/MS and GC-ECD. The working conditions are described below.
LC-MS/MS operating conditions
For operation in the MS/MS mode, the following parameters are set: curtain gas (N2) at 7 bar; temperature 500 °C (López Dávila, Houbkaren et al., 2020). Samples are analyzed using a Waters ACQUITY UPLC™ equipped with a quaternary pump. The separation column, an Acquity UPLC BEH C18 (130 Å, 1.7 µm, 2.1 mm X 50 mm), is maintained at 40 °C. An automatic injector is set to inject 10 µL per sample. The mobile phase components are (A) Milli-Q water with 0.1 % with formic acid and (B) acetonitrile with 0.1 % formic acid. The gradient used is adjusted to a flow rate of 0.4 mL/min with 98 % mobile phase A for 0.25 min. From 0.25 min to 7 min, a linear gradient to 98 % mobile phase B is applied, which is maintained for 1 min. Then, a linear gradient of 98 % mobile phase A is used and maintained for 1 min. The assay is performed on a triple quadrupole system with electrospray ionization (detection mass spectrometer, Waters Xevo® TQD; Waters, Zellik, Belgium). The capillary needle is maintained at +2kV.
For operation in MS/MS mode, the following parameters are set: cut-off gas (N2) at 7 bar; temperature 500 °C (López Dávila, Houbkaren et al., 2020). The a.i. is monitored and quantified using multiple reactions monitoring (MRM). Two different m/z transitions are selected for each analyte. The MS/MS-transitions, ionization mode, cone voltage, and collision energy are detailed in Table 1. Generally, the limit of quantification (LOQ) is set at 0.001 mg/kg and the limit of detection (LOD) at 0.0003 mg/kg, respectively.
Table 1 Tandem mass spectrometry (MS/MS) transitions, applied collision energy, and running time for the active ingredient (a.i.) of pesticides used in tobacco cultivation in Sancti Spíritus province, Cuba, analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS).
| Pesticide | Pesticide ion (m/z) | Fragment ion (m/z) | Ionization mode (/) | Cone voltage (eV) | Collision energy (eV) | Residence time (ms) |
| methomyl | 163 | 88 | ES | 20 | 10 | 0.017 |
| 163 | 106 | ES | 20 | 10 | 0.017 | |
| acephate | 184.1 | 125.1 | ES | 11 | 18 | 0.052 |
| 184.1 | 143 | ES | 11 | 8 | 0.052 | |
| pyrimethanil | 200 | 82 | ES | 45 | 24 | 0.015 |
| 200 | 107 | ES | 45 | 24 | 0.015 | |
| thiodicarb | 355 | 87.9 | ES | 20 | 16 | 0.015 |
| 355 | 107.9 | ES | 20 | 16 | 0.015 | |
| difenoconazole | 406 | 111.1 | ES | 40 | 60 | 0.015 |
| 406 | 251.1 | ES | 40 | 25 | 0.015 | |
| malathion | 331 | 99 | ES | 20 | 24 | 0.013 |
| 331 | 127 | ES | 20 | 12 | 0.013 | |
| dimethoate | 230.1 | 125 | ES | 18 | 20 | 0.012 |
| 230.1 | 199 | ES | 18 | 10 | 0.012 | |
| tebuconazole | 308 | 70.1 | ES | 40 | 22 | 0.015 |
| 308 | 125 | ES | 40 | 40 | 0.015 | |
| chlorpyrifos | 349.9 | 97 | ES | 30 | 32 | 0.037 |
| 349.9 | 198 | ES | 30 | 20 | 0.037 | |
| imidacloprid | 256.1 | 175.1 | ES | 34 | 20 | 0.038 |
| 256.1 | 209.1 | ES | 34 | 15 | 0.038 | |
| parathion | 291.9 | 110 | ES | 30 | 33 | 0.017 |
| 291.9 | 236 | ES | 30 | 14 | 0.017 | |
| diazinon | 305 | 96 | ES | 31 | 35 | 0.017 |
| 305 | 169 | ES | 31 | 22 | 0.017 | |
| propiconazole | 342 | 69 | ES | 40 | 22 | 0.017 |
| 342 | 159 | ES | 40 | 34 | 0.017 | |
| methamidophos | 142 | 93.9 | ES | 28 | 13 | 0.163 |
| 142 | 124.9 | ES | 28 | 13 | 0.163 |
*ES: electrospray. / ES: electropulverización.
Gas chromatography with electron capture detection
Pesticides were analyzed using an Agilent Technologies 6890N gas chromatograph equipped with an Agilent Technologies 7683 Series automatic injector coupled to an electron capture detector (GC-ECD). Separation was carried out on an HP-5MS capillary column (phenyl methyl siloxane 5 %, 30 m × 0.25 mm, 0.25 μm). The operating conditions were as follows: initial column temperature set at 60 °C, then increased at a rate of 20 °C/min until 150 °C. Subsequently, it was increased at a rate of 15 °C/min up to 250 °C, held for 2 min at 250 °C, further increased at a rate of 30 °C/min up to 270 °C and held constant for 10 min at 270 °C. Finally, it was increased at a rate of 30 °C/min to 280 ° and held for 11 min. The injector and detector temperatures were maintained at 200 °C and 250 °C, respectively. Helium was used as the carrier gas at a flow rate of 1.1 mL/min, and injections were made in the cut-off mode at a ratio of 52.7:1 (López Dávila, Houbkaren, et al., 2020).
The retention times of the active ingredients of interest analyzed in the GC are as follows: chlorothalonil at 11.4 min., captan at 13.6 min, endosulfan at 14.2 min, and cypermethrin at 24.2 min. To assess selectivity, individual solutions were injected, followed by a mixture of the normal solutions and a mixture of the formulated a.i. Blank tests (n=3) were performed following the analytical extraction method to check for the absence of interference peaks under the same conditions regarding degradation products, impurities, and matrix effects. Accuracy was evaluated using the placebo recovery method (European Commission Directorate General for Health and Food Safety (DG SANTE), 2021). The tobacco reference is injected at a concentration of 100 µL x 10 mg/L to determine the recovery at the maximum expected amount of a.i. The samples were analyzed under the same conditions, and the ratio of the calculated amount to the expected amount expressed as a percentage was used to evaluate the recovery.
The linearity of each a.i. was determined through linear regressions of the calibration curve for five concentration levels between 0.004 and 0.1 mg/kg for each a.i., and the coefficient of determination (R2) was calculated. Repeatability is evaluated by the coefficient of variation (CoV) of the measurement of each standard concentration and each a.i. The limits of detection (LOD) and quantification (LOQ) shown in Table 2 were determined by the method of the t99sLLMV (Bernal, 2014).
Table 2 Limit of detection (LOD), the limit of quantification (LOQ), and coefficient of determination (R2) of the active ingredients used in tobacco cultivation, tested by gas chromatography with electron capture detector (GC-ECD). Sancti Spíritus, Cuba. 2016-2019.
| Active ingredients | (LOD (µg L-1) | LOQ (µg L-1) | R2 |
| chlorothalonil | 0.003 | 0.01 | 0.9998 |
| alachlor | 0.003 | 0.01 | 0.9998 |
| endosulfan | 0.003 | 0.01 | 0.9994 |
| bifenthrin | 0.003 | 0.01 | 0.9995 |
| λ-cyhalothrin | 0.003 | 0.01 | 0.9999 |
| cypermethrin | 0.003 | 0.01 | 0.9982 |
Tobacco recoveries are consistently low and require correction. The obtained values of percent recoveries of the a.i. analyzed by GC-DCE and LC-SM/SM were adjusted for recoveries below 70 % or above 120 %. The dilution made during sample preparation is also factored into the calculations. Pesticide recoveries in tobacco were generally low due to interference or matrix effects (DG SANTE, 2021; López-Dávila, Houbkaren, et al., 2020).
Description of the applied survey
Fifty farmers representative of the municipality of Cabaiguán (selected from farmers with average yields across all tobacco cooperatives in the region and references from the municipality’s tobacco growers’ association) were interviewed. A summary of the main questions is described below in Table 3. It should be noted that to the author’s knowledge, and in the absence of bibliographical evidence of any previous study, this is the first study on the subject to be conducted not only in the municipality under study but in the country as a whole.
Table 3 Main questions of the survey on risk perception and pesticide use among tobacco (Nicotiana tabacum) growers in the province of Sancti Spiritus, Cuba.
| Question |
| Socio-demographic background of study farmworkers |
| What is your: age/gender/education level/family members / total hectares / Did you receive training to apply pesticides? |
| Knowledge and use of pesticides |
| Where do you store pesticides? Do you follow the label instructions? |
| What outfit do you usually wear during pesticide application? |
| How close to a water body, do you spray pesticides? |
| Pesticide application |
| What do you mainly spray against? / Which products do you use? |
| How many hours do you work in the field? |
| How many pesticides do you apply per month? |
| What type of equipment do you use to apply pesticides? |
| Farmers’ risk perception of human and environmental health by the use of pesticides |
| What is the field re-entry interval? What is the pre-harvest interval? |
| Do you consider that the use of pesticides can affect your health & the environment? / What health risks are related to working with pesticides? |
Results
Generalities of the active ingredients (a.i.) used in the province of Sancti Spíritus on the tobacco crop in the period 2016-2019
In this initial section, the aspects of the active ingredients (a.i.) used during the studied period are presented with the support of a database containing all the pesticides assigned by the Provincial Department of Plant Protection of Plant Health in the province of Santi Spíritus for the tobacco crop. Figure 1 depicts the total consumption of a.i. for tobacco cultivation by the municipality during the period under studied.

Figure 1 Total consumption of active ingredients (a.i) (kg) in tobacco (Nicotiana tabacum) cultivation by the municipality in the period studied. Source: Pesticide allocations to the province of Sancti Spíritus, Cuba. 2016-2019
Just the municipalities of Cabaiguán and Sancti Spíritus, depicted in Figure 1, account for 74 % of the consumption of the a.i. allocated to the province. This aligns with the agricultural strategy of the province, where Taguasco, Sancti Spíritus, and Cabaiguán collectively represent 87 % of the total a.i. used during the studied period. The municipalities of Trinidad and La Sierpe are not included, as they are not part of the provincial strategy for tobacco cultivation. Moreover, Trinidad historically focuses on coffee and livestock production, while La Sierpe is known for livestock and rice production, being considered the second rice pole in the country.
Fungicides are the most utilized chemical family at the municipal level, followed by insecticides and herbicides (Figure 1), except for Fomento, which consumed more insecticides. This variation could be attributed to Fomento’s primary crop being coffee, which is susceptible to attack by Broca (Hypothenemus hampei), necessitating the use of insecticides. At the provincial level, 66.2 t of fungicides, 20.4 t of insecticides, and 5.29 t of herbicides were used, representing 71 %, 22 %, and 7 % of the total consumption (91.9 t), respectively.
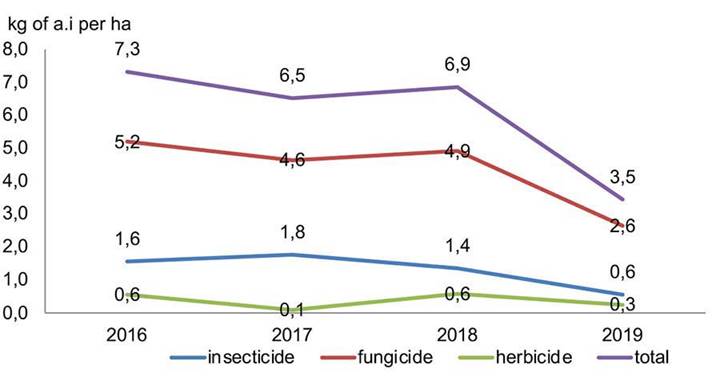
In general terms, as illustrated in Figure 2, there was a 50 % decrease in pesticide consumption during the studied period. This decline corresponds to the national policy of phytosanitary protection, aiming to reduce the toxic pollutant load and mitigate potential effects on the environment and human health. While herbicides and insecticides show relativity stable trends due their lower a.i. usage per unit area, fungicides exhibit a more pronounced reduction owing to their higher a.i. usege.
Results of the interview with farmers
A total of 67 active ingredients (a.i.) were reported by the farmers during the interview, and 45 % of these did not match those assigned by the Provincial Plant Health Department. Considering that the list of a.i. assigned by the Provincial Department of Plant Health comprises only 59 a.i., it can be inferred that the interviewed farmers utilized additional a.i. not officially designated for tobacco crop. The use of a.i. in non-assigned crops poses a significant risk to the health of the crop, as well as to the applicator and agricultural worker, besides the potential risk of pest resistance.
Among the most commonly used a.i., as detailed in Table 4, five (acephate, cypermethrin, fosetyl aluminium, imidacloprid, and mancozeb) are common between those declared by farmers and those assigned by plant health. Furthermore, there is substantial correspondence between the 10 most used chemical families reported by farmers and those assigned by plant health (Table 4).
Table 4 Main active ingredients and chemical families reported by farmers and assigned by the Provincial Department of Plant Health for the cultivation of tobacco (Nicotiana tabacum) in Sancti Spíritus, Cuba. 2016-2019.
| Declared by the farmers | Assigned at Provincial Level | |||||
| Active ingredient | Frequency | % | Active ingredient | Frequency | % | |
| 2,4-D | 3 | 2.5 | acephate | 19 | 7.9 | |
| acephate | 4 | 3.3 | cypermethrin | 8 | 3.3 | |
| bifenthrin | 5 | 4.1 | diazinon | 7 | 2.9 | |
| cypermethrin | 4 | 3.3 | folpet | 8 | 3.3 | |
| chlorothalonil | 4 | 3.3 | fosetyl aluminium | 8 | 3.3 | |
| difenoconazole | 3 | 2.5 | imidacloprid | 7 | 2.9 | |
| fosetyl aluminium | 3 | 2.5 | mancozeb | 22 | 9.1 | |
| imidacloprid | 6 | 4.9 | methomyl | 7 | 2.9 | |
| mancozeb | 8 | 6.6 | novaluron | 10 | 4.1 | |
| tebuconazole | 6 | 4.9 | thiodicarb | 6 | 2.5 | |
| Chemical family | Frequency | % | Chemical family | Frequency | % | |
| phenoxy acetic acid derivative | 2 | 1.7 | aryloxyphenoxypropionates | 6 | 2.5 | |
| aryloxyphenoxypropionates | 4 | 3.3 | benzoylurea | 18 | 7.5 | |
| avermectins | 2 | 1.7 | carbamates | 16 | 6.6 | |
| carbamates | 3 | 2.5 | inorganic compound | 6 | 2.5 | |
| inorganic compound | 3 | 2.5 | dithiocarbamates | 29 | 12 | |
| dithiocarbamates | 3 | 2.5 | phthalimide | 9 | 3.7 | |
| neonicotinoids | 4 | 3.3 | neonicotinoids | 7 | 2.9 | |
| organophosphates | 5 | 4.2 | organophosphates | 45 | 19 | |
| pyrethroid | 5 | 4.2 | pyrethroid | 16 | 6.6 | |
| triazoles | 4 | 3.3 | triazoles | 8 | 3.3 | |
The predominant chemical families include carbamates, triazoles, aryloxyphenoxypropionates, organophosphates, neonicotinoids, inorganic compounds (copper oxychloride), dithiocarbamates, and pyrethroids. While a total of 39 chemical families were reported by farmers, Plant Health assigned only 35. This mirrors the situation observed with a.i., where farmers report a higher number of chemical families compared to those assigned by Plant Health.
A decentralized analysis by the municipality revels that the a.i. methyl parathion (Ia) and methomyl (Ib), classified as extremely and highly hazardous to humans, respectively, were used in five out of the six municipalities cultivating tobacco. Methamidophos (Ib) was exclusively used by the municipality Sancti Spíritus in 2016. Among the moderately toxic (II) classification for humans, insecticides used during the study period pose the greatest risk to human health at the provincial level.
During the study period, certain a.i. exhibited a degree of toxicity (extremely and/or highly hazardous) to the humans and the environment. Some of these a.i. are either no longer used or their usege is restricted in several countries, particularly in Europe and North America. Table 5 list the a.i. assigned by the Provincial Plant Health Department that are prohibited for use and/or restricted by the European Food Safety Agency and the Environmental Protection Agency of the United States of America.
Table 5 List of active ingredients banned for use by the European Food Safety and the Environmental Protection Agency of the United States.
| Active ingredient | Prohibited in EC and/or USA | Restricted | Cancerogenic | Endocrine disruptor |
| acephate | x | |||
| bifenthrin | x | x | ||
| captan | x | |||
| β-ciflutrina | x | |||
| chlorothalonil | x | |||
| deltamethrin | x | x | ||
| diazinon | x | x | ||
| diquat | x | |||
| phenthoate | x | |||
| fluazifop-p-butyl | x | |||
| glyphosate | x | |||
| glufosinate - ammonium | x | |||
| imidacloprid | x | |||
| lufenuron | x | |||
| malathion | x | x | ||
| maneb | x | x | x | |
| methamidophos | x | |||
| methomyl | x | x | ||
| paraquat | x | x | ||
| parathion methyl | x | |||
| quizalofop -p- ethyl | x | |||
| tetraconazole | x | |||
| tiodicarb | x | |||
| zineb | x | x | x |
As evident, approximately 19 % of the assigned a.i. are prohibited for use, with a similar percentage being restricted. Moreover, certain a.i. are classified as possible or probable carcinogens and/or endocrine disruptors, predominantly organophosphates, pyrethroids, and dithiocarbamates. Analyzing these findings suggests that the tobacco crop can be regarded as a crop of moderate toxicity and ecotoxicity, primarily based on the assigned compounds and their impact on human health and the environment.
To evaluate the possible risk of toxicity to humans and ecotoxicity to fish and bees, a characterization of the a.i. used during the study period is provided below.
Toxic and ecotoxic assessment of tobacco cultivation
The examination of pesticide toxicology and ecotoxicity is crucial for comprehending the risks posed to the environment and human health. By identifying the most concerning molecules, initiatives can be devised to eliminate or replace them with less toxic alternatives, thereby mitigating their adverse impact on the environment and human well-being. Upon analyzing Figure 3, it becomes apparent that although fungicides were the most widely utilized pesticides in the province (as indicated in Figure 1), their impact on humans and the environment did not rank the highest.

Figure 3 The trend of toxicity and ecotoxicity by biological function of the products used in tobacco (Nicotiana tabacum) cultivation in Sancti Spíritus, Cuba, over the study period. 2016-2019.
The active ingredients of insecticides (organophosphates, carbamates, pyrethroids, neonicotinoids) have a greater impact on the environment and human health compared to the main chemical families of fungicides and herbicides. Consequently, insecticides exert significant pressure on the POCER, which calculates the risk of total intended pesticide use. Despite a slight trend towards decreasing kilograms of a.i. per hectare during the study period (Figure 1), the POCER indicator indicates contrasting results. Overall, the toxic and ecotoxic pressure from both fungicides and herbicides exhibited an increasing tendency. While Insecticides showed an irregular decrease, their values remained quantitatively higher than the pressures exerted by fungicides and herbicides.
Quantitatively, the toxic pressure outweighs the toxic pressure on humans. This perspective is supported by the results depicted in Figures 3 and 4 (below). Often, humans fail to recognize the damage that synthetic pesticides can inflict on the environment, where natural pest controllers coexist, causing losses and damage to crops. Pesticides thus become double-edged weapons: while they control pests, they also eliminate their natural controllers, leaving crops dependent on synthetic chemicals for future pests control.

Figure 4 The sum of toxic and ecotoxic pressure calculated by POCER for the products used in tobacco (Nicotiana tabacum) cultivation in Sancti Spíritus, Cuba, for the period 2016-2019
Correct use of personal protective equipment (PPE) such as gloves, masks, and respiratory filters can reduce risk rates in treated field re-entry activities. A practical example of these benefits is shown in Figure 5, where the POCER indicator was used to model two scenarios; the first (a) without PPE and the second (b) with the appropriate PPE depending on the activity carried out. This figure demonstrates that the sole use of appropriate protective equipment can reduce the pressure on human health by more than 50 % in most scenarios.

Figure 5 Use of the POCER indicator in evaluating the benefit of personal protective equipment (scenario b) in reducing toxic pressure (scenario a) from products used in tobacco (Nicotiana tabacum) cultivation for the period 2016-2019 in Sancti Spíritus, Cuba.
The objective of POCER is to evaluate the pressure, from low (0) to high risk (1), exerted by a pesticide in each of the evaluated modules. This information enables decision-makers to determine whether to prohibit the use of certain high-risk products or replace them with others that fulfill the same phytosanitary function but with less risk. Figure 6 illustrates this concept. As shown, organophosphates (methamidophos, diazinon, acephate, methyl parathion) present the highest values of polluting pressure and exert pressure on a greater number of modules compared to other i.a. used in pest control for tobacco cultivation. By simply substituting this chemical family, a significant reduction in contaminant pressure would be achieved (Figure 6).
Chemical residues
In total, 16 different a.i. were detected (Tables 6 & 7). A minimum of six a.i. was detected in the samples analyzed. The highest frequencies of detection were eight a.i. (30 %) and ten a.i. (30 %). Up to 12 a.i. were detected in one sample. Forty-five percent of these residues was quantifiable. Tebuconazole and chlorothalonil do not appear on the Cuban list of pesticides authorized for use in tobacco cultivation. Such unauthorized use of certain a.i. can contribute to the development of pest resistance. Four a.i. of the 16 detected (thiodicarb, methyl parathion, methamidophos, and endosulfan) are banned for use in Europe. Endosulfan is also listed in Annex III of the Rotterdam Convention as a chemical banned or restricted for use. This exemplifies how developing countries, like Cuba, continue to use of some banned products.
Table 6 Active ingredients detected in tobacco (Nicotiana tabacum) leaves sampled from forms of the farmers interviewed. Sancti Spíritus, Cuba. 2016-2019.
| Sample | Active ingredients (mg/kg) | |||||||
| cypermethrin | tebuconazole | captan | chlorothalonil | metamidofós | pyrimethanil | valifenalate | difenoconazole | |
| Tobacco 1 | < LC | 0.01 | < LD | < LC | < LC | |||
| Tobacco 2 | 0.04 | < LD | ||||||
| Tobacco 3 | 0.04 | 0.41 | 0.01 | < LD | < LC | <LD | ||
| Tobacco 4 | < LC | 0.41 | 0.02 | < LD | < LC | |||
| Tobacco 5 | < LC | 0.41 | 0.01 | < LD | ||||
| Tobacco 6 | 0.10 | < LC | 0.41 | 0.01 | < LD | < LC | ||
| Tobacco 7 | 0.01 | < LC | 0.01 | < LD | < LC | < LC | ||
| Tobacco 8 | 0.02 | < LC | 0.41 | 0.01 | < LD | < LC | 0.05 | |
| Tobacco 9 | < LC | 0.01 | < LD | |||||
| Tobacco10 | 0.07 | 0.41 | 0.01 | < LD | ||||
| % recovery | 48.4 | 57.0 | 56.4 | 54.3 | 50.2 | 40.9 | 45.2 | 46.9 |
| (CV) | (5.72) | (2.32) | (14.0) | (8.67) | (10.36) | (8.62) | (12.5) | (14.0) |
Table 7 Continuation of the active ingredients detected in the tobacco (Nicotiana tabacum) leaves sampled from the farms of the farmers interviewed. Sancti Spíritus, Cuba. 2016-2019.
| Sample | Active ingredients (mg/kg) | |||||||
| parathion methyl | thiodicarb | ethoxysulfuron | fipronil | chlorpyrifos | thiamethoxam | endosulfan | phenthoate | |
| Tobacco 1 | <LC | 0.04 | <LD | |||||
| Tobacco 2 | 0.11 | <LC | < LC | <LC | 0.12 | <LD | ||
| Tobacco 3 | 0.06 | <LC | 0.02 | <LC | 0.09 | <LD | ||
| Tobacco 4 | 0.05 | 0.03 | <LC | 0.06 | <LD | |||
| Tobacco 5 | <LC | 0.077 | 0.05 | <LD | ||||
| Tobacco 6 | <LC | 0.04 | <LD | |||||
| Tobacco 7 | 0.03 | <LC | 0.10 | <LD | ||||
| Tobacco 8 | <LC | 0.09 | <LD | |||||
| Tobacco 9 | <LC | 0.04 | <LD | |||||
| Tobacco 10 | 0.02 | 0.04 | <LD | |||||
| % recovery (CV) | 42.0 (11.8) | 42.0 (3.79) | 49.6 (13.1) | 49.3 (6.05) | 49.4 (10. 6) | 54.3 (9.63) | 47.5 (6.66) | 53.7 (6.93) |
Discussion
The authors of the study recommend increased supervision, advice, and vigilance by phytosanitary signalers to reduce infractions and incorrect use of chemicals, which could cause severe damage to crop production and the farmer’s health. A similar amount (41 chemical families) was applied in other provinces of equal agricultural importance in the country (Pérez-Consuegra, 2018).
One active ingredient (a.i.), methyl parathion, is classified by the WHO (2020) as extremely hazardous (Ia). Two others a.i.s (methomyl and methamidophos) are classified as highly hazardous (Ib). Additionally, another 16 compounds (27 %) fall into the moderately hazardous category (II). Approximately 37 % of the products show some degree of toxicity to bees, posing an important environmental risk that could lead to decrease bee populations and thus impact ecosystems (Claus et al., 2021; Claus & Spanoghe, 2020; Fevery et al., 2016). Furthermore, 71 % of the a.i.s have some degree of toxicity in fish.
In agreement with the results obtained in Figure 4, several authors have noted that due to the persistence of some pesticides in the soil and their capacity to leach into groundwater and water bodies, aquatic organisms, according to the POCER indicator, are the main modules at risk from the use of highly toxic herbicides such as paraquat, and prometryn, as well as organophosphate insecticides (Bozdogan et al., 2015; Fevery et al., 2016; Yarpuz-Bozdogan & Bozdogan, 2016). For instance, in a citrus region of Spain, chlorpyrifos (organophosphates) were the pesticides most commonly found to be ecotoxic to aquatic organisms (Bueno & Da Cunha, 2020; Cunha et al., 2012). It is known from previous studies that about 90 % of the farmers in the province do not use personal protective equipment (PPE) (López-Dávila, Torres, et al., 2020).
Communication about risks and the use of PPE is facilitated by pictograms on pesticide labels (Bagheri et al., 2021; FAO & WHO, 2020). It is recommended that the farmers be informed about the toxic risks they are exposed to, as well as the importance of using PPE to minimize exposure to pesticides and prevent health damage (Damalas et al., 2019; Yarpuz-Bozdogan & Bozdogan, 2016).
Since the tobacco plant is not considered human food or animal feed, there are no Maximum Residue Limit values available in Cuba, nor in the North American or European Environmental Protection Agency (López Dávila, Torres, et al., 2020). In 1979, the Cuban delegation to the Codex Committee on Pesticide Residues highlighted the need to discuss pesticide residues in tobacco crops due to their importance in international trade (FAO, 1979). Today, there are maximum residue level guidelines for most a.i.s used in tobacco leaf production from the Center for Cooperative Research in Tobacco Science and Technology (CORESTA) (Papenfus, 2017). However, many values vary between countries, and there remains a need for recognition by international regulatory bodies and authorities such as EFSA and U.S. EPA.
Some a.i.s found in the tobacco leaf samples have the same mode of action (methamidophos, chlorpyrifos, phenthoate and methyl parathion, plus cypermethrin; tebuconazole, and difenoconazole) and should be alternated with a.i.s with different modes of action to prevent resistance development (Fungicide Resistance Action Committee, 2018; Insecticide Resistance Action Committee, 2018).
It is relevant to note that the a.i.s (endosulfan, ethoxysulfuron, fipronil, thiamethoxam, chlorpyrifos and phenthoate) found in the tobacco leaves sampled were not assigned to the tobacco crop. This highlights the negligence and poor agricultural practices of some farmers who use certain chemical formulations on non-assigned crops, increasing the risks of pests resistance and compromising crop health, the environment, and human health. Endosulfan, chlorpyrifos, phenthoate, cypermethrin, methamidophos, and chlorothalonil are the most prevalent in the samples analyzed. Future actions should be focus on raising farmers’ awareness of these poor practices and systematically controlling the pest control products used in tobacco cultivation and other crops.
Conclusions
The interview with local farmers revealed the incorrect use of pesticides not assigned to tobacco cultivation. Utilizing the POCER indicator allows for more precise analyses of the toxicity and ecotoxicity of synthetic pesticides. Eliminating or substituting higher-pressure active ingredients with lower-pressure ones will significantly reduce the risk exerted on human health and the environment. A wide range of pesticides was detected in samples collected from tobacco.
The authors consider that phytosanitary control should be increased in rural areas to prevent the inappropriate use of synthetic products on unauthorized crops. Additionally, there is a need to increase the knowledge of agricultural producers through a training system aimed at the rational use of pesticides in production systems. It is also important to promote the implementation of Integrated Pest Management (IPM) and Ecological Pest Management (EPM) programs.