Introduction
Andinia (Luer) Luer was re-circumscribed by Wilson et al. (2017) to reflect phylogenetic affinities and to reduce taxonomic confusion. The new circumscription incorporated species of uncertain affinity previously assigned to various different genera, including Lueranthos Szlach. & Marg., Masdevalliantha (Luer) Szlach. & Marg., Neooreophilus Archila, Pleurothallis R.Br., Stelis Sw. and Xenosia Luer. Wilson et al. (2017) conducted the phylogenetic analyses based only on nuclear internal transcribed spacer (nrITS) sequences and the plastid matK gene. However, a study by Doucette (2017) incorporated the low-copy nuclear gene phyC and the plastid sequences trnL-F and ycf1 and also found strong support for the clade representing Andinia sensuWilson et al. (2017). While there are no rules for generic re-circumscription and the outcomes can sometimes be contentious, Wilson et al. (2017) circumscribed a genus representing a strongly supported monophyletic clade that encompassed similar phylogenetic depth to that occurring in the related genera Dryadella Luer, Muscarella Luer, Platystele Schltr., Specklinia Lindl., Scaphosepalum Pfitzer and Teagueia (Luer) Luer (Chumová et al. 2021, Doucette et al. 2017, Karremans 2016, PérezEscobar et al. 2017, Wilson et al. 2017).
A recent criticism of the genus as circumscribed by Wilson et al. (2017) was not the absence of a synapomorphy per se, but the lack of a consistent morphology for all members of Andinia (Szlachetko et al. 2022). In particular Szlachetko et al. (2022) contrasted the Lepanthes-like flowers and pendent vegetative morphology of species formerly assigned to Neooreophilus, currently assigned to subgenus Brachycladium, with the caespitose vegetative morphology of the species assigned to the other subgenera of Andinia. However, neither a synapomorphy nor a consistent vegetative or floral morphology are required in generic circumscriptions and they have not been employed in recent circumscriptions in Pleurothallidinae. Further, the circumscription by Wilson et al. (2017) appears to have general acceptance by the botanical community. Apart from the one species described by Szlachetko et al. (2022), most new pendent taxa have been described in Andinia rather than Neooreophilus (Karremans & Vieira-Uribe 2016, Karremans & Vieira-Uribe 2020, Ocupa-Horna et al. 2021) and Neooreophilus is listed as a synonym of the accepted genus Andinia in the World Checklist of Selected Plant Families (WCSP 2022).
Despite their preference for a broadly defined genus Andinia, Wilson et al. (2017) did suggest the recognition of five monophyletic subgenera: subgenus Aenigma (Luer) Karremans & Mark Wilson; subgenus Andinia; subgenus Brachycladium (Luer) Karremans & S.Vieira-Uribe; subgenus Masdevalliantha (Luer) Karremans & Mark Wilson; and subgenus Minuscula Karremans & Mark Wilson. However, although subgenera Brachycladium and Masdevalliantha were well sampled, subgenera Andinia, Aenigma, and Minuscula were less well sampled, and, therefore, several species were assigned to subgenera based on morphological similarity alone. In the current study additional species were sampled, primarily in subgenus Aenigma, to provide phylogenetic confirmation of subgeneric assignments of some of the taxa previously assigned solely based on morphology.
Luer (1986) originally created subgenus Aenigma in genus Pleurothallis to include P. dalstroemii Luer, P. ibex Luer, P. schizopogon Luer, P. trimytera Luer & R. Escobar and P. vestigipetala Luer. To these were added P. hystricosa Luer, P. pentamytera Luer and P. pogonion Luer (Luer 1994); P. panica Luer & Dalström (Luer 1996); and P. lappacea Luer (Luer 2000). At that time Andinia consisted of only two species, A. pensilis (Schltr.) Luer and A. dielsii (Mansf.) Luer (Luer 2000). However, following the inclusion of P. lappacea in their phylogeny, Pridgeon and Chase (2001) transferred all these species to the genus Andinia. With the addition of A. hirtzii Luer by Luer (2005), this brought the number of species in the genus to 13. However, neither Pridgeon (2005) nor Chase et al. (2015) proposed an infrageneric taxonomy.
Of the 13 species assigned to genus Andinia at that time, Wilson et al. (2017) sequenced all but A. hystricosa, A. hirtzii, A. ibex, A. panica and A. pentamytera. In their nrITS phylogeny, species A. dalstroemii, A. pogonion and A. schizopogon clustered with 100% bootstrap support. Since Luer (1986) designated Pleurothallis schizopogon as the type of subgenus Aenigma, this clade in Andinia was designated by Wilson et al. (2017) as subgenus Aenigma. Based on morphology, the subgenus was provisionally circumscribed to additionally include A. ibex and A. pentamytera, plus the newly described species A. sunchubambensis A.Doucette & Janovec and A. uchucayensis A.Doucette & J.Portilla.
In this study we describe and illustrate the new species Andinia peruviana, hypothesized to belong to subgenus Aenigma based on morphological similarity to A. schizopogon. The nrITS region of A. peruviana was sequenced and phylogenetic analyses performed to test this hypothesis. Furthermore, additional nrITS sequences of the previously described species A. hirtzii and A. uchucayensis were incorporated in these phylogenetic analyses to determine whether those species had been correctly assigned to subgenus Aenigma. And, additional nrITS sequences of A. trimytera and A. vestigipetala, each only represented by a single sample in the analyses of Wilson et al. (2017), were incorporated to determine whether their exclusion from subgenus Aenigma, in contradiction to the taxonomy of Luer (1986), was correct or not. To confirm or refute inclusion in subgenus Aenigma, these limited objectives are adequately achieved using nrITS sequencing alone and do not require the sequencing of multiple nuclear and plastid genes as would a complete re-assessment of the circumscription of the genus Andinia.
Materials and methods.
Phylogenetic methods.- For genetic analysis, plant material was legally purchased from the commercial nurseries Ecuagenera, Gualaceo, Ecuador; Mundiflora, Cuenca, Ecuador; or EquaflorA, Cuenca, Ecuador. Genomic DNA of the Andinia plants grown in the Colorado College living collection was extracted, the nrITS region amplified by PCR, and the PCR product purified as described in Wilson et al. (2017). Purified PCR products were sequenced with primers 17SE, 26SE, ITS1 and ITS4 (Wilson et al. 2017) at Azenta/GeneWiz (New Jersey, USA). The two forward and two reverse sequences were aligned and a consensus generated using Geneious Prime v. 2022.1.1 (Dotmatics, USA). Consensus ITS sequences truncated in front of CGG GCG GTT at the 5' end and after CCA CCC G at the 3' end were uploaded to GenBank (Table 1). ITS sequences of these species, those sequenced by Wilson et al. (2017), and others available from GenBank (Table 1) were aligned using MUSCLE (Edgar 2004) with default parameters in MEGA 11 (Tamura et al. 2021).
Maximum parsimony (MP) and maximum likelihood (ML) analyses of the nrITS matrix were conducted in MEGA 11 using default parameters and 1000 bootstrap replicates (Tamura et al. 2021). The nrITS matrix was then analyzed by Bayesian inference (BI) using MrBayes (Huelsenbeck & Ronquist 2001, Ronquist & Huelsenbeck, 2003) on XSEDE v. 3.2.7a through the CIPRES Science Gateway v. 3.3. (Miller et al. 2010) using the GTR+G+I evolutionary model (Ponert et al. 2020; Szlachetko et al. 2022). Two parallel runs of four chains were run, with 10 million generations per run and a sampling frequency of 1000 trees. The first 25% of trees were discarded as burnin. Effective sample size (ESS) was checked in Tracer v. 1.7.1 in BEAST 2 and found to be sufficient. The trees were imported into Geneious Prime v. 2022.1.1 and a consensus tree generated. Nodes with less than 0.60 posterior probability were collapsed. The resulting tree was exported as a Newick file and imported into MEGA v. 11 to allow formatting consistent with the MP and ML trees.
Taxonomic methods.- The type specimen was collected in northern Peru under permit RDG N° 247-2016-SERFOR/DGGSPFFS and the extension Resol. 430-2017-SERFOR/DGGSPFFS. Vouchers were dried as herbarium material and were deposited in the HUT and USM herbaria. Living and preserved specimens were examined for morphological and taxonomic comparisons. In addition, original descriptions of related species were reviewed and compared, and specimens from the following herbaria were consulted online: HA, MO, PRC and SEL, and no additional material of the new species was found.
The description and drawings were prepared from dissected living specimens. Floral and vegetative structures of living plants were photographed with a Canon Rebel 80D camera equipped with a Canon EF 100mm f/2.8L Macro USM lens and a pressurized Raynox DCR-250 mm super-macro lens. The images were used to create a Lankester composite digital plate (LCDP) using Adobe Photoshop CC 2020. In addition, a digital composite line drawing was made in the Procreate illustration application with an iPad 8th generation tablet. The botanical terminology used in the manuscript was consulted in Beentje (2016) and Stearn (2004).
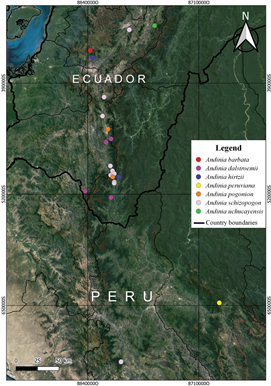
Preparation of species occurrence map.- A map showing the occurrence of species of the subgenus Aenigma was prepared using QGIS 3.10 software (Development Team 2022), using as reference the protologues and the localities of the species vouchers in the online databases (GBIF.org 2022, Tropicos 2022).
Table 1 List of ingroup taxa; collection/voucher numbers; and GenBank accession numbers for nuclear ITS sequences; and origin of sequences.
| Taxon | Collection-Voucher | GenBank Accession Number | Source |
|---|---|---|---|
| Andinia barbata J.Ponert, M.Portilla, Chumová & P.Trávn. | P998 | MT249823 | Ponert et al. (2020) |
| Andinia dalstroemii (Luer) Pridgeon & M.W.Chase | AN005 | KP012339 | Wilson et al. (2017) |
| Andinia dalstroemii (Luer) Pridgeon & M.W.Chase | AN068 | KP012339 | Wilson et al. (2017) |
| Andinia aff. dielsii | AK5429 | KC425739 | Karremans et al. (2012) unpubd. |
| Andinia hirtzii Luer | AN097 | ON391672 | This study |
| Andinia lappacea (Luer) Pridgeon & M.W.Chase | AN022 | KP012345 | Wilson et al. (2017) |
| Andinia lappacea (Luer) Pridgeon & M.W.Chase | AP108 | KC425837 | Pridgeon & Chase (2001) |
| Andinia lappacea (Luer) Pridgeon & M.W.Chase | LO1428 | KP012343 | Wilson et al. (2017) |
| Andinia longiserpens (C.Schweinf.) Karremans & Mark Wilson | AK5724 | KC425744 | Karremans et al. (2012) unpubd. |
| Andinia longiserpens (C.Schweinf.) Karremans & Mark Wilson | AN013 | KP012354 | Wilson et al. (2017) |
| Andinia longiserpens (C.Schweinf.) Karremans & Mark Wilson | AN021 | KP012356 | Wilson et al. (2017) |
| Andinia longiserpens (C.Schweinf.) Karremans & Mark Wilson | LO4515 | KP012353 | Wilson et al. (2017) |
| Andinia longiserpens (C.Schweinf.) Karremans & Mark Wilson | --- | MK294822 | Doucette (2017) |
| Andinia lynniana (Luer) Karremans & S.Vieira-Uribe | AN065 | KR827587 | Wilson et al. (2017) |
| Andinia nummularia (Rchb.f.) Karremans & S.Vieira-Uribe | AN042 | KR827578 | Wilson et al. (2017) |
| Andinia nummularia (Rchb.f.) Karremans & S.Vieira-Uribe | AN044 | KR827579 | Wilson et al. (2017) |
| Andinia nummularia (Rchb.f.) Karremans & S.Vieira-Uribe | AN045 | KR827580 | Wilson et al. (2017) |
| Andinia nummularia (Rchb.f.) Karremans & S.Vieira-Uribe | AN048 | KR827581 | Wilson et al. (2017) |
| Andinia nummularia (Rchb.f.) Karremans & S.Vieira-Uribe | AN051 | KR827584 | Wilson et al. (2017) |
| Andinia ortiziana (S.Vieira-Uribe & Thoerle) Karremans & S.Vieira-Uribe | AN071 | KP012378 | Wilson et al. (2017) |
| Andinia pensilis (Schltr.) Luer | AN002 | KP012336 | Wilson et al. (2017) |
| Andinia pensilis (Schltr.) Luer | AP200 | KP012344 | Pridgeon et al. (2001) |
| Andinia pensilis (Schltr.) Luer | --- | MK294774 | Doucette (2017) |
| Andinia pensilis (Schltr.) Luer | MWC8007 | AF262826 | Pridgeon et al. (2001) |
| Andinia peruviana Ocupa, S.Vieira-Uribe & Mark Wilson | AN135 | ON391673 | This study |
| Andinia pilosella (Rchb.f.) Karremans & S.Vieira-Uribe | AN063 | KP012375 | Wilson et al. (2017) |
| Andinia pogonion (Luer) Pridgeon & M.W.Chase | AN003 | KP012337 | Wilson et al. (2017) |
| Andinia pogonion (Luer) Pridgeon & M.W.Chase | AN110 | ON391674 | This study |
| Andinia pogonion (Luer) Pridgeon & M.W.Chase | AN117 | ON391675 | This study |
| Andinia pogonion (Luer) Pridgeon & M.W.Chase | LO3845 | KP012342 | Wilson et al. (2017) |
| Andinia pogonion (Luer) Pridgeon & M.W.Chase | LJ8293 | KP012335 | Wilson et al. (2017) |
| Andinia schizopogon (Luer) Pridgeon & M.W.Chase | AK5783 | KC425740 | Karremans et al. (2012) unpubd. |
| Andinia schizopogon (Luer) Pridgeon & M.W.Chase | AN004 | KP012338 | Wilson et al. (2017) |
| Andinia schizopogon (Luer) Pridgeon & M.W.Chase | AN069 | KP012347 | Wilson et al. (2017) |
| Andinia schizopogon (Luer) Pridgeon & M.W.Chase | AN076 | KP012350 | Wilson et al. (2017) |
| Andinia schizopogon (Luer) Pridgeon & M.W.Chase | DCI610 | MN551415 | Gutiérrez Morales et al. (2020) |
| Andinia schizopogon (Luer) Pridgeon & M.W.Chase | LO2004 | KP012341 | Wilson et al. (2017) |
| Andinia sp. | AN006 | KP012340 | Wilson et al. (2017) |
| Andinia spiralis (Ruiz & Pav.) Karremans & Mark Wilson | AN007 | KP012351 | Wilson et al. (2017) |
| Andinia spiralis (Ruiz & Pav.) Karremans & Mark Wilson | AN070 | KP012357 | Wilson et al. (2017) |
| Andinia stalactites (Luer & Hirtz) Karremans & S.Vieira-Uribe | AN024 | KP012359 | Wilson et al. (2017) |
| Andinia stalactites (Luer & Hirtz) Karremans & S.Vieira-Uribe | LO2248 | KP012374 | Wilson et al. (2017) |
| Andinia trimytera (Luer & R.Escobar) Pridgeon & M.W.Chase | AN073 | KP012349 | Wilson et al. (2017) |
| Andinia trimytera (Luer & R.Escobar) Pridgeon & M.W.Chase | AN136 | ON391676 | This study |
| Andinia uchucayensis A.Doucette & J.Portilla | AN132 | ON391677 | This study |
| Andinia vestigipetala (Luer) Pridgeon & M.W.Chase | AN075 | KR827588 | Wilson et al. (2017) |
| Andinia vestigipetala (Luer) Pridgeon & M.W.Chase | --- | MK294775 | Doucette (2017) |
| Andinia vestigipetala (Luer) Pridgeon & M.W.Chase | AN093 | ON391678 | This study |
| Andinia vieira-pereziana (P.Ortiz) Karremans & S.Vieira-Uribe | AN072 | KP012379 | Wilson et al. (2017) |
| Andinia werneri (Luer) Karremans & S.Vieira-Uribe | AN053 | KR827585 | Wilson et al. (2017) |
| Andinia xenion (Luer & R.Escobar) Karremans & Mark Wilson | AN008 | KP012352 | Wilson et al. (2017) |
| Andinia xenion (Luer & R.Escobar) Karremans & Mark Wilson | AN074 | KP012358 | Wilson et al. (2017) |
| Andinia xenion (Luer & R.Escobar) Karremans & Mark Wilson | AP250 | KP012355 | Wilson et al. (2017) |
Results
Phylogenetic analysis.-
The clade corresponding to genus Andinia, as circumscribed by Wilson et al. (2017), was strongly supported in all three analyses of nrITS sequence data: MP = 98% (Fig. 1); ML = 93% (Fig. 2); and BI = 0.96 (Fig.3). Within Andinia, all three analyses recovered the same 6 clades as those previously reported by Wilson et al. (2017), with one exception (see below). As expected, these clades correspond to the five subgenera and two sections circumscribed by Wilson et al. (2017): Aenigma, Andinia, Brachycladium section Amplectentes, Brachycladium section Brachycladae, Masdevalliantha and Minuscula. The only significant topological difference between the phylogenies was the relationships among the clades (Fig. 1 -3).
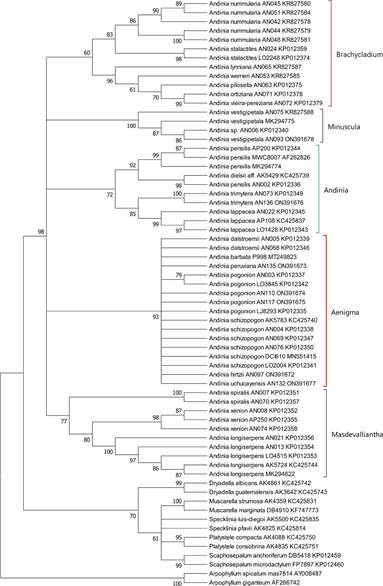
Clades corresponding to subgenera proposed by Wilson et al. (2017) are labeled.
Figure 1 Bootstrap consensus phylogenetic tree inferred from nrITS sequence data set using maximum parsimony analysis with 1000 bootstrap replicates in MEGA 11 using default parameters. Values at each node represent percent bootstrap support. Nodes with less than 60% bootstrap support are collapsed.
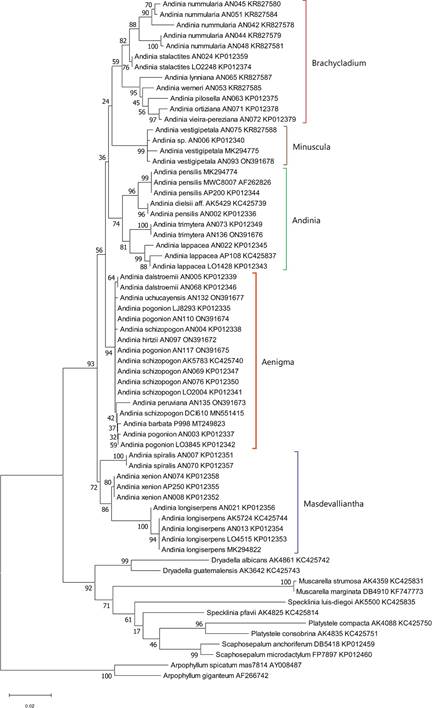
Clades corresponding to subgenera proposed by Wilson et al. (2017) are labeled.
Figure 2 Phylogenetic tree inferred from the nrITS sequence data set using maximum likelihood analysis with 1000 bootstrap replicates in MEGA 11 using default parameters. The tree with the highest log likelihood is shown. Values at each node represent percent bootstrap support.
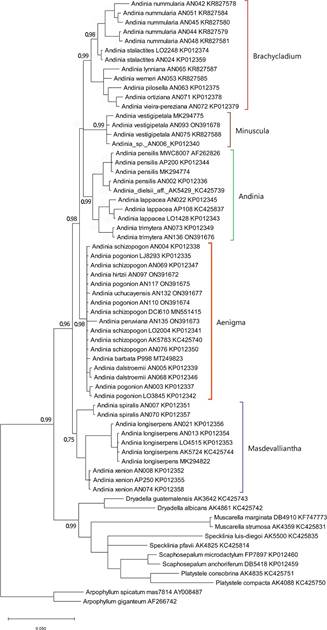
Clades corresponding to subgenera proposed by Wilson et al. (2017) are labeled.
Figure 3 Consensus phylogenetic tree inferred from nrITS sequence data using MrBayes. Values at nodes represent posterior probabilities of pertinent clades. Nodes with less than 0.6 posterior probability are collapsed.
Unexpectedly, adding two new sequences of Andinia vestigipetala and one new sequence of A. trimytera revealed an unfortunate error in the prior analyses by Wilson et al. (2017), which was the accidental switch of the sequences for A. trimytera AN073 and A. vestigipetala AN075. The error is corrected in the current analyses (Fig. 1). Consequently, the position of each of these species in the clade is different than in the prior study.
The clade corresponding to subgenus Aenigma, the focus of this study, is strongly supported in all three analyses: MP = 93% (Fig. 1); ML = 94% (Fig. 2); and BI = 0.98 (Fig. 3). The clade includes the seven species Andinia barbata, A. dalstroemii, A. hirtzii, the new species A. peruviana, A. pogonion, A. schizopogon and A. uchucayensis.
The clade corresponding to subgenus Andinia is moderately-to-strongly supported in the three analyses: MP = 72% (Fig. 1); ML = 74% (Fig. 2); and BI = 0.99 (Fig. 3). The clade includes the A. dielsii, A. lappacea, A. pensilis and now, additionally, A. trimytera.
The clade corresponding to subgenus Brachycladium section Brachycladae, including Andinia nummularia (Rchb.f.) Karremans & S.Vieira-Uribe and A. stalactites (Luer & Hirtz) Karremans & S.VieiraUribe, is moderately-to-strongly supported: MP = 83% (Fig. 1); ML = 82% (Fig. 2); and BI = 0.98. The clade corresponding to subgenus Brachycladium section Amplectentes, includes all species previously assigned to Neooreophilus other than A. nummularia and A. stalactites, is strongly supported in all three analyses: MP = 96% (Fig. 1); ML = 95% (Fig. 2); and BI = 0.99 (Fig. 3).
The clade corresponding to subgenus Masdevalliantha has low-to-moderate support: MP = 77% (Fig. 1); ML = 72% (Fig. 3); and BI = 0.75 (Fig. 3). The clade includes the species Andinia longiserpens (C.Schweinf.) Karremans & Mark Wilson, A. spiralis (Ruiz & Pav.) Karremans & Mark Wilson and A. xenion (Luer & R.Escobar) Karremans & Mark Wilson.
The clade corresponding to subgenus Minuscula has high support in all three analyses: MP = 100% (Fig. 1); ML = 99% (Fig. 2); and BI = 0.99 (Fig. 3). The clade includes only A. vestigipetala and an unflowered and now deceased Andinia, accession AN006, presumed initially to be A. trimytera based solely on its position in the phylogeny of Wilson et al. (2017), but which was probably A. vestigipetala.
The only topological difference between these analyses is that in a consensus tree, MP and ML analyses recover a polytomy of five clades corresponding to the five subgenera. In comparison, BI recovers a polytomy of four clades corresponding to subgenera Aenigma, Andinia, Brachycladium, and Minuscula, which is found to be sister to the clade corresponding to subgenus Masdevalliantha.
Taxonomic treatment
Andinia peruviana Ocupa, S.Vieira-Uribe & Mark Wilson, sp. nov. (Fig. 4-5)
TYPE: PERU. Amazonas: Prov. Bongará, Distrito Florida, comunidad campesina San Lucas de Pomacochas, camino de San Lorenzo a Yambrasbamba, 3130 m, 23 October 2020, L. Ocupa 267 (Holotype: HUT!).
Diagnosis: Andinia peruviana is most similar to Andinia schizopogon (Luer) Pridgeon & M.W.Chase (Fig. 6) but differs in the lateral sepals connate for ca. ⅔ of its length (vs. connate to less than ½ of their length), covered with hairs up to 5 mm long (vs. longpubescent-spiculate), with strongly revolute sides (vs. revolute sides), petals with tortuous cilia on the margins (vs. microscopically irregular margins), the apical mid-lobe of the lip with lacerate to filiform-papillose margins (vs. shortly muriculate), basal lobes of the lip with papillose margins (vs. entire margins).
Epiphytic herb, caespitose, repent or ascending, up to 11 cm tall. Roots white, thick, flexuous, fleshy, 1.5 mm diameter. Rhizome stout, up to 8 mm between ramicauls. Ramicauls abbreviated, terete, ascending, unifoliate, 3-7 mm long, enclosed by 1-2 tubular, membranaceous, semi-translucent, ribbed sheaths. Leaf suberect to inclined, obovate to narrowly obovate, coriaceous, foveolate on both sides, adaxially green, occasionally with dark-purple lines parallel to the mid-vein, abaxially very pale green with purple splashing, adaxially canaliculate in the central vein, margin irregularly crenulate, marginate, apex obtuse to rounded, tridenticulate, 16-35 × 8.5-9.5 mm, the base cuneate to attenuate into a canaliculate petiole 10 mm long. Inflorescence a loose, successively 1-2 flowered raceme, to 4-6 cm long including a very slender filiform peduncle, borne from the apex of the ramicaul; floral bract infundibuliform, acuminate, membranous, 1 × 2 mm. Ovary pedicellate, greenish-yellow, terete, densely villose, ca. 3-7 mm long, including the pedicel. Flowers semi-translucent, with sepals and petals greenish yellow; lip greenish yellow with burgundy papillae; column greenish yellow, becoming vinaceous towards the apex. Dorsal sepal ovate, apex long-acuminate, arched forward, shallowly concave and subcordate at the base, margins irregular and sparsely ciliated, 3-veined, abaxially tricarinate, adaxially sparsely villose, 27-28 × 4.4-4.7 mm. Lateral sepals connate for ca. ⅔ of their length into a narrowly ovate, bifid synsepal, apex long-acuminate, cordate at the base, 4-veined, adaxially covered with hairs up to 5 mm long, abaxially bicarinate, with the sides strongly revolute, 30-32 × 5-7 mm. Petals spreading, oblong, apex gradually long-acuminate, 1-veined, with tortuous cilia on the margins, 26 × 1.3 mm. Lip 3-lobed, thick, adnate to the base of the column, 2.6 × 4.1 mm unexpanded, deflexed 90 degrees near the middle, 3-veined; the central, basal part sub-quadrate, concave, extending into the apical lobe as an orbicular depression, 1.4 × 1.5 mm; the apical mid-lobe widely ovate, the apex broadly rounded, minimally obtuse, cellular-papillose, with lacerate to filiform-papillose margins, with a shallow rounded central depression, 3.56 × 1.8 mm, 3-carinae along the veins on the abaxial surface, with a high, bellshaped callus on the abaxial surface at the apex of the mid-vein; the basal lobes sub-circular, broadly rounded, erect, with papillose margins, surrounding the column. Column semi-terete, arched, dilated at base and apex, 4.5 mm long; stigma sub-apical, transversely elliptic, rostellum crescent-shaped, apical, acuminate, antrorse. Anther apical, sub-orbicular, cream purple tinged. Pollinia two, yellow, narrowly pyriform. Capsule not seen.
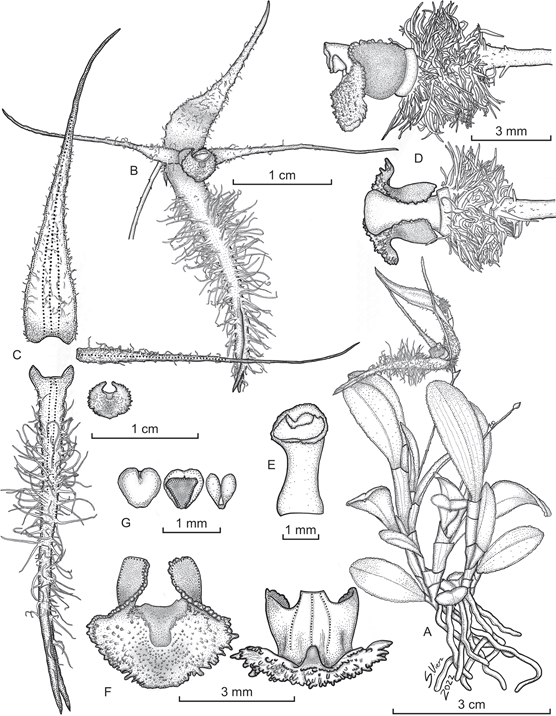
A. Habit.
B. Flower.
C. Dissected perianth.
D. Lip, column and ovary, lateral and dorsal view.
E. Column, – view.
F. Lip, adaxial (left) and abaxial (right) views.
G. Anther cap and pollinia.
Prepared by S. Vieira-Uribe from the plant that served as type.
Figure 4 Illustration of Andinia peruviana Ocupa, S.Vieira-Uribe & Mark Wilson.
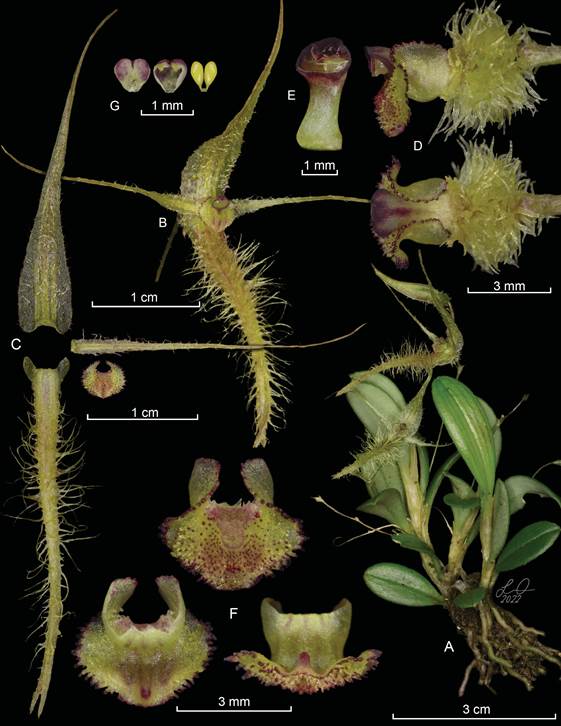
A. Habit.
B. Flower.
C. Dissected perianth.
D. Lip, column and ovary, lateral and dorsal views.
E. Column, – view.
F. Lip, adaxial (above) and abaxial (two views, below).
G. Anther cap and pollinia.
Prepared by L. Ocupa Horna from the plant that served as type.
Figure 5 LCDP of Andinia peruviana Ocupa, S.Vieira-Uribe & Mark Wilson.

A. Habit.
B. Flower.
C. Dissected perianth.
D. Lip, column and ovary, lateral view.
E. Lip, adaxial (above, left), abaxial (above, right) and lateral (below) views.
Prepared by L. Ocupa Horna using photos from A. Diaz (A. Diaz s.n. (HUT)).
Figure 6 LCDP of Andinia schizopogon (Luer) Pridgeon & M.W.Chase.
Additional Material Examined: Ecuador. Legally purchased from Mundiflora, Cuenca, Ecuador, as Andinia schizopogon ''xanthina'', without collection information, flowered in cultivation in Colorado. M. Wilson & I. Acaro AN135 (COCO-spirit!). Peru. Amazonas: Prov. Bongará, Distrito Florida, ruta de San José a Nuevo Horizonte, en un bosque montano, 2720 m, 20 November 2021, L. Ocupa 270 (USM!).
Etymology: The specific name is in reference to the country where the type was first collected, Peru.
Distribution and Habitat: To date Andinia peruviana is only known from a small area located on the eastern side of the northern branch of the Andes, in the department of Amazonas, northern Peru (Fig. 7), at an elevation of ~3100-3200 m. The type specimen was found in a very humid secondary forest, growing as an epiphyte on the branches of Weinmannia ovata Cav. (Cunoniaceae) at ~1.5 m above the ground, on a trail on the way to Yambrasbamba (Fig. 8). The flowering of this species occurs at the beginning of the rainy season, between October and November.

A. Close-up of the flower.
B. Detail of the plant growing on moss-covered tree trunk.
C. Habitat.
Prepared by L. Ocupa Horna.
Figure 8 Andinia peruviana Ocupa, S.Vieira-Uribe & Mark Wilson in situ.
Conservation Status: This species is currently known from a single area in northern Peru. While the population occurs within the San Lorenzo Private Conservation Area, district of Florida, province of Bongará, Amazonas department, anthropogenic activities, such as deforestation and the expansion of crops, may endanger its small population. Therefore, according to the IUCN Red List (IUCN 2012), it may be listed as critically endangered (CR, criteria B1/extent of occurrence and C1/small population size and decline).
Taxonomic comments: Among the species of subgenus Aenigma, Andinia peruviana is most similar to A. schizopogon (Fig. 6), sharing characteristics such as: obovate to narrowly obovate leaves, sepals ovate with long acuminate apices, petals oblong and long acuminate, lip 3-lobed, deflexed 90° near the middle, the central, basal part sub-quadrate with a cellular-papillose mesochile, the apical lobes broadly ovate, callus on the under surface, the basal lobes erect, surrounding the column. However, A. peruviana has smaller leaves (16-35 mm long vs. 25-65 mm long), a shorter petiole (10 mm vs. 25 mm long), inflorescences up to 6 cm long (vs. 15 cm long) laterals sepals connate to 22 mm (vs. connate to 10 mm), column up to 4.5 mm long (vs. 3 mm long). A. peruviana is easily distinguished from the other species of the subgenus Aenigma by the characteristics shown in Table 2.
Table 2 Comparison of Andinia peruviana with closely related species.
| Andinia hirtzii | Andinia peruviana | Andinia pogonion | Andinia schizopogon | Andinia uchucayensis | |
|---|---|---|---|---|---|
| Dorsal sepal size | 18 × 3 mm | 27-28 × 4.4-4.7 mm | 7-8 × 2 mm | 20-30 × 6-8 mm | 11 × 2.9 mm |
| Synsepal size | 18 × 5 mm | 30-32 × 5-7 mm | 7-8 x 2.5 mm | 20-30 × 6-7 mm | 12 × 1.9 mm |
| Connation of synsepal | 13 mm | 22 mm | 2.5 mm | 9-10 mm | 2 mm |
| Synsepal ornamentation | minutely papillose | hairs up to 5 mm long | long-pubescentspiculate | long-pubescentspiculate | spiculate |
| Synsepal sides | Revolute | strongly revolute | - | revolute | - |
| Petals (mm) | 15 × 1 | 26 × 1.3 | 6-8 × 0.6-1 | 20-25 × 1.5-2 | 9.3 × 0.4 |
| Lip (mm) | 3 × 3 | 4 × 4 | 1.5 × 1.5 | 3 × 3 | 1.5 × 1.1 |
| Column (mm) | 3 | 4.5 | 1.5 | 3 | 1.2 |
It is important to mention that the leaves of A. peruviana are foveolate on both sides, are whitishgreen with purple splashes on the abaxial side, have irregularly crenulate and marginate margins, the apex is obtuse-to-rounded, tridenticulate, and the base is cuneate-to-attenuate into a canaliculate petiole (Fig. 9), additional characteristics that could be useful to distinguish among species in subgenus Aenigma.
Discussion
As in previous studies (Doucette et al. 2017, Pérez-Escobar et al. 2017, Wilson et al. 2017), the species of Andinia analyzed here by MP, ML and BI form a strongly supported, monophyletic clade. Although a thorough re-evaluation of the circumscription of genus Andinia and infrageneric groupings is beyond the scope of this study and the nrITS data therein, we are not in agreement with the proposal of Szlachetko et al. (2022) to divide Andinia into 9 separate genera. We prefer to retain the five subgenera and two subsections previously proposed by Wilson et al. (2017) pending a far more comprehensive genetic analysis. In the future, with additional data from multiple nuclear genes (Chumová et al. 2021, Eserman et al. 2021, Pérez-Escobar et al. 2021); with plastid genomes (Chumová et al. 2021, Mauad et al. 2021, Pérez-Escobar et al. 2021, Serna-Sánchez et al. 2021); and with greater taxon sampling (including pertinent missing species, such as A. barba-caprina, A. condorensis, A. hystricosa, A. ibex, A. panica, A. pentamytera, A. sunchubambensis, and A. tingomariana), it may, at that time, be appropriate to propose alternate generic circumscriptions if indicated by the data.
This study was focused on one of those five subgenera, Andinia subgenus Aenigma. This clade was strongly supported in all three analyses by MP, ML and BI (Fig. 1-3). The clade corresponding to subgenus Aenigma in each case was found to include seven species: Andinia barbata, A. dalstroemii, A. hirtzii, the new species A. peruviana, A. pogonion, A. schizopogon and A. uchucayensis. While A. hirtzii, A. peruviana, and A. uchucayensis were previously presumed to be members of this subgenus based on morphology, this is the first confirmation of their phylogenetic inclusion in the Aenigma clade. Furthermore, A. barbata, described and sequenced recently by Ponert et al. (2020), is also confirmed to belong to subgenus Aenigma. Therefore, at this time, the phylogenetically-confirmed members of subgenus Aenigma are: Andinia barbata, A. dalstroemii, A. hirtzii, A. peruviana, A. pogonion, A. schizopogon and A. uchucayensis (Fig. 10-11). However, we disagree with Szlatchetko et al. (2022) that subgenus Aenigma should be segregated from Andinia as genus Aenigma (Luer) Szlach. & Kolan. at this time, so species in that genus are here listed as synonyms (see list below).
Species of Andinia subgenus Aenigma (Fig. 10-11) are distributed from southern Ecuador (which includes the provinces of Azuay, Loja, and Zamora-Chinchipe) to the montane forests located between southwestern Cajamarca and northern Amazonas, in northern Peru (Fig. 7). Apparently, the center of richness for these species is southeastern Ecuador, in the provinces of Loja and Zamora-Chinchipe, with only two species occurring in Peru. Why this group of species exhibits such a relatively narrow distribution, at least in comparison to subgenus Brachycladium which ranges from Colombia to Peru, is unknown. Biogeographic studies by Szlachetko et al. (2022) did not reveal a unique ecological niche for these species, but the sampling in that study may not have been adequate. Perhaps the distribution is constrained by either pollinator or mycorrhizal symbiont availability.
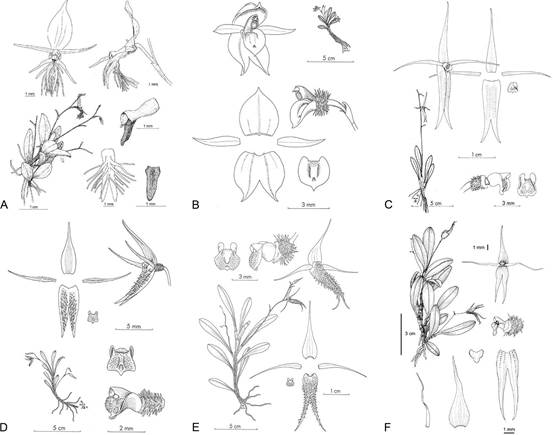
A. A. barbata.
B. A. dalstroemii.
C. A. hirtzii.
D. A. pogonion.
E. A. schizopogon.
F. A. uchucayensis.
Drawings A (reproduced with permission of the author and the journal), B-E (reproduced with permission of Missouri Botanical Garden Press, St. Louis) and F (reproduced with permission of the author and Phytotaxa).
Figure 10 Drawings of the Andinia species subgenus Aenigma.
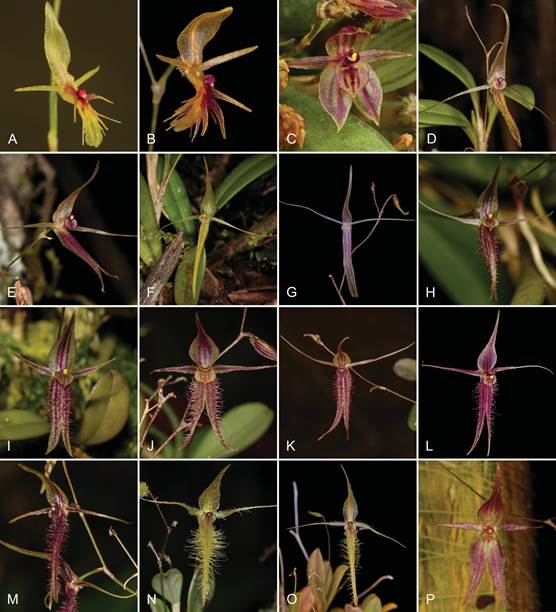
A-B. A. barbata.
C. A. dalstroemii.
D-G. A. hirtzii.
H-J. A. pogonion.
K-M. A. schizopogon.
N-O. A. peruviana.
P. A. uchucayensis.
Photographs by J.M. Pallandre (A), J. Ponert (B, G, O), Y. Dewèvre (C), M. Wilson (D, E, F, J, K, L, P), K. Olszowski (H), Kilian Zuchan (I), A. Diaz (M), L. Ocupa (N).
Figure 11 Photographs of the Andinia species subgenus Aenigma.
There remains a question as to whether the sympatric Andinia schizopogon and A. pogonion are distinct species. In the description of Pleurothallis pogonion, Luer (1994) says, ''because P. schizopogon is variable in size, this species was at first thought to be merely a small form, but in retrospect the two taxa appear distinct. Except for size, the floral morphology of the two are similar.'' Despite the inclusion of multiple accessions of both species in this study, no consistent sequence differences in nrITS were observed, suggesting either that A. schizopogon and A. pogonion are the same species with a wide variation in floral dimensions or that they are sympatric sister species and that nrITS is not sufficiently variable to distinguish between them. Only sequencing of more variable nuclear and plastid genes/regions, accompanied by a floral morphometric study, will resolve this question with certainty. For now, they are retained as distinct species following Luer (1994).
Since only one accession each of Andinia trimytera and A. vestigipetala were included in the analyses of Wilson et al. (2017), a second sample of both species was included in this analysis to confirm the previously reported phylogenetic affinities and that the species were not part of subgenus Aenigma as originally circumscribed by Luer (1986). A third sequence of A. vestigipetala was obtained from GenBank (Doucette 2017). The analyses in this study revealed the very unfortunate switching of the sequences for A. trimytera AN073 and A. vestigipetala AN075 in Wilson et al. (2017). In the current corrected analyses, A. vestigipetala is now the sole representative of the clade corresponding to subgenus Minuscula and A. trimytera is recovered as a member of the clade corresponding to subgenus Andinia. Most unfortunately, this mistake impacted the proposed alternate taxonomy of Szlachetko et al. (2022), which those authors may now wish to revise.
Given the unusual floral morphology of A. vestigipetala, recognized by Luer (1994) when he segregated Pleurothallis vestigipetala from other members of subgenus Aenigma with the creation of section Vestigipetalae, the recovery of a clade consisting of this species alone is not surprising. However, we do not agree with Szlachetko & Margonska (2001) and Szlachetko et al. (2022) that A. vestigipetala should be segregated from Andinia in the monotypic genus Lueranthos Szlach & Marg. The species is embedded phylogenetically in Andinia; the vegetative morphology of A. vestigipetala is very similar to that of subgenus Aenigma; and the unique floral morphology likely reflects adaptation to a unique pollination syndrome.
The recovery of Andinia trimytera as sister to A. lappacea in the clade corresponding to subgenus Andinia, suggests the possibility that the other species with tri-lobed lips, including A. hystricosa, A. ibex, A. panica, A. pentamytera and A. sunchubambensis (syn. A. wayqechensis) (Fig. 12) also belong in subgenus Andinia. This would be different than their provisional infrageneric assignments by Wilson et al. (2017). Szlachetko et al. (2022) also noted the affinity of A. hystricosa, A. ibex, A. panica, A. pentamytera and A. sunchubambensis with A. trimytera. However, we are not in agreement with their proposal that these six species (Fig. 12) should be segregated from genus Andinia until their phylogenetic relationships with the other members of subgenus Andinia (i.e. A. dielsii, A. pensilis and A. lappacea) can be unequivocally determined. Sequencing of duplicate accessions of each of these difficult to acquire species will be required to confirm their infrageneric phylogenetic affinities prior to any taxonomic re-assignments.
Two additional species described since Wilson et al. (2017), Andinia tingomariana (Diaz-Hernández et al. 2018) and A. barba-caprina (Ocupa-Horna et al. 2021) have been hypothesized to be members of subgenus Andinia. Both A. tingomariana and A. barbacaprina exhibit unique floral morphologies reminiscent of the Stelis species formerly attributed to genus Salpistele. Further sequencing will allow the determination of their phylogenetic affinities and whether they belong in subgenus Andinia or not.
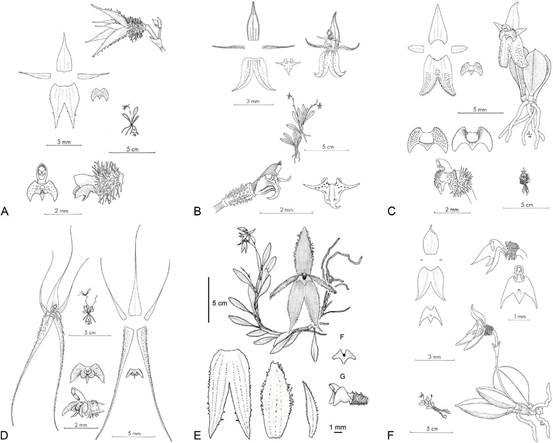
A. A. hystricosa.
B. A. ibex.
C. A. panica.
D. A. pentamytera.
E. A. sunchubambensis.
F. A. trimytera.
Drawings A, B, C, D and F (reproduced with permission of Missouri Botanical Garden Press, St. Louis) and E (reproduced with permission of the author and Internet Orchid Sp. Photo Encycl. Nomencl. Notes).
Figure 12 Drawings of the Andinia species with a tri-lobed lip.
Phylogeny-Confirmed members of Andinia subgenus Aenigma
Andinia subgen. Aenigma (Luer) Karremans & Mark Wilson, Phytotaxa 295(2): 121. 2017.
Andinia barbata J.Ponert, M.Portilla, Chumová & P.Trávn., Phytotaxa 439(1): 79. 2020.
Andinia dalstroemii (Luer) Pridgeon & M.W.Chase, Lindleyana 16(4): 251. 2001. Basionym: Pleuro-
thallis dalstroemii Luer, Orchideer 5: 52. 1984. Synonym: Aenigma dalstroemii (Luer) Szlach. & Kolan., Diversity 14. 2022.
Andinia hirtziiLuer, Monogr. Syst. Bot. Missouri Bot. Gard. 103: 275. 2005. Synonym: Aenigma hirtzii (Luer) Szlach. & Kolan., Diversity 14. 2022.
Andinia peruviana Ocupa, S.Vieira-Uribe & Mark Wilson, Lankesteriana 22(3): 249. 2002.
Andinia pogonion (Luer) Pridgeon & M.W.Chase, Lindleyana 16(4): 251. 2001. Basionym: Pleuro- thallis pogonion Luer, Monogr. Syst. Bot. Missouri Bot. Gard. 52: 61. 1994. Synonym: Aenigma pogonion (Luer) Szlach. & Kolan., Diversity 14. 2022.
Andinia schizopogon (Luer) Pridgeon & M.W.Chase, Lindleyana 16(4): 251. 2001. Basionym: Pleurothallis schizopogon Luer, Selbyana 5(2): 179. 1979. Synonym: Aenigma schizopogon (Luer) Szlach. & Kolan., Diversity 14. 2022.
Andinia uchucayensis A.Doucette & J. Portilla, Orchids (Lindleyana), 86(1): 72. 2017. Synonym: Aenigma uchucayensis (A.Doucette & J.Portilla) Szlach. & Kolan., Diversity 14. 2022.












 uBio
uBio