Introduction
Paphiopedilum originates from the Greek word 'Paphian' an epithet for Aphrodite, the Roman goddess known as Venus, and ''pedilon'' which means slipper (Cash 1991, Cribb 1998). Orchids in this genus are commonly known as slipper orchids because of the unique slipper or shoe-like flowers (Cash 1991, Cribb 1998, McGough et al. 2006). The genus Paphiopedilum Pfitzer comprises about 167 species, with distribution extending from Southern China to Tropical Asia (Braem 1988, Cribb 1998, Chen et al. 2005, Govaerts et al. 2021). Paphiopedilum gained pits popularity and investment value in the horticulture industry through its exotic appearance and production of large flowers on small plants (Cribb 1998). Most of the species are regarded as endangered and threatened with extinction due to habitat destruction, over- collection and illegal trading. They are amongst the plants listed on the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES - Appendix 1). Within this list, one can find well-known Malaysian species Paphiopedilum barbatum (Lindl.) Pfitzer (Bearded Paphiopedilum) (Rankou 2015a), Paphiopedilum callosum (Rchb.f.) Stein (Callus Paphiopedilum) (Rankou et al. 2015), Paphiopedilum niveum (Rchb.f.) Stein (Snow-White Paphiopedilum) (Rankou 2015b), Paphiopedilum rothschildianum (Rchb.f.) Stein (Rothschild's Paphiopedilum) (Rankou 2015c), Paphiopedilum sanderianum (Rchb.f.) Stein (Sander's Paphiopedilum) (Rankou 2015d) and Paphiopedilum stonei (Rchb.f.) Stein (Rchb.f.) Stein (Stone's Paphiopedilum) (Rankou & O'Sullivan 2015).
Systematically, Paphiopedilum is considered an early branch group due to its geographical distribution and relatively unspecialized floral structures (Rosso 1966). The subfamily Cypripedioideae is unusual amongst the Orchidaceae because of the presence of two fertile stamens, the disposition of these stamens in the inner staminal whorl lateral to the style, and the incomplete adnation of stylar and staminal tissues (Rosso 1966). A saccate labellum is usually present and is responsible for the common name ''slipper orchids'' so often applied to these plants (Seidenfaden & Wood 1992, Cribb 1998). Taxonomically, Paphiopedilum is classified based on morphological, cytological, and molecular phylogenetic data into three subgenera; Parvisepalum, Brachypetalum and Paphiopedilum (Cribb 1998, Chochai et al. 2012). Until now, only subgenus Brachypetalum and subgenus Paphiopedilum are recorded for Peninsular Malaysia. We investigate four aspects to identify an orchid species: general morphology, chromosome numbers, leaf and floral anatomy, and DNA barcoding. Species delimitation based on general floral morphology for Paphiopedilum species found in Peninsular Malaysia shows a clear resolution for most of the species, except for the highly resemblant ones, for instance, P. barbatum and P. callosum var. sublaeve (Rchb.f.) P.J.Cribb belong to subgenus Paphiopedilum (Seidenfeiden & Wood 1992, Cribb 1998, Leong 2014). A work on DNA Barcoding of Endangered Paphiopedilum species of Peninsular Malaysia using four DNA barcode loci and their combinations (rbcL, matK, ITS, trnH-psbA) published by Rajaram et al. (2019) clusters each species as a monophyletic clade. The matK sequences discriminate the closely related P. barbatum and P. callosum var. sublaeve, therefore supporting the species circumscription by Cribb (1998) (Rajaram et al. 2019). Nevertheless, slipper orchids are infamously variable, and unusual plants may sometimes be natural hybrids, especially when the putative parents grow sympatrically (Averyanov et al. 2007, Leong 2014, van der Ent et al. 2015). Natural hybridizations between two confusable Paphiopedilum species occur in Peninsular Malaysia, e.g. in between P. barbatum and P. callosum var. sublaeve - where the chloroplast matK sequence matched that of P. barbatum and the nuclear ITS sequence matched that of P. callosum var. sublaeve (Khew in prep. cited in Leong 2014).
Cytologically, the genus is characterized by significant chromosome variation, ranging from 2n = 26 to 42 (Duncan & Macleod 1949, Karasawa 1979, Karasawa & Aoyama 1988). Pollen studies and anatomy observations on the leaf, root, stem, and inflorescence for members of subfamily Cypripediodeae are enumerated in Pfitzer (1903), Holm (1904), Cheadle (1942), Rosso (1966) and Atwood (1984). The systematic significance of inner and outer cuticular micromorphology of mottled and xeromorphic leaves of Paphiopedilum species is unclear for either taxonomical or ecological purposes (Guan et al. 2011). The floral micromorphology of this genus, on the other hand, has not been thoroughly examined, except for pollen morphology. Pollens of some Paphiopedilum species, including P. barbatum, P. callosum and P. niveum, were studied under the microscope by Williams & Broome (1976), Newton & Williams (1978), and Burns-Balogh & Hesse (1988), are taxonomically useful at the intergeneric level. The exine of P. callosum is formed by isolated sporopollenin particles of the thick, peripherally channelled intine. Paphiopedilum niveum differs by having the foveolate exine with small pits. However, studies on the micromorphology of other floral parts of the genus Paphiopedilum are lacking. Given the above, we employed scanning electron microscope (SEM) observations to evaluate surface microstructures' applicability in taxonomic delimitation and physioecological functions.
Materials and methods
Species selection
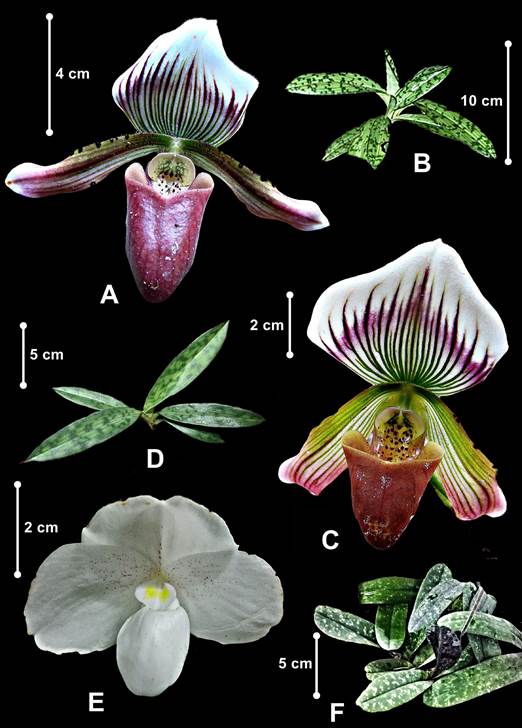
Three Paphiopedilum species from Peninsular Malaysia were selected to predict the congeneric contrasts (Fig. 1): Paphiopedilum barbatum and P. callosum var. sublaeve belong to section Barbata in subgenus Paphiopedilum with mottled leaves, mostly spotted warty petals and thick-textured labellum, and P. niveum, the only representative of subgenus Brachypetalum in Peninsular Malaysia, with mottled leaves, concolourous white flowers and thin-textured labellum. Paphiopedilum barbatum thrives under deep dark valleys, open areas or rocky boulders covered with humus, leaf litters or carpets of thick moss at streamside from about 200 m to 1200 m a.s.l. Both P. callosum var. sublaeve and P. niveum confined to the northern part of Peninsular Malaysia differ in habitat types. Paphiopedilum callosum var. sublaeve occurs in mossy forest or open vegetation with the ground covered with sphagnum mosses or coarse white sand, whereas, P. niveum is a calcicoles congener inhabiting limestone cliff shaded from direct sunlight at about 300 m a.s.l.
Sample collection and processing.
One individual for each species was obtained through field sampling conducted in three different localities in Peninsular Malaysia, allowed by a permit. Acomplete specimen for each species was processed as an herbarium specimen following techniques outlined in Bridson & Forman (2000) and deposited in the Herbarium of Universiti Putra Malaysia (UPM). The voucher numbers and attributes are listed in Table 1. Two flowers of each species were used in macro- and micromorphology examinations. The flower specimens were dissected and photographed under AM4113ZT Dino-Lite Digital Microscope. Species identification was accomplished by morphological assessment by referring to the published taxonomic monographs and the botanical illustrations of Seidenfaden & Wood (1992) and Leong (2014). The currently accepted names of the orchids were validated through the KEW World Checklist of Selected Plant Families (Govaerts et al. 2021).
Table 1 Paphiopedilum species examined including their locality, habitat and voucher.
| Species | Type Locality | Habitat | Voucher Deposited |
|---|---|---|---|
| P. barbatum | Terengganu | Peaty areas and rocky boulder in waterfall in lower montane forest | EDW060 (UPM) |
| P. callosum var. sublaeve | Kedah | Highland heath forest with ground made up of granite, quartzite and sandstone | RG4574 (UPM) |
| P. niveum | Perlis | Limestone hill forest | WY125 (UPM) |
Micromorphology examination.
The microstructural study was carried out in Microscopy Unit (EM) in the Institute of Biological Sciences (IBS), UPM, Malaysia. The floral parts examined were dorsal sepal, synsepal, lateral petals, pouch or labellum and staminode. For SEM, the samples were processed according to a modified protocol by IBS explained in Besi et al. (2020): First, fragments about 1 cm × 1 cm were excised from the margin, basal, apex and middle portions of the floral parts, except for the staminode which was used entirely. The excised samples were put into separate vials and soaked in fixative (4% glutaraldehyde) for two days at 4oC. After two days, samples were washed with 0.1 M sodium cacodylate buffer for three changes of 30 min each and post- fixed in 1% osmium tetraoxide for 2 h at 4oC. Then, samples were rewashed with 0.1 M sodium cacodylate buffer (three times 30 min each) before dehydration with series of acetone: 35% (30-45 min), 50% (30-45 min), 75% (30-45 min), 95% (30-45 min), and 100% (1 h for three changes). The samples were further dried using the critical dryer Leica EM CPD 030 for about 30 min. Lastly, the samples were mounted on stubs using double-sided carbon adhesive tabs and then sputter-coated with gold in auto fine coater Baltec SCD 005 Sputter Coater. The coated samples were examined under the Jeol JSM 6400 SEM (Beam voltage: 15 kV). The surface of each floral part was observed under various magnifications (15x-4000x). All the stubs prepared are housed in the EM unit in IBS, UPM, Malaysia.
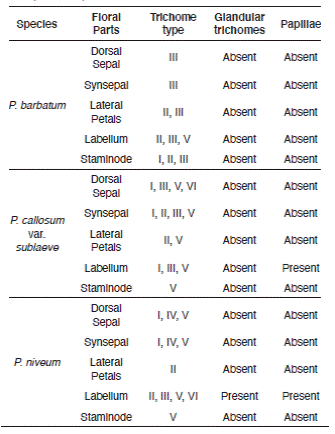
The microstructures observed on the floral parts were trichomes and papillae, pustular glands, stomata, epicuticular ornamentation and waxes. Classification of stomata was according to Wilkinson (1979) and Carpenter (2005) based on shapes and patterns of the stomatal ledges flanking aperture, guard cells and peristomatal striae, and arrangement of the contact cells. Here, we have adopted the term 'contact cell' to take the place of the subsidiary cell and neighbouring cell, to refer to any cell, specialized or not, that is adjacent to the stoma (Upchurch 1984). The studied Paphiopedilum species have some stomata where contact cells' patterns were not shown clearly in the SEM micrographs. Thus, the stomata type was omitted from the analysis and these stomata were described based on guard cells, stomatal ledges and peristomatal striae. For the individual stomatal parameters, stoma length and width, a magnification of 500x and a measurement method in Savvides et al. (2011) were employed in the current study. Stoma width was chosen instead of guard cell width since the latter changes up to 50% as stomata close (Shope & Mott 2006). Meanwhile, trichomes were described and classified based on Theobald et al. (1979), Adedeji et al. (2007), and Angulo & Dematteis (2014). Comprehensive terminologies of trichome morphology follow Angulo & Dematteis (2014). The parameter measurements were done using a ruler and the values obtained were multiplied with the magnification scales. Surface's cuticular ornamentation was described following Piwowarczyk (2015), Ghimire et al. (2018), and Kong & Hong (2018), and description on epicuticular waxes was based on Wilkinson (1979). Assessment of the examined species and the comparative study were conducted following Ghazalli et al. (2019).
Results
Epicuticular ornamentation was observed on the floral parts of the selected Paphiopedilum species. Six different features of simple and uniseriate trichomes, vary in structure, distribution, and number of cells, except branched trichomes. Description of the epicuticular ornamentation and trichomes are in Tables 2 and 3.
Table 2 Trichomes types on the floral parts Paphiopedilum barbatum, P. callosum var. sublaeve and P. niveum, including description on the morphology.
| Type | Morphology description |
|---|---|
| I | Simple, uniseriate, non-glandular, unicellular, rugose, ca. 100-200 µm, narrowly clavate |
| II | Simple, uniseriate, non-glandular, multicellular, long, ca. 200-1,000 µm, moniliform |
| III | Simple, uniseriate, non-glandular, multicellular, elongated, ca. 200-2,000 µm, moniliform with topmost cell very narrow |
| IV | Simple, multiseriate, non-glandular, bicellular, multiseriate base, short, ca. 100-400 µm |
| V | Simple, uniseriate, non-glandular, bicellular or multicellular, short, ca. 100-400 µm |
| VI | Simple, uniseriate, glandular, unicellular, sessile, ca. 5-20 µm, barrel-shaped |
| Papillae globular or tall, striated |
Table 3 Epicuticular ornamentation on the floral parts Paphiopedilum barbatum, P. callosum var. sublaeve and P. niveum, including description on the morphology.
| Type | Morphology description |
| I | Foveate outer periclinal wall; furrowed, straight and rounded anticlinal wall |
| II | Foveate outer periclinal wall; fibrillary, straight and rounded anticlinal wall |
| III | Flat outer periclinal wall; reticulate, fibrillary, straight and rounded anticlinal wall |
| IV | Laevigate and often striated outer periclinal wall; undulate and furrowed anticlinal wall |
| V | Outer periclinal wall with a complex network of undulate striae; fibrillary, straight and rounded anticlinal wall |
| VI | Entirely covered by hairs (Type I non-glandular trichomes) |
| VII | Laevigate-with-seams outer periclinal wall; furrowed, straight and rounded anticlinal wall |
Species assessment under SEM.
Paphiopedilum barbatum (Fig. 2).
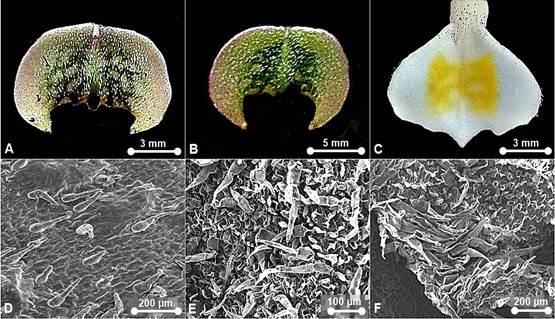
Waxes: scattered, warty- granulated and flake-like. Epicuticular ornamentation: Type III, IV and VII. Stomata formation: same level with the epidermal wall, in parallel or random formation. Stomata distribution: sparsely occurred on dorsal sepal, synsepal, lateral petals, labellum and staminode. Stomata ornamentation: comprise a defined rosette of five to seven contact cells with radial elongation of some cells but not others or characterized by four lateral contact cells. Guard cells and ledges indistinguishable from the neighbouring stomatal apparatus in the staminode. Stomatal cuticular striation: smooth or slightly striated. Stomatal size: L (11.11-41.67 μm) × W (4.44-33.33 μm). Trichome distribution: present on dorsal sepal, synsepal, lateral petals, labellum and staminode. Trichome type: non-glandular--Type I, II, III, and V; glandular--absent. Pustular glands: absent. Papillae: absent.
A. Warty-granulated wax. B. Warty-granulated wax. C. Warty- granulated wax. D. Flake-like wax. E. Type III epicuticular ornamentation. F. Type IV epicuticular ornamentation. G. Type VII epicuticular ornamentation. H. Type VII epicuticular ornamentation. I. Aperture from by detachment of trichome on dorsal sepal. J. Aperture from by detachment of trichome on synsepal. K. Stoma on synsepal - characterized by four lateral contact cells. L. Stoma on synsepal - comprise a defined rosette of five to six contact cells. M. Nectarostoma on staminode. N. Trichomes on dorsal sepal - Type II. O. Trichomes on petal - Type II. P. Trichomes on dorsal sepal and staminode - Type II. Q. Trichomes on labellum - Type II. R. Trichomes on petal and labellum - Type II.
Photographs by Edward Entalai Besi.
Figure 2 SEM observations of epicuticular waxes (A-D), epicuticular ornamentation (E-H), stomata (I-M) and trichomes (N-R) on floral parts of Paphiopedilum barbatum.
Paphiopedilum callosum var. sublaeve (Fig. 3)
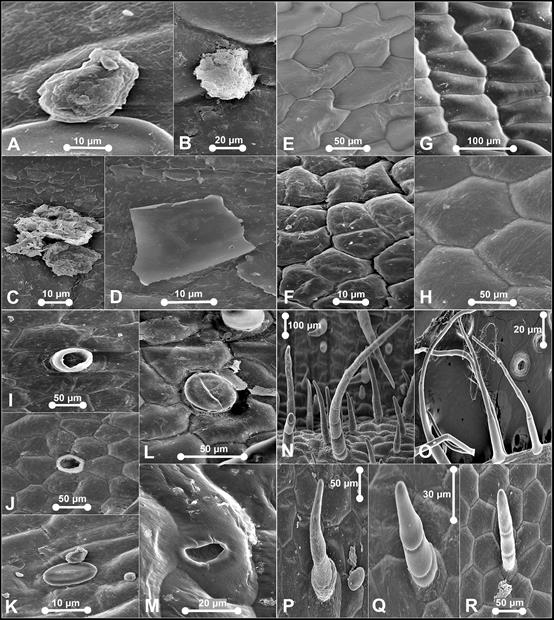
Waxes: scattered, warty-granulated and flake-like. Epicuticular ornamentation: Type II, III, IV, VI, and VII. Stomata formation: superficial, raised from the epidermal wall. Stomata distribution: sparsely occurred on dorsal sepal, synsepal and lateral petals. Stomata ornamentation: narrowly elliptical outer stomatal ledges with prominent guard cells. Contact cells indistinguishable. Stomatal cuticular striation: radiating peristomatal striae in irregular orientation from ledge cells. Stomatal size: L (41.38-52.38 μm) × W (22.79‒31.21 μm). Trichome distribution: present on dorsal sepal, synsepal, lateral petals and labellum. Two major groups of trichomes were observed on the dorsal sepal; non-glandular and glandular trichomes. The long and non-glandular trichomes were mostly located marginally and glandular trichomes on the dorsal sepal. Trichome type: non-glandular-- Type I, II, III, and V. Pustular glands: sessile, widely-scattered on sepals. Papillae: congregated on labellum, globular, striated and connected by radiating striae.
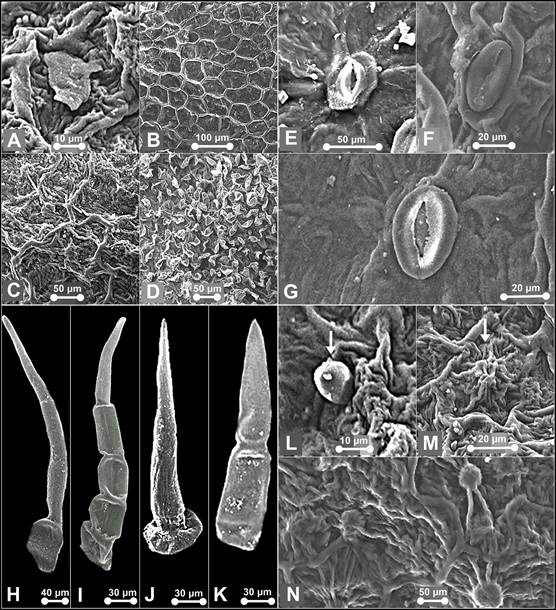
A. Flake-like wax on the pouch of P. niveum. B. Type II epicuticular ornamentation. C. Type V epicuticular ornamentation. D. Type VI epicuticular ornamentation. E. Stoma on dorsal sepal of P. callosum var. sublaeve. F. Stoma on lateral sepals of P. niveum. G. Stoma on dorsal sepal of P. niveum. H. Trichomes on synsepal of P. callosum var. sublaeve. I. Trichome on dorsal sepal of P. callosum var. sublaeve. J. Trichome on dorsal sepal of P. niveum - Type IV. K. Trichome on synsepal of P. callosum var. sublaeve - Type IV. L. Trichome on the pouch of P. niveum - Type VII. M. Papillae on the pouch of P. niveum - tall and striated. N. papillae on the pouch of P. callosum var. sublaeve - globular and striated.
Photographs by Edward Entalai Besi and Lam Shun Jia.
Figure 3 SEM observations of epicuticular waxes (A), epicuticular ornamentation (B-D), stomata (E-G), trichomes (H-L) and papillae (M-N) of Paphiopedilum callosum var. sublaeve and P. niveum.
Paphiopedilum niveum (Fig. 3)
Waxes: scattered, warty-granulated and flake-like. Epicuticular ornamentation: Type II, III, V and VI. Stomata formation: paraficial, semi-raised from the epidermal wall. Stomata distribution: sparsely occurred on dorsal sepals and synsepal. Stomata ornamentation: narrowly elliptical outer ledges and distinct irregular quadrilateral guard cells. Contact cells indistinguishable from the neighbouring stomatal apparatus. Stomatal cuticular striation: long radiating buttressed striae. Stomatal size: L (37.98-45.45 μm) × W (30.32‒38.66 μm). Trichome distribution: presence on dorsal sepal, synsepal, lateral sepals, labellum and staminode. Non-glandular trichomes were dense in petals and sepals. Glandular trichomes occasionally occur on the labellum. Trichome type: non-glandular--Type I, II, III, IV, and V; glandular--Type VI. Pustular glands: occur sparsely on sepals, sessile to subsessile, resemble subsessile trichomes, except the former commonly striated or connected by striae, or resemble papillae, except the former not prominently protruding. Papillae: congregated on labellum, tall striated.
Comparative study on the floral-surface micromorphology
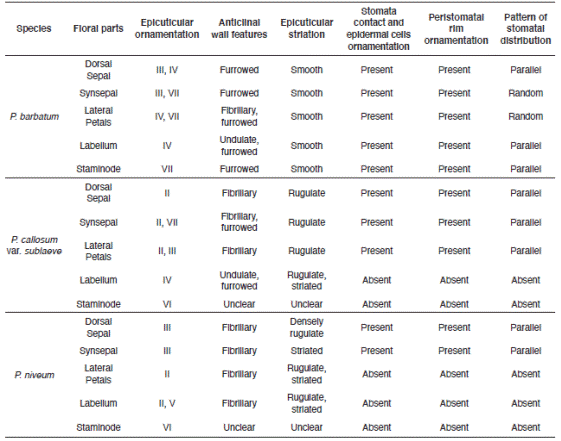
Prominent cuticular sculpturing was clearly observed on the epidermal surface of the selected species and varied significantly in anticlinal and periclinal wall characteristics. Stomata were present in floral parts of P. barbatum but only occurred occasionally for P. callosum var. sublaeve and P. niveum. Trichomes were observed in all studied species. All had diverse types of trichomes on their floral parts. The features and occurrence of each micromorphology are shown in Table 4 and 5.
Table 4 Features and distribution of floral-surface micromorphology characteristics of Paphiopedilum barbatum, P. callosum var. sublaeve and P. niveum (epidermal, stomata).
Discussion
Questions have arisen over the usefulness of floral-surface micromorphology in the recircumscription of confusable Paphiopedilum species found in Peninsular Malaysia, P. barbatum and P. callosum var. sublaeve. At first, we discuss the taxonomic significance and then the physioecological importance of epicuticular ornamentation, stomata and trichomes. Non-glandular trichomes are classified as non-glandular for not functioned as secretory structures (Peterson & Vermeer 1984). The non- glandular trichomes occur on various floral parts (Ko et al. 2007, Baran et al. 2010). Glandular trichomes, papillae and floral stomata play essential roles in fragrance and metabolite release which offers food to ensure pollinators revisit (Davies & Turner 2004, Choi & Kim 2013, Stpiczyñska et al. 2018).
Taxonomic aspects.
The invariable presence of warty-granulated and flake-like epicuticular waxes without any unique types on each floral part suggests no significance systematics value for the studied species. Contrariwise, the multi-pattern epicuticular ornamentation on the floral parts offers a significant taxonomic value to discriminate the infrasubgeneric P. barbatum and P. callosum var. sublaeve. The epicuticular sculptures are also consistent to differentiate them from their congener P. niveum (Table 4).
Stomata were found in all three studied Paphiopedilum species. The contact cells obscurity could be a characteristic of a genus. Nevertheless, the stomata can be clearly distinguished based on the prominence of the guard cells and their shape. Solereder (1908) and Carpenter (2005) strongly emphasized the diagnostic importance of the morphology of the guard cells and their cuticular ledges. The outline of the pair of guard cells as seen in surface view is usually constant in the examined specimens and is also possible a characteristic of a genus. Also, stomata in P. barbatum differs significantly from P. callosum var. sublaeve and P. niveum by having clear and noticeably contact cells, epidermal cells and peristomatal rim but rather obscure guard cells. Here, we can also deliberately compare between P. barbatum and P. callosum var. sublaeve based on the stomata and stomatal formation when observed from the top view. In comparison, stomata in P. callosum var. sublaeve were superficial and standout distinctly with prominently raised guard cells. P. niveum had stomata slightly raised and irregular-shaped guard cells that may provide a unique diagnostic character at the species level. Based on the general designation of the stomatal size provided in Wilkinson (1979), the stomata present on the slipper orchids are termed as 'large', similar to Corybas holtumii and Corybas selangorensis (see Besi et al. 2019).
Dominance of simple non-glandular trichomes and occurrence of variegated stomata on the floral surface of the Paphiopedilum species may separate genus Paphiopedilum from other genera within the Cypripedioideae subfamily. In many cases, such trichomes were living cells whereas in others they were dead, and the protoplasm was replaced by air spaces (Fahn 1988) and easily distorted or torn as observed on the labellum of P. callosum var. sublaeve. Different types of trichomes possess varies morphological characteristics were distinctively occurred on these floral surfaces of Paphiopedilum species (Table 5). The simple non-glandular trichomes were dominant on the floral surface. In contrast, the papillae were scarce, localized and only occurred on the labellum of
P. callosum var. sublaeve (globular and striated) and P. niveum (tall and striated). This suggests the presence of papillae with varied morphology on the labellum of Paphiopedilum are of systematic significance and can be used as a diagnostic character to distinguish them further morphologically. There were pustular glands observed on the sepals and petals that resemble either subsessile trichomes or papillae. Short and rugose non- glandular trichomes were formed by two to five cell tiers. The trichomes occurred at different length ranged from 61.11 µm to 1533.3 µm for P. barbatum, 48 µm to 190 μm for P. callosum var. sublaeve and 100 µm to 240 μm for P. niveum.
The presence of different types of simple non- glandular trichomes on the floral parts of the studied Paphiopedilum species denotes species specificity. It provides a piece of useful evidence for delineation of the confusable P. barbatum and P. callosum var. sublaeve. Morphologically, P. barbatum differs only by having dorsal sepal broadly ovate, petals with warts on upper or both margins and sometimes on the petals blades too, whereas P. callosum var. sublaeve has dorsal sepal broadly ovate to suborbicular and petals with warts on upper margin only (Seidenfaden & Wood 1992, Leong 2014). Clearly, these diagnostic characters are inconspicuous without a definite boundary to discriminate and sometimes misleading. Therefore, here, floral-surface micromorphology serves as a steadfast advanced technique for the taxonomic circumscription of the confusable P. barbatum and P. callosum var. sublaeve. Micromorphologically, P. barbatum varies in the diversity of non-glandular trichomes on its floral parts compared to its complex, P. callosum var. laeve (Table 5). Conspicuously, the former species has the longest Type III non-glandular trichomes (1233.3-1533.3 µm) on the margin of its lateral petals, noticeably elongated and moniliform with topmost cell very narrow, which such trichomes were absent in the latter species. Also, glandular trichomes occurred in P. callosum var. sublaeve but lacking in P. barbatum. Variability of the micromorphology observed on the staminode is systematically insignificant at infrasubgeneric level. Notwithstanding, a combination of the micromorphological characteristics on staminode separates P. barbatum and P. callosum var. sublaeve in subgenus Paphiopedilum.
The existence of certain trichome types allows differentiation of the Paphiopedilum species from different subgenera. Unlike P. barbatum and P. callosum var. sublaeve, P. niveum contrasts by having dense hairs (Type I non-glandular trichomes) along the margin of the staminode (Fig. 4). Besides having distinctive diversity of non-glandular trichomes, the confined distribution of different types of papillae found only on the labellum for P. callosum var. sublaeve and P. niveum are also distinguishing. The trichomes' length and papillae' diameters were not much diverse between the studied species.
Overall, the present research suggests floral- surface features to be very useful in delimitation of the infrageneric taxa from different subgenera of the genus by epicuticular ornamentation, stomata and trichomes. The data from this study laid evidence for delimiting two confusable Paphiopedilum species. It provides conclusive proof to support the molecular phylogenetic analyses and validates the possibility of natural hybridization occurrence in between P. barbatum and P. callosum var. sublaeve. Moreover, it demonstrates that the former is indeed distinct from the latter. The floral-surface characteristics differentiate species from two different subgenera to some extent based on the presence of different types of epicuticular ornamentation and papillae on the labellum, and the diverse variation and distribution of the non-glandular trichomes on sepals and petals. Also, the occurrence of different formation, cuticular striation and ornamentation of stomata is of taxonomic interest in this study and can be used to identify the species.
Physioecological aspects.
The presence of dense epicuticular waxes on the floral surface of the selected Peninsular Malaysian Paphiopedilum species raises questions. One clear role of waxes is to protect the plant from desiccation and herbivorous insects (Davies & Turner 2004). It may or may not offer food rewards. In Maxillaria, one of the important ways insect attraction is achieved involves the secretion of wax- like material rich in lipids and protein (van der Pijl & Dodson 1966, Davies et al. 2003). It is also reported that wasps may also collect wax from the labella of Maxillaria (Dressler 1993). Dense waxes on the labellum of P. callosum var. sublaeve and P. niveum may attract potential pollinators. Male Bactrocera fruit flies are often observed to probe the labellum, sepals and petals of Bulbophyllum species. The probing and licking behaviours displayed by the flies suggests that the pollinators' reward may be compounds released by the flower (Ong et al. 2011).
Orchid floral stomata are non-functional and practically closed in orchid flowers (Hew et al. 1980). Our finding supports this claim as the stomata found in the studied species were closed (Fig. 2K,L and Fig. 3F), or opened with a small aperture (Fig. 3E,G). Also, there were nectarostomata without a presence of guard cells (Fig. 2M), which might indicate modified stomata, cavities where the waxes are exuded through on the cuticular surface, known to occur and are of great diagnostic value in some plant species (Pant & Mehra 1965, Wilkinson 1979, Chattopadhyay et al. 2014, Prashanta Kumar & Krishnaswamy 2014, Baruah 2017, Verma et al. 2018, Besi et al. 2019, Besi et al. 2020). Notably, apertures formed by the detachment of the trichomes which could have been mistakenly identified as stomata in plant specimens (Fig. 2I,J). Waxes observed on floral surface indicates an active function of the unspecialized osmophores on the floral parts of orchid species, the regular epidermal cells secreting volatile oils (Toh et al. 2017). Identical to our previous finding on Corybas anatomical profiling work, the trichomes and stomata of the Paphiopedilum species offer more values on anatomical adaptions in defence and pollination rather than for the release of fragrance (Besi et al. 2019). The densely hairy staminode may mimic an aphid mimicry as aphidophagous hoverflies lay eggs on false brood sites on their flowers (Bänziger et al. 2012, Jin et al. 2014). Paphiopedilum flowers are postulated rewardless or nectarless to the pollinators and luring hoverflies or bees by deceit (Bänziger 1996, 2011, Bänziger et al. 2012). This is supported by the lack of glandular trichomes, papillae and stomata occurring on the labellum and reproductive parts. However, thorough observations are lacking for Malaysian species (Leong 2014). The low occurrence of glandular trichomes, papillae and stomata on the floral parts explains the unscented flowers of P. barbatum and P. callosum var. sublaeve. Except, the labellum of P. callosum var. sublaeve and P. niveum, although lacking trichomes, are heavily clothed with papillae. Though no odour is detectable to the human nose in P. niveum, when a live flower is wrapped in a plastic bag for a couple of hours, P. niveum release a faint, pleasant fragrance (Bänziger et al. 2012). Therefore, the papillae may function as osmophores for P. niveum.
Conclusions
Features of floral parts surfaces, such as epicuticular ornamentation, stomata (formation, distribution, ornamentation and size), trichome (distribution and type) are recognized as useful to differentiate highly confusable species and delimit species from different subgenera of Paphiopedilum. SEM analysis of floral-surface micromorphology supports a segregation of a narrowly distributed P. callosum var. sublaeve from P. barbatum, a widespread species in Peninsular Malaysia. The latter species is known to produce a wide range of flower morphology and colouration along the elevation gradients. All these diagnostic characters based on floral-surface morphology of these selected species should be used with care at intergeneric and intersubgeneric levels. It should be noted that these characters are far from being enough at this time to fully discriminate Paphiopedilum species in Peninsular Malaysia. A larger sampling is required to know the level of variation of the analyzed characters and to be able to make stronger conclusions. The usefulness of these floral microcharacters in biological and ecological aspects is difficult to predict based on the current preliminary finding. A further investigation on chemical compound released by Paphiopedilum flowers in relation to pollination mechanism is highly recommended.












 uBio
uBio