Introducción
The transition of plants to the earth, 470 million years ago, was based on the emergence of cooperation between plants and soil microorganisms, supported according to fossil records and the conserved mechanism for recognition between organisms (1).
Globally, in over 80% of land plants, roots associated with the soil fungi, forming chimeric organs called mycorrhizae, and numerous ecosystems are dominated by mycorrhizal plants, in a gram of soil can be meters of mycorrhizal hyphae. This suggests mycorrhizal fungi may represent the most abundant plant mutualist (2). The word mycorrhiza is the union of the Greek terms associated with “fungi” and “root”. Mycorrhizal fungi create an extensive net of hyphae in the soil, the place called the internet of plants, which can be related to plant plants to offer an efficient horizontal transfer of nutrients (3).
Mycorrhizal fungi classification cannot be realized easily, since, mycorrhizal fungi are a variable aggregate of species distributed in various fungal taxa (4). Commonly, mycorrhizae are classified in ectomycorrhizae (EM) when the hyphae form networks in the intercellular spaces of the root, and endomicorrhizae when they colonize the inside of the cells. Endomycorrhizae is divided into orchids, ericoids and arbuscular mycorrhizae (AM) (3).
The life cycle of mycorrhizal fungi has a stage in which these are not associated with the root; however, the rest of the time, they are always associated with higher plants, such as forest species and grasses, among others. They have an important role in the cycle of nutrients through the specific activity of their mycelium to absorb nutrients from the soil and provide them to the plant (4).
This review seeks to provide an overview of mycorrhizal interactions between the fungus and the plant, the connection between plants mediated by mycorrhizal networks and special emphasis on the transfer of carbon through mycorrhizae, conducting a compilation of updated research on the subject.
Plant-mycorrhizal interaction
The plant-mycorrhizal interaction can occur mainly in two ways: by ectomycorrhizae (EM) or by arbuscular mycorrhizae (AM).
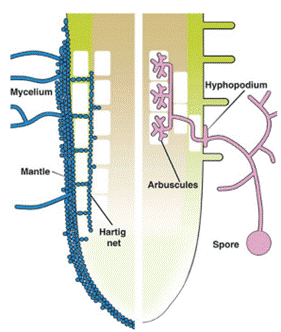
To begin with, ectomycorrhizae interactions are described. Mechanism of formation of the ectomycorrhizal fungus begins when a layer of thick and closely formed hyphae surrounds the tip of the root, then, the Hartig network, which are hyphae surrounding the epidermal cells, forms inside the root (see figure 1). EM fungi have a dual lifestyle: they live as symbionts when they colonize the lateral roots of trees with mycorrhizae coating and can also live in plant roots, as facultative saprotrophs in the soil, in addition, their growth capacities in Petri dishes have been demonstrated (4).
On the other hand, arbuscular mycorrhizae do not colonize the tip of the root. Since only a hifopodium forms in the epidermis of the root, which comes from a hypha generated from a spore. Finally, colonization occurs both between the intercellular spaces and within the cell where arbuscules are formed, which are hyphae in the shape of a small tree (4).
Influence of the mycorrhizal interaction in the nutrition of plants
The factors that regulate the diversity of plant species are not yet determined. However, an important factor is the relationships established between the plant and other organisms, whether beneficial or not.
A relevant relationship is the symbiosis between plant and fungi, which directly influences the nutrition of the plants and therefore their development. Nevertheless, the benefit of this interaction depends on the type of mycorrhiza that forms, in particular between dominant arbuscular mycorrhizae (AM) and ectomycorrhizae (EM).
For instance, EM interaction provides better access and transfer of nitrogen to its associates, so that adult trees with EM more systematically facilitate the recruitment of seedlings than trees with AM (5). This makes EM mycorrhizae more beneficial in systems with nitrogen limitation (6).
In addition, it has been confirmed that EM fungi offer greater protection against patogens than AM fungi because they form an envelope at the tip of the root (7). By the above mechanisms, the survival of young EM plants is greater than that of AM seedlings. Therefore, plant-soil feedback may be more negative for plants with AM mycorrhizae than those of EM plants.
Research conducted by (8), indicates; first, most species of EM have shown positive plant-soil feedback and, on the contrary, most AM species shows negative feedback. In addition, the phylogenetic distribution of mycorrhizae and not of trees determines phylogenetic influences in plant-soil feedback, however, this does not occur in all environment. Plant-soil feedback continued negative for AM plants and positive for EM plants, regardless of the substrate used for growth. This indicates that plant-soil feedback strongly influences the type of mycorrhiza present. However, there is a possibility that the plant-soil feedback for the different types of mycorrhizae is influenced by other factors that are not controlled or measured as the availability of nitrogen in its different forms.
Second, the EM roots had greater mycorrhizal colonization and maintained the size of the radicular lesions in the different duels, which indicates a certain specificity in the EM relationship. On the contrary, the colonization of the AM roots was similar in different soils but with more radicular lesions. In addition, greater lesion density than the colonization density for the two types of mycorrhizae was observed, possibly due to competition for space at the root of the resources, although the densities of the lesions for the AM species remained high, except for the trees with more mycorrhizae.
By combining the above results, it could be inferred that young EM plants benefit more from the abundance of specific EM fungi that protect them more from antagonists, whereas compatible AM fungi are less specific and appear everywhere, AM seedlings are more damaged by the pathogens, probably because AM colonization provides less protection.
Therefore, EM fungi improve the survival of plants by reducing root damage. However, to reach a conclusion, more extensive tests are needed to establish if this fact shown in all plants with mycorrhiza EM. Since, the improvement in plants could be due to indirect effects such as improvement in nutrition and not direct protection against antagonists.
Evolutionary stability of mycorrhizal interaction
One of the great questions regarding mycorrhizae is how this symbiosis has evolved successfully, despite the diversity of resources provided by fungi (protection against pathogens, tolerance to drought, trace elements and vitamins) (9). Accordingly, until now, it is not known how the plant can evaluate and favor a specific fungus according to the benefits offered.
Second, giving resources to another agency is considered a high expense, however, fungi often reduce the root:shoot proportion of the host plants, which reduces the carbon assigned to the roots and reduces the cost of associating with AM fungi. Furthermore, in EM associations organic carbon may be in greater quantity than is necessary for the plant, thus, it is not an expensive resource for plants (10). The exchange of these surpluses could be beneficial for both partners if none the carbon for the seedling nor the nutrients for the fungus are limited. In complex associations, plants are not necessarily able to discriminate among the associated fungi. Therefore, the carbon can be distributed in a non-directional way between the fungal partners present in the root system. In this scenario, carbon is considered to be a good of everyone, enjoyed by the most and least beneficial symbiotes (11). It has been shown that the exchange of such commons is evolutionarily stable (12). Similarly, if the cost of relating to cheaters is low, the regulation of biological markets is less important for evolutionary stability.
Third, the costs and benefits of symbiosis differ greatly with the temporal and environmental context (13).To cite an example, the relative accessibility of phosphorus and nitrogen in the substratum greatly influences the beneficial effects of the symbiosis. Soils limited in phosphorus, it has been shown that fungi are beneficial for plants, whereas in soils limited in nitrogen the same fungi can suppress growth (14). Consequently, the symbiotic relationship should be considered as a “conditional mutualism” since it can not be beneficial in all situations. Therefore, for a symbiote, it is complicated to estimate the benefit of a current relationship, since investing now in a nonbeneficial partner may be important for future survival under different environmental situations (15). To cite an instance, the exchange of resources does not immediately match in time but is done on the basis of “receive now, give later” (16). In conclusion, the identity of the partners and factors such as the environment, the efficient acquisition of complementary resources, strict reciprocity and multifunctionality directly influence cooperation in the mycorrhizal symbiosis.
Plant-to-plant interactions
Mycorrhizal networks that colonize plants roots can cause rapid changes in their behaviour, in response to biochemical communication that fungi transmit through them. In plants, behaviour is defined as the response to environmental stimuli, expressed by changes in morphology or physiology that are not part of the basic development processes (17). This plant-to-plant dialogue leads to changes in root and shoot growth, photosynthetic rate, nutrition, defense against plagues and pathogens, drought resistance, among others; and the relation can be between individuals of the same species, with a specific response if they are the same kin or not, and between unrelated species.
The behaviour of plants connected to a mycorrhizal network depends on the nature of the organisms involved, the environmental conditions and the specific needs of each of participants in the communication, including the mycorrhizal fungus (18). For instance, if the network receives more carbon than it requires from a plant, the excess could be transferred to another plant in need, to protect a potential carbon source for the future. For the fungus, having a greater variety of plants populations brings more stability and lower risk than rely on a single group of similar organisms (19), as a broader range of stress response signaling and nutrients donors can benefit the whole connected community, therefore the fungus (20). Even though the mycorrhiza prefers a variety of species in the network, the plants expose different responses to the net whether the connected plants are related or not, as highlighted in the following sections.
Intraspecific communication: protection of seedlings and kin plants
Despite the widespread belief that trees relations in a forest are exclusively competition, conspecific plants have developed strategies to protect each other and promote their development, and mycorrhizal networks represent an important tool for this. For instance, the resource transfer through fungus has an important impact in the regeneration of mono-specific forests, as it has been demonstrated in Douglas-fir forests (Pseudotsuga menziesii) by many authors. It was found by (21) that older trees transfer carbon, nitrogen, and water to seedlings to encourage rapid development of their photosynthetic rates, while (22) alleged that when humidity is limited, redistributed water through mycorrhiza allows shallow-rooted seedlings to maintain the hydraulic potential of the stand structures. This behaviour present plasticity, i.e., the transference of water and nutrients are not directly related to each other, instead, it happens according to the specific needs of the ecosystem in a place and moment. This intelligence in the management of resources leads to improved seedling survival, increasing the regenerative capacity of the forest even under conditions modified by climate change, as drought stress, by transferring water from replete to stressed individuals (23).
Besides water and nourishment, mycorrhizal networks also convey biochemical signals when a plant is under the attack of a plague or parasite. This signaling triggers a behavioural response in foliar defense chemistry or pest resistance, as reported by (24), they discovered that when a plant of broad beans (Vicia faba) connected to a network is attacked by aphids, it sends a message to neighbouring plants, which respond by producing methyl salicylate, to cause repellency to the attackers and attraction to aphid-enemies like parasitoids.
The relatedness of the plants joined by the mycorrhizal fungus determines their behaviour. It was discovered that individuals of common ragweed (Ambrosia artisifolia L.) showed better foliar nutrition when they were grown with siblings than when growth with conspecific strangers and this improvement was directly related with higher root colonization and fungi growth (25). This plant kin recognition is supposed to be related to root exudates that give the mycorrhizal fungus information about the genetic identity of the plant (26), and mycorrhizal fungi can identify and transfer this information to the plants. Other studies over the Douglas-fir mycorrhizal network found that more carbon is transmitted to younger kin plants than to stranger seedlings, giving the kin buds more opportunities to grow and development (27).
Interspecific cooperation in favor of the community
Although plants conduct efforts to favour the survival of kin individuals, for the fungi this relatedness lacks importance provided that it has a durable carbon source. This causes that mycorrhizal networks generate pathways between plants for transferring nutrients, water, and biochemical signaling, creating interspecific relations. As mentioned before, mixed species contribute to the stability of the mycorrhiza, due to the lower risk of losing the whole carbon source in comparison to monocultures (17). These situations do not seem to be equally beneficial to the involved plants, for example, it was found that in a mixed culture, flax received huge percentages of nitrogen and phosphorus from the mycorrhiza, and it gave in return small amounts of carbon. On the other hand, sorghum plants invested significant amounts of carbon and gained few nutrients, resulting in no positive growth effects for sorghum. In spite of the seemingly obvious disadvantage for the sorghum, both crops were more productive when they were mixed, although one of them received more resources from the network (28).
It was discussed before that Douglas-fir trees donate resources to protect conspecific seedlings, but in addition, they can transfer photosynthates to nearby plants belonging to the unrelated spices paper birch (Betula papyrifera), and these neighbours pass the same amounts of carbon back in a different season (29). Another investigation conducted by (30) found that Douglas-fir cooperates with ponderosa pine (Pinus ponderosae) when one of them result damaged. Defoliation of Douglas-fir caused by western spruce budworm triggered the transference of carbon from the damaged tree to neighbouring ponderosa pines, and also the recipient of the message increased the activity of defense-related enzymes. Although the damaged tree does not seem to receive a direct benefit, the permanence and strength of the mycorrhizal network and the other trees will benefit the health of the forest.
These examples shed light upon the reasons that encourage a plant to donate to the mycorrhiza carbon that may be transferred to a plant that is not related to it, and send defense signals when suffering the attack of a pathogen or herbivores. Being connected to a diverse mycorrhizal network represents an evolutionary advantage because the mycorrhiza acquires more adaptive capability due to the variety of responses of the various species, and simultaneously gives the plants tools to cope with the variability of the environment effectively (17).
As well as beneficial compounds and signaling can travel through fungus mycelium, mycorrhizal networks can distribute allelochemicals and herbicides in the soil, increasing the negative effect in exposed plants. Tomato plants have shown reduced growth due to the strong contribution of mycorrhiza to the transport of naturally released juglone, an allelochemical produced by Juglans regia (31). Another study (32) reports the facilitation of allelochemicals and herbicides through mycorrhizal networks, affecting the development of receiver plants. Another competitive response is the increase in the production of the allelopathic compounds conducted by wheat (Triticum aestivum) when close neighbours are detected through the mycorrhizal network (33).
Carbon Exchange
Over the years the importance of the carbon cycle has been known, but knowledge about the reservoirs that exist on the planet is very limited, interest in this topic has been growing due to the risk in which biodiversity is found in the forests, therefore, its importance to understand the evolution and biogeography of mycorrhizal fungi and their fundamental role in the carbon cycle and the earth’s climate system (34). Over time, the benefits of mycorrhizae have been studied from a nutrient supply perspective, but until recent years research has focused on how these underground roads formed by large amounts of mycorrhizal fungal hyphae are able to transfer considerable quantities (> 1 g) of mobile carbon compounds between networks established between trees (35). According to (36) up to 20% of the carbon fixed by the plant is transferred to the mycorrhizae.
In the last decades the developed studies have sought convincingly to determine that one of the most important functions of mycorrhizae is the ability to connect trees of the same or different species. These functional associations have been demonstrated in species such as Alnus, Betula, Larix, Picea, Pinus and Pseudotsuga interconnected by mycorrhizae that belong to the genders Amanita, Paxillus, Pisolithus, Scleroderma, Suillus and Thelephora (37). But until August 1997, ecology professor Suzanne Simard and her collaborators suggested for the first time that mycorrhizal networks connected different tree species and that the carbon transfer that occurred between them was bidirectional (38).
In order to suggest that this exchange between trees or plants occurs, a series of questions were raised, including how much carbon was transferred, since if only small quantities were involved, this process did not have a relevant importance at the ecological and physiological level. Therefore, whether the transferred carbon was moved to the cells of the plant or remained in the fungal structures, this was due to the fact that if it remained inside the fungus it could not be used by the plant as a carbon source, so it would not influence the competitive relationships between neighboring plants. Another important question is the direction of the transfer, if this occurred in one direction could be a parasitism and not a symbiotic relationship and finally, focuses on the role played by the links between hyphae, if the transfer of Carbon was produced mostly through the soil would relate to the normal carbon cycle and it would not be necessary to think that mycorrhizal networks played an important role in the process, otherwise if it was proven that mycorrhiza greatly influenced the transfer, converting them into physiologically and ecologically important elements.
Stable labeling of carbon isotopes
To answer the series of questions established by Simard, research has focused on procedures for stable labeling of carbon isotopes, it is necessary to clarify that the labels have been given several decades ago with various isotopes in order to demonstrate the capacity of the mycorrhizae to mobilize water, carbon, phosphorus and nitrogen in interconnected plants or trees but recently the procedures are based on demonstrating and quantifying the transfer of carbon between the same or different species of plants or trees that are in the same area.
Isotopes are different forms of the same chemical element that have the same atomic number of protons but different number of neutrons (39), the process that is part of the labeling is the isotopic fractionation, which is based on the mass differences between the isotopes of the same element causing them to behave differently in many environmental and physiological processes, producing variations in their relative abundance causing an increase or decrease in proportions (40).
The isotopes used in the investigations have been the stable isotope of carbon dioxide C-13 and the radioactive carbon dioxide gas C-14 (41). According to (38) the process consists of taking at least three different species of trees as samples, covering them with plastic bags and injecting them with a different isotope to each one, this is done in order to prove if there is a twoway communication between the species, it is estimated that in an hour the samples will absorb CO2 through photosynthesis, transform them into sugar and send it to the roots. It was checked through the isotopes, that if one of the samples was blocked preventing it from absorbing sunlight, the other species connected to the network was going to transfer the carbon it required, affirming the communication or bidirectional transfer that Simard indicated, while if any of the three species was not part of that connection there would be no transfer.
Interaction between trees
One of the factors that most influence this transfer is the season of year, since according to the species that are interconnected these will have morphological and physiological changes seeking to adapt the environment, which will have greater or lesser need for carbon, therefore, they will take on the role of emitter or receiver (42), which would make the trees cooperative rather than competitors as has always been believed.
From this it began to be known that the huge network formed by mycorrhizas were the cause of this communication, which worked as the internet, where everyone could communicate with everyone, it was discovered that there were mother trees or nuclei that fed the youngest (43), in a single forest these nuclei could be connected with thousands of trees and even small seedlings, making these last ones have a higher survival rate thanks to the large amount of nutrients and carbon that are transferred to them.
These nuclei or mother trees have a greater connection with those of the same species, send larger amounts of carbon to the seedlings that are their relatives, even colonize them with larger mycorrhizal networks and reduce the competition of their own roots by creating their children a “protection” framework (44). When the mother trees are wounded or dying they send wisdom signals to their seedlings, increasing the carbon transferred and the defense signals, teaching the future generations to resist all the factors that threaten their survival, increasing all the resistance in that community.
All this leads us to think about the importance not only of the trees but also of the network that they make up and how the constant felling attacks against all that wisdom, all that feedback and resistance that the larger trees and their network of mycorrhizae provide.
Conclusions
It is necessary to visualize forests as complex systems with a high capacity for self-regeneration thanks to all that information that crosses the mycorrhizal networks, how they allow a diversity of species, genes and genotypes to interlace to form a community resistant to the constant change that suffers the planet. This research gave a general vision, starting from the small unit called mycorrhiza, to a huge network formed by thousands of trees and plants connected to each other, forming a cooperative relationship to guarantee their survival. It is imperative to propose solutions to the threat of losing that communication and that important balance generated by this network, consider actions such as becoming more involved with the importance of forests to preserve all natural processes correctly, conserve mother trees and networks since these are genetic repositories that allow knowledge to be transmitted, we must cut down less and reforest more, but in an intelligent and diverse way and thus guarantee that all this varied interaction establishes a resistance to the imminent damage that the human being is generating to the Earth.