Introduction
Sea urchin aquaculture has shown great potential for growth in recent years (Rubilar & Cardozo, 2021) with research focused on the development of feed formulations that can optimize growth and gonad development in commercially available species (Lawrence et al., 2013). Formulated dry feeds have become an essential component of aquaculture practices for marine species, offering several advantages over traditional feeds such as consistency in quality and composition, water stability, and ease of use. The use of formulated feeds in sea urchin aquaculture is crucial for the establishment of large-scale production facilities and the commercial viability of the industry. However, the nutritional content and ingredient quality of the feeds play a significant role in the success of sea urchin aquaculture, with research indicating that specific feed formulations can have a significant impact on gonad development (Martínez-Pita et al., 2010; Raposo et al., 2019).
In addition to the importance of feed quality, feed costs can represent a significant portion of the total production cost in aquaculture. To address this issue, feeds formulated with viable nutritional levels of by-products have been developed, reducing production costs and contributing to environmental sustainability (Vizzini et al., 2019; Ciriminna et al., 2021). By-product feeds, such as shrimp and squid meals, have been successfully used as ingredients for sea urchin feed formulations, improving feed efficiency and reducing organic waste generated in the fish and seafood processing industry (Gunathilaka et al., 2021). These developments have contributed to the circular economy, promoting the sustainable use of resources and reducing waste.
Shrimp is an important economic resource in the Argentinean Patagonian region (Bondad-Reantaso et al., 2012). Shrimp processing generates large amounts of waste, including heads, shells, and other by-products, which can be a source of environmental pollution if not properly managed. In Patagonia, for instance, the shrimp industry generates an estimated 60 000 tons of waste per year (González-Zevallos et al., 2020; Moriondo & de la Garza, 2020). In this context, the development of shrimp-based feed formulations has the potential to contribute to both the economic and environmental sustainability of the region.
Several studies have investigated the use of shrimp waste as a source of protein and other nutrients for the production of aquaculture feeds (Abuzar et al., 2023; Canseco et al., 2015; Espinosa-Chaurand et al., 2015). For example, Espinosa-Chaurand et al. (2015), formulated a shrimp-based feed that achieved similar growth rates and feed conversion ratios to those of a commercial feed. Similarly, Canseco et al. (2015) found that shrimp waste could replace up to 50 % of fishmeal in the diet of juvenile white shrimp. Shrimp meal has also been used as a protein source in sea urchin feed, with studies showing that it can improve feed efficiency and reduce the cost of feed. These findings suggest that the use of shrimp waste in feed formulations may reduce the cost of feed production while contributing to the circular economy by reducing waste.
Although much research has been conducted on the effect of feed on gonads in commercially available sea urchin species, little is known about the effect of feed on Arbacia dufresnii (Blainville, 1825) a novel commercial species (Lawrence et al., 2013). This species is attractive to consumers due to the production of nutraceutical products manufactured by a Start Up EriSea S.A. One of the products has high levels of polyunsaturated fatty acids and high polyunsaturated fatty acids (PUFAs and HUFAs) from their gonads (Díaz de Vivar et al., 2019). Since PUFAs and HUFAs are important for human health and have been shown to have anti-inflammatory, anti-cancer, and cardiovascular benefits (Jobling, 2012; Simopoulos, 2010; Trenzado et al., 2012) and the fatty acid composition of sea urchin gonads and eggs can be influenced by feed quality and composition (David et al., 2020; Kozhina et al., 1978); it is important to investigate the effect of feed on the fatty acid composition of A. dufresnii gonads.
The most important HUFA are docosahexaenoic acid (DHA) and Arachidonic acid (ARA), since they are components of cell membrane phospholipids and play important roles in cell division, differentiation, and signaling (National Research Council, 2011). Besides eicosapentaenoic acid (EPA), which is also an essential HUFA, is present in high proportions in marine organisms; (Bell et al., 2003). Therefore, the incorporation of HUFAs in the diet of A. dufresnii may have a positive impact on gonad development and fatty acid composition. Therefore, this study aims to evaluate the effect of feed on the fatty acid composition of A. dufresnii eggs. Understanding how feed affects the fatty acid composition of this novel commercial species can provide valuable information for the development of feed formulations that optimize growth and gonad development, and increase the commercial viability of sea urchin aquaculture.
Materials and methods
Specimens’ collection: Mature individuals of the species A. dufresnii were collected by scuba diving during the winter and spring of 2021, as well as at the end of summer in 2022. The collection took place in Puerto Madryn (42º 43.6” S & 65º 1.2” W), Gulf Nuevo Bay, Northern Patagonia, Chubut, Argentina. Subsequently, the individuals were transported in seawater to the Erisea S.A. pilot aquaculture facility. Once there, the individuals were sorted by sex through the induction of spawning using an injection of KCl (0.5 M). The individuals were then kept in a fasting state for a minimum period of five days after spawning.
Experimental design: Four different fatty acids concentrations in feed were tested. Their concentrations in gametes were analyzed before and after 60 days. A total of 2 240 females were separated into the four treatments, each treatment with four replicates (140 individuals per replicate).
Individuals of A. dufresnii were randomly distributed into baskets within a production tank. Throughout the 60-day duration of the experiment, environmental conditions were kept constant, including temperature (14 ± 2 ºC) and photoperiod (12:12 h) (Sepúlveda et al., 2021), and periodic monitoring of ammonia, nitrite, nitrate, dissolved oxygen, and salinity values (35 ppm) was conducted to maintain them within recommended ranges (Rubilar & Crespi-Abril, 2017). The animals were fed ad libitum with 400 mg feed per individual every three days, following the protocol described by (Rubilar et al., 2016).
Feed: Four balanced feeds were developed with different concentrations of fatty acids for aquaculture. Each diet shared 76 % of its basic formulation, while the remaining 24 % was differentiated by the addition of flours from different parts of Pleoticus muelleri (Spence Bate, 1888) shrimp: pulp (TA), cephalothorax (TD), or a combination of both with exoskeleton (TB or TC; 12 %: 12 % respectively). The formulation of each diet was based on theoretical nutritional information of the ingredients obtained from an aquaculture formulators’ database (Table 1) and the concentration of essential fatty acids (Table 2). Furthermore, analyses were conducted on the feeds to verify the actual FA concentration in each ingredient.
Table 1 Proximal composition (%) for each feed.
| Nutrient (%) | TA | TB | TC | TD |
| Lipids | 4.17 | 4.83 | 5.47 | 5.49 |
| Soluble protein | 12.18 | 15.86 | 17.67 | 19.55 |
| Insoluble protein | 16.48 | 21.10 | 23.04 | 25.73 |
| Fiber | 7.05 | 8.56 | 10.80 | 10.07 |
| Ashes | 15.96 | 18.94 | 22.46 | 21.91 |
Abbreviations from balanced feeds treatments’ labeled: TA, 24 % of P. muelleri pulp; TB, combination pulp and exoskeleton of P. muelleri (12 %: 12 %); TC, combination cephalothorax and exoskeleton of P. muelleri (12 %: 12 %); TD, cephalothorax of P. muelleri (24 %).
Table 2 Specific differentiation in the composition of fatty acids in feed.
| Fatty acids (%) in feed | TA | TB | TC | TD |
| LA | 1.66 | 1.58 | 1.51 | 1.51 |
| ALA | 0.62 | 0.52 | 0.44 | 0.41 |
| ARA | 0.30 | 0.31 | 0.30 | 0.32 |
| EPA | 0.36 | 0.99 | 1.90 | 1.62 |
| DHA | 2.56 | 2.14 | 1.96 | 1.72 |
Abbreviations from balanced feeds treatments’ labeled: TA, 24 % of P. muelleri pulp; TB, combination pulp and exoskeleton of P. muelleri (12 %: 12 %); TC, combination cephalothorax and exoskeleton of P. muelleri (12 %: 12 %); TD, cephalothorax of P. muelleri (24 %). Fatty acids abbreviated names: LA, Linoleic acid; ALA, α-Linolenic acid; ARA, Arachidonic acid; EPA, Eicosapentaenoic; DHA, Docosahexaenoic acid.
Gametes collection: The oocytes of A. dufresnii females were collected before the start of the experiment and at its conclusion, after being fed with the treatments. They were induced to spawn by injecting 0.3 mL of KCl (0.5 M) into the peristomal membrane (Sun & Chiang, 2015). Once injected, the females were placed in direct contact with previously filtered and UV-treated seawater, and a pool of oocytes was collected from each experimental replicate. Subsequently, the oocyte sample was allowed to settle in a settling funnel for a maximum of 12 h. at 4 ºC. Ten milliliters of oocytes from each treatment were stored at -20 ºC under the N2 atmosphere until further processing, while maintaining light protection throughout the process.
Fatty acid (FA) determination: Gametes from A. dufresnii females, as well as meal from P. muelleri and the four feeds used in the experiment, were employed. To obtain the FAME (fatty acid methyl ester), a 100 mg dry sample (dried at 40 ºC) was taken and subjected to transmethylation using the Lepage & Roy (1986) method, with tricosanoic acid as the standard (51.04 μg). The separation and quantification of FAME were performed using gas chromatography with mass spectrometry detection (GC-MS) on a Thermo Focus ISQ instrument. Peaks corresponding to each detected FAME molecule were identified by comparing their relative retention times with authentic standards (a mixture of 37 FAME species from Supelco 47885-U). Additionally, mass spectra were analyzed and compared to the NIST library (National Institute of Standards and Technology, USA) and The Lipid Web database for confirmation (Christie, 1998; Fahy et al., 2007).
The concentration of fatty acids (FA) was calculated in the four formulated feeds and A. dufresnii gametes. The values are presented as mean (± SD) in μg·g-1 of dry sample. FA were expressed using their I.U.P.A.C nomenclature and corresponding abbreviated names, which include: Linoleic acid (LA, C18:2 (n-6)), α-Linolenic acid (ALA, C18:3 (n-3)), Arachidonic acid (ARA, C20:4 (n-6)), Eicosapentaenoic acid (EPA, C20:5 (n-3)), and Docosahexaenoic acid (DHA, C22:6 (n-3)). The FA were grouped according to the abundance (%) of the nutritional metabolic pathway in (ω-3) (ALA→EPA→DHA) and (ω-6) (LA→ARA). The (ω-3) /(ω-6) ratio was calculated.
Statistical analysis: Analysis of variance (ANOVA) was used to test the significance of the concentration of each FA and the abundance (%) grouped according to the metabolic pathways, (ω-3) and (ω-6), in the gametes of A. dufresnii females from the treatments, including the wild animals. The assumptions of normality and homogeneity of variances were verified using the Welch method, α = 0.05 (© Minitab, 2017), and were checked before all ANOVA analyses.
Results
Fatty acids concentration: Based on the data obtained from GC-MS chromatography, the concentration was calculated for the FA in the four food types (TA, TB, TC, TD). TA showed the highest concentration of the studied FAs, being 50 times richer in LA, 60 times richer in ALA, EPA, and DHA, and 11 times richer in ARA. Although treatments TB, TC, and TD have similar concentrations among themselves, there is a decreasing trend in the concentration of FAs, especially in LA. The concentration of FAs, expressed as (μg·g-1), in the four foods (TA, TB, TC, TD) is detailed in Table 3.
Table 3 Fatty acids concentration (μg·g-1) in the four foods.
| Feed | LA | ALA | ARA | EPA | DHA |
| TA | 298 865.46 | 92 441.77 | 9 131.53 | 78 770.08 | 88 959.84 |
| TB | 5 118.59 | 1 642.88 | 837.83 | 1 331.61 | 1 518.34 |
| TC | 5 014.57 | 1 620.38 | 48.01 | 1 358.97 | 1 591.51 |
| TD | 4 707.16 | 1 476.31 | 169.66 | 1 251.24 | 1 455.72 |
Abbreviations from balanced feeds treatments’ labeled: TA, 24 % of P. muelleri pulp; TB, combination pulp and exoskeleton of P. muelleri (12 %: 12 %); TC, combination cephalothorax and exoskeleton of P. muelleri (12 %: 12 %); TD, cephalothorax of P. muelleri (24 %). Fatty acids abbreviated names: LA, Linoleic acid; ALA, α-Linolenic acid; ARA, Arachidonic acid; EPA, Eicosapentaenoic; DHA, Docosahexaenoic acid.
The fatty acids composition: FA composition in the female gametes presented significant differences among treatments. They were also different from the wild individuals analyzed in this study (Fig. 1). TA and TB exhibited higher values of LA (5 026 ± 435 μg·g-1; 4 903 ± 1 199 μg·g-1), respectively, in the diets. On the other hand, from wild animals’ values showed the lowest FA concentration (1 346 ± 468 μg·g-1) (F (4, 21; 0.05) = 19.3, P = 0.000). TC had the lowest values of ALA (1 142 ± 301 μg·g-1), similar to the values found in wild gametes (1 192 ± 374 μg·g-1) (F (4, 21; 0.05) = 5.3; P = 0.005). Although the ARA values in TC seem to be the highest (2 532 ± 1 764 μg·g-1), there were no significant differences (1 496 ± 471 μg·g-1) (F (4, 21; 0.05) = 2.57; P = 0.073). TD had the highest values of EPA (4 909 ± 816 μg·g-1) among the treatments, which were very similar to the wild gametes (5 256 ± 729 μg·g-1), however, no significant differences were observed (4 541 ± 946 μg·g-1) (F (4, 21; 0.05) = 1.66; P = 0.203). TA and TB exhibited the highest values of DHA (2 643 ± 261 μg·g-1; 2 479 ± 39 μg·g-1), respectively (F (4, 21; 0.05) = 14.64; P = 0.000).
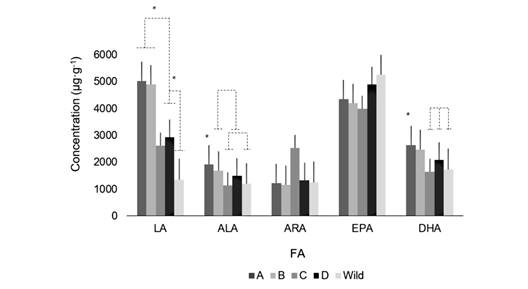
Fig. 1 Concentration of fatty acids (μg·g-1) in female gametes of A. dufresnii. A. gametes from treatment A; B. gametes from treatment B; C. gametes from treatment C; D. gametes from treatment D; and gametes from Wild animals spawning before treatments.
Fatty acids abundance: There were significant differences in the abundance (%) of both (ω-3) and (ω-6). The (ω-3) metabolic pathway was higher in the gametes of wild animals and those fed with TD (F (4, 21; 0.05) = 8.56; P = 0.000). On the other hand, the (ω-6) metabolic pathway was less relevant in this group (F (4, 21; 0.05) = 8.56; P = 0.000) (Fig. 2). The (ω-3) /(ω-6) ratio showed the highest values in the gametes from wild animals (F (4, 21; 0.05) = 12.71; P = 0.000), with increasing values observed from TA to TD treatments (Table 4), and the wild had the highest ratio.
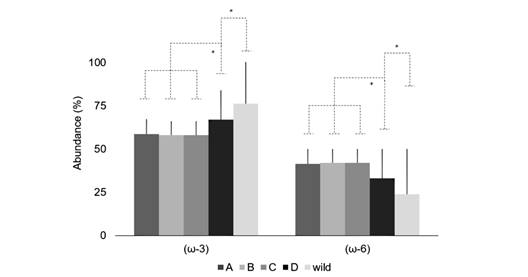
Fig. 2 Abundance (%) of FA according to their metabolic pathway in A. dufresnii gametes. A. gametes from treatment A; B. gametes from treatment B; C. gametes from treatment C; D. gametes from treatment D; and gametes from Wild animals spawning before treatments.
Table 4 The (ω-3) /(ω-6) ratio of the abundance (%) in gametes of A. dufresnii.
| Feed | TA | TB | TC | TD | Wild |
| Ratio | 1.43 | 1.44 | 1.52 | 2.10 | 3.37 |
Abbreviations from balanced feeds treatments’ labeled: TA, 24 % of P. muelleri pulp; TB, combination pulp and exoskeleton of P. muelleri (12 %: 12 %); TC, combination cephalothorax and exoskeleton of P. muelleri (12 %: 12 %); TD, cephalothorax of P. muelleri (24 %).
Discussion
In this study, the female gamete fatty acid composition was studied using different concentrations of fatty acids in the feed. The origin of the fatty acid were different parts of the shrimp P. muelleri. The cephalothorax was identified as the most abundant part in (ω-3) fatty acids, particularly EPA and DHA (Cretton et al., 2020; Hop et al., 1990). Conversely, the exoskeleton is considered a by-product of the shrimp industry, resulting from the processing for human consumption (De Carli et al., 2012). The utilization of this shrimp waste in the feed provided to A. dufresnii demonstrates an interesting strategy for resource utilization and the generation of organic inputs in aquaculture.
The feed composition used in this study is highly complex, incorporating ingredients from diverse sources such as terrestrial vegetable meals, fish oil, and algae meal. This complexity may impact the bioavailability and potential oxidation of fatty acids during the pelletization process of the feeds. However, it is noteworthy that LA and ALA are essential fatty acids primarily originating from terrestrial plant sources (Prato et al., 2018), while ARA, EPA, and DHA are predominantly derived from marine sources. These findings align with previous research analyzing the fatty acid composition in different feeds with varied nutrients that are better assimilated (Espinoza et al., 2022; Fernandez & Boudouresque, 1998; Lawrence et al., 2013; Spirlet et al., 2001).
The different fatty acid composition in the feed fed to A. dufresnii revealed that the composition of the feeds used in the treatments maintained is in an appropriate range of proteins; considered of high nutritional quality for echinoderms (Wilson, 2003). These findings are consistent with previous research highlighting the importance of maintaining adequate nutritional quality in the diet of sea urchins (Lawrence et al., 2013).
The availability of fatty acids in the feeds allowed for their bioaccumulation in the gametes, reaching concentrations equal to or higher than those observed in wild animals for LA, ALA, and DHA. ARA and EPA concentrations remained stable. Our results indicate that the duration of the 8-week experiment and the selection of feeds were appropriate for obtaining values equivalent to those observed in animals in their natural environment.
During the gametogenesis stage, there is an increase in the accumulation of (ω-3) fatty acids, particularly EPA and ARA, in the gametes of wild animals (Rahman et al., 2014; Rocha et al., 2019; Sanna et al., 2017; Wang et al., 2020; Zárate et al., 2016). Diet plays a significant role in the bioavailability of these fatty acids, and it has been demonstrated that balanced feeds can enhance the bioaccumulation of EPA and DHA in gametes studies investigating fatty acid concentrations in gametes have reported elevated levels of EPA and ARA in wild animals (Díaz de Vivar et al., 2019; García & Pita, 2010; Qi et al., 2018); and studies of modulation in the FA accumulated in the eggs (Carboni et al., 2013; Zhou et al., 2011). The availability of fatty acids in the feeds allowed bioaccumulation in the gametes, reaching concentrations equal to or higher than those observed in wild animals for LA, ALA, and DHA. ARA and EPA concentrations remained stable in A. dufresnii. Therefore, this study demonstrated that diet modulated the fatty acid composition in female A. dufresnii gametes.
The effect of overfeeding in the aquarium, as mentioned by Guillou et al. (2000), is a phenomenon that occurs when animals have low energy expenditure and accumulate excess energy in their tissues. The excessive contribution of fatty acids from the TA diet, compared to LA and ALA, may be related to this overfeeding effect, which was reflected in a disproportionate accumulation of EPA and DHA. Therefore, the formulation of balanced diets and regulation of dosage to meet the nutritional needs of A. dufresnii is crucial to avoid imbalances in fatty acid metabolism.
In conclusion, this study demonstrated significant differences in fatty acid composition and metabolic pathways in A. dufresnii gametes under different feeding treatments. Diets rich in (ω-3) fatty acids had a notable effect on the (ω-3) metabolic pathway, while diets rich in (ω-6) fatty acids deviated significantly from the natural metabolic pathways of the organisms. The TD diet, formulated with shrimp waste, resulted in gametes with fatty acid values similar to those found under natural feeding conditions, making it a suitable option for maintenance diet in aquaculture. The study also highlighted the potential of utilizing shrimp waste as a resource in aquaculture and promoting the development of a sustainable blue economy.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio 


