Introduction
The pelagic sea snake Hydrophis platurus (Serpentes: Elapidae) has the broadest distributional range of all the snake species (Boundy, 2020; Greene, 1997; Heatwole, 1999; Wallach et al., 2014), and is the only occurring in tropical waters off the west coast of the Americas (Campbell et al., 2004; Kropach, 1975). In this region, H. platurus ranges in tropical and subtropical waters from the Gulf of California southward through Ecuador, including the Galapagos and Easter islands, to Northern Peru (Campbell et al., 2004). It is common along the Pacific coast of Costa Rica, especially on gulfs and bays, usually between 1 and 20 km from the coast (Lillywhite et al., 2015; Savage, 2002; Solórzano, 2004; Solórzano, 2022).
The color pattern of H. platurus varies from the extremely rare entirely or almost completely yellow specimens recorded in the Eastern Pacific (Campbell et al., 2004; Kropach, 1975) to the more common bicolored or tricolored individuals: a black dorsum with cream, brown or yellowish flanks in the belly (Campbell et al., 2004). The yellowish morphs, once considered extremely rare in early population assessments of the species, were often interpreted as the result of rare recessive alleles or the adverse effect of natural selection (Campbell et al., 2004; Kropach, 1975; Savage, 2002; Tu, 1976; Voris, 1983). However, a recent discovery has challenged this notion. A population composed entirely of yellow specimens was reported for Golfo Dulce in the Southern Pacific of Costa Rica (Solórzano, 2011), suggesting that infrequent alleles may not be sufficient to explain the prevalence of that phenotype there.
In fact, the restricted geographical area where this population is located suggests that it may experience selective and environmental pressures different from those found in other populations throughout the distribution of the species. Evaluating ecological aspects in this yellow-morph population could help clarify its enigmatic origin. Since 2009, we began a series of studies on these unusual yellow pattern snakes to better characterize this population’s morphological and ecological patterns and compared them with those of the bicolored population inhabiting the Pacific coast outside the gulf (from now on referred to as the oceanic population). Here, we present some observations on the natural history, density, and morphometrics of H. platurus within the Golfo Dulce region in Costa Rica.
Material and methods
Study site: The Golfo Dulce is a deep tropical estuary like a fjord (Wolff et al., 1996), surrounded by steep mountains, located on the South Pacific coast of Costa Rica (8°27’-8°45’ N & 83°07’-83°30’ W, Fig. 1). This ecosystem represents the only anoxic basin on the Pacific coast of the Western Hemisphere and is one of the four systems with these characteristics that exist in the tropics (Quesada-Alpízar & Cortés, 2006; Quesada-Alpízar & Morales-Ramírez, 2004; Svendsen et al., 2006). The gulf is about 50 km long and has an approximate surface area of 680 km2, with a width of 10 to 15 km (Quesada-Alpízar & Cortés, 2006). The maximum depth reaches 215 m in the inner part of the gulf, with a shallow entrance of about 60 m deep (Fig. 2). These characteristics tend to limit water circulation in the internal basin of this gulf, and most of the circulation results from the entry of fresh water from rivers, effect of local winds, and its interaction with the topography (Svendsen et al., 2006). There are two layers of water in the Inner Gulf: one superficial with high temperatures, low salinity, and moved mainly by the winds, and another deeper, colder, and with higher salinity, with a slower flow (Svendsen et al., 2006).
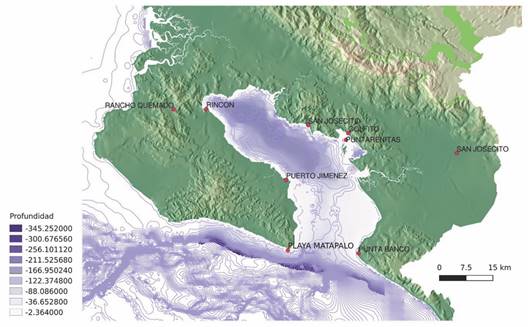
Fig. 2 Bathymetry in Golfo Dulce and the position of current meters. Depth measures in meters. (Courtesy of Omar Lizano, Centro de Investigaciones en Ciencias del Mar y Limnología, CIMAR).
Snake surveys and capture: We searched for H. platurus from a low-speed boat during the morning (7:00-11:00) and night (19:30-23:00) from February 2009 to July 2018. Surveys started at Playa Nicuesa (8°66’084’’ N & 83°28’098’’ W) following random routes across the gulf, moving between the sectors of Puerto Jiménez, Rincón, Playa Blanca, Punta Mogos, Punta Esquinas and San Josecito (Fig. 1). We also searched in the gulf entrance, between Playa Matapalo and Punta Banco (Fig. 1). A total of 34 visits were made for three to four days each month to the Golfo Dulce (102 days in total) to sampling the abundance, dial activity patterns, and external morphology (morphometry and lepidosis).
Snakes were collected manually using a circular net (Fig. 3A). Sea surface conditions and GPS coordinates were recorded at sight. The sea surface condition was recorded on an ordinal scale with four categories: a) mirror-effect (i.e., water without surface movement, Fig. 4A); b) clean and calm surface (minor undulations or weak surface waves, Fig. 4B); slightly disturbed surface (evident water movement and moderate waves, Fig. 4C); d) turbulent surface (strong waves and evident movement of the surface water mass, Fig. 4D). We also registered the behavior and surface activity of the snakes prior to capture.

Fig. 3 A. Collecting specimens of Hydrophis platurus using a circular net. B.-C. Specimen floating during the day time on the surface in the interior of Golfo Dulce, Puntareas province. D.-E. Specimen floating during the night time on the surface in the interior of Golfo Dulce, Puntarenas province (E. Photo by Jaime Culebras, Photo Wildlife Tours). F. A group of 62 individuals of Hydrophis platurus colected at night on a monitoring trip of May 13, 2013, in Golfo Dulce, Puntarenas province.

Fig. 4 Usual condition of the surface layer in the interior of Golfo Dulce. A. Note the effect of flat or ¨mirror¨ surface. B. Clean and calm surface. C. Slightly disturbed waters. D. Usual turbulent surface on the entrance of Golfo Dulce in Cabo Matapalo.
Body measurements: The captured snakes were sexed manually, using the inversion of the hemipenis in males. For all specimens, body lengths (total length, snout-vent length, head length, and width) were taken to the nearest millimeter using a tape measure. The animal’s weight is also estimated using a 500 g electronic scale. For comparative purposes, we took body measurements from oceanic bicolored specimens captured in both Golfo Dulce and Playa del Coco, Guanacaste Province, in the Northern Pacific of Costa Rica, kept at the Museo de Zoología, Centro de Investigaciones en Biodiversidad y Ecología Tropical, Universidad de Costa Rica. To avoid duplication of information, captured individuals were marked by cutting a tissue clip from the end of the tail (approximately 3 mm long). This clip was saved for future DNA analysis.
Female reproductive status (gravid vs non-gravid) was examined by palpation of the lower abdomen. Sometimes, stomach contents and freshly ingested prey were collected after manually forcing regurgitation.
Statistical analyses: Morphometric data were analyzed using means comparisons using ANOVA or non-parametric analogs. The comparisons were implemented in SPSS 20.0 (IBM Institute).
Research and collection permit issued by the Instituto de Pesca y Acuacultura (INCOPESCA), Resolution A.J.D.I.P 138/2010.
Results
Surface conditions during captures: Within Golfo Dulce, we found 482 yellow-pattern specimens and four bicolored or tricolored individuals during our surveys. Most of these snakes were encountered in the gulf’s internal sector, which coincides with its deepest area (Fig. 2). The snakes were found floating day and night on the surface, mostly (> 90 %) in clean, calm, and mirror-effect waters (Fig. 3B, Fig. 3D, Fig. 4A, Fig. 4B). However, a few individuals were observed in slightly disturbed waters (Fig. 4C). In contrast, none were observed in the shallower and usually turbulent surface area at the entrance to this gulf around Cabo Matapalo (Fig. 4D). We rarely find individuals within the flotation strips or smooth points (drift lines or foam cords) with accumulations of debris and organic remains such as twigs, branches, and leaves. In most cases, the snakes were found at rest, with their bodies wavy or slightly coiled (Fig. 3E). Rain and strong wind breezes that cause high turbulence significantly affect the presence of snakes on the surface, as no individuals were observed under these conditions.
Diel activity: The number of snakes observed during the day and night differs. The highest sightings occurred during nocturnal surveys, with an average of 7.8 individuals (2-62) per trip and a maximum of 122 observed for three nights (62, 16, and 44 specimens) on 13, 14, and 15 May 2013 (Fig. 3F). In contrast, the average number of individuals observed during the day was 3.7 individuals (1-16).
We could not find individuals during the censuses on a full moon, so this condition influences their activity on the surface. However, we observed and collected specimens that approached the boat several times at night. These facts prove the version of local fishermen who claim that snakes are attracted to boats’ light when anchored at various points fishing at night.
Body size: In Golfo Dulce, yellow morph females have snout-vent body lengths between 285 and 555 mm, while those of the captured males varied between 275 and 473 mm. Consequently, the mean body length of females is significantly larger than that of males (Table 1, SVL: U = 176.0, df = 1, p = 0.026). In this population, females have more robust and elongated heads, recording head lengths between 20 and 34 mm, while for males, this measurement varies between 19.7- and 29.1 mm. Means for this variable also differ between sexes (U = 164, df = 1, p = 0.029).
Table 1
Body size ( ± S.E.) for adult males and females from Golfo Dulce and the bicolored oceanic population.
Population/ Sex
SVL
TL
Head length
Golfo Dulce
Female
452.05 ± 12.02*
506.45 ± 9.68*
27.90 ± 0.63*
Male
415.43 ± 13.62
477.54 ± 8.83
25.75 ± 0.72
Oceanic
Female
507.54 ± 17.96
570.40 ± 20.16
31.47 ± 0.90
Male
526.75 ± 19.92
596.37 ± 21.30
29.75 ± 1.19
*Sex differences, p < 0.05.
On the other hand, snout-vent body lengths of oceanic bicolored females varied between 241 and 730 mm, while in males from that same population, the measurement was between 456 and 635 mm. No differences were retrieved between sexes in mean body size in our sample from the oceanic population (SVL: U = 217, df = 1, P = 0.95; TL: U = 216, df = 1, p = 0.93). Furthermore, for this population, the head length ranges from 18.0 to 44.0 mm for females, while that of males varies between 25 and 34 mm. Again, this variable does not show dimorphism in this population (Head length: U = 177, df = 1, p = 0.38).
Thus, the values of body and head measurements reported here indicate that yellow-morph adults from the Golfo Dulce population are significantly smaller than the bicolor adults of the oceanic population (Fig. 5, SVL: Mann Whitney U = 1031.5, df = 1, p = 0.001; TL: U = 1648, p < 0.001).

Fig. 5 Comparative body size of females (above) and males (below) Hydrophis platurus from bicolor oceanic population (left) and yellow population of Golfo Dulce (right).
Color pattern variation: Individuals in the Golfo Dulce population exhibit an entirely yellow or yellow pattern combined with spots (black, dark brown, or greenish), and dark or light circles or lines predominate. In addition, there is variation in the intensity of the yellow tones (Fig. 6A). In the entire research period, only four bicolor or tricolor specimens (the typical color patterns of the oceanic population) were found within the gulf (Fig. 6B), which represents less than 0.9 % frequency.

Fig. 6 A. Variation in the intensity of the yellow tone in the population of Hydrophis platurus in Golfo Dulce, Puntarenas province. B. Oceanic color specimen of Hydrophis platurus found in the inner part of the Golfo Dulce during a monitoring trip of May 13-17, 2013.
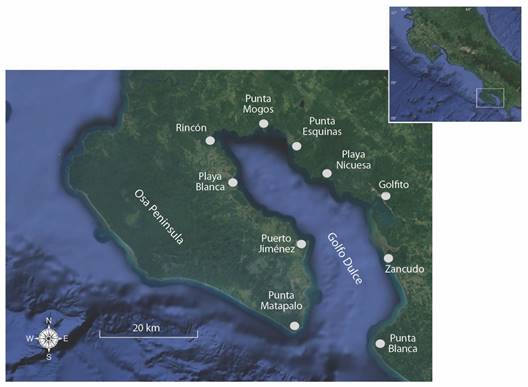
Diet and feeding behavior: Stomach contents and prey found in the snakes’ mouths before ingestion reveal the exclusive presence of small fish (< 30 % snake length, Fig. 7A) from three families: Carangidae (Caranx sexfasciatus), Clupeidae (Ophistonema sp.) and Priacanthidae (possibly Pristigenys serrula). Nocturnal observations show snakes passively floating on the surface with the body undulating and coiled and with the head slightly downward and the mouth sometimes ajar, stalking their approaching prey.
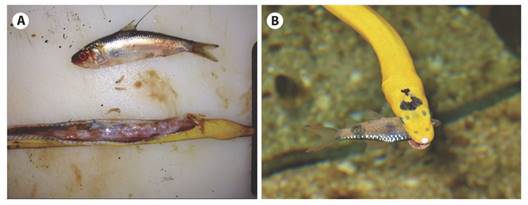
Fig. 7 A. Stomach contents and prey found in specimens of Hydrophis platurus from Golfo Dulce, Puntarenas province (A-photo by Alejandra Rojas-Barrantes). B. Yellow specimen of Hydrophis platurus with a small fish caught laterally and using their venom to subjugate it.
Reproduction: We only found three juveniles between March and April 2009 with sizes between 305 and 336 mm in total length. On February 16, 2011, a mating pair was observed on the surface between 10 and 11 in the morning, made up of a yellow and a bicolor specimen. The male (yellow) was coiled tightly around the female’s tail (Fig. 8A). Between March 20 and 21, 2018, two gravid females were collected at night, one of them with two almost fully developed embryos of 204 and 200 mm, respectively (Fig. 8B).
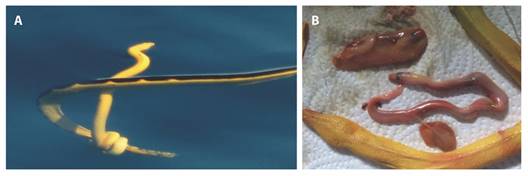
Fig. 8 A. Pelagic seasnakes (Hydrophis platurus), a yellow male and a bicolor oceanic female, mating in the interior of Golfo Dulce in February 2011 (Photo courtesy of Gerardo Sequeira). B. Dissection of gravid yellow female of Hydrophis platurus, with two embryos with advanced development, February 2013, Golfo Dulce, Puntarenas province.
Behavior and surface activity: Observed specimens within the Golfo Dulce generally remain floating passively on the surface, and most of the specimens were found in the central part within the perimeter of greater depth of the gulf. In the surveys during the day, we noticed that most individuals tend to be more evasive when approaching the boat and tend to sink quickly. However, a shift in their behavior occurs at night. They become much more passive, allowing observers to get closer before showing signs of evasive behavior. This unique nocturnal behavior was a significant finding of our research. During our surveys, we observed a few individuals close (< 2 km) to the coast. Local fishermen reported that sea snakes are rarely seen stranded on inland Gulf beaches, and we did not observe any during this investigation. Even so, we were able to register some reports of specimens found lifeless in the sand, but for the local inhabitants, these encounters are infrequent.
Predation: We did not observe any predation case on this population of yellow snakes, and none has been documented to date. However, in May 2013, we found a specimen with the tail cut and scarred by apparent bites, like what was observed in the oceanic bicolor individuals in Golfo de Papagayo in the Northwest of the province of Guanacaste (Fig. 9).

Fig. 9 A.-C. Adults specimens of oceanic Pelagic seasnakes (Hydrophis platurus) from Golfo de Papagayo in Northwestern Guanacaste province, with tails cut off and body scarred. D. Adult yellow specimen from Golfo Dulce, Puntarenas province, with tail cut and scarred tail, all probably from an attempted predation.
Discussion
The discovery of a population of H. platurus established in the interior of the Golfo Dulce basin of Costa Rica, where yellow morphos predominate, was an unexpected event (Bessessen, 2012; Solórzano, 2011). On the one hand, the species is remarkably pelagic, and in the Eastern Pacific, it is distributed uninterruptedly close to the American continental shelf, where bicolored morphotypes dominate. These bicolored morphs resemble those observed in the Western Pacific and Indian Ocean populations (Heatwole, 1987). The recent report of a population dominated by yellow-pattern snakes from Golfo Dulce (Solórzano, 2011) was also surprising due to the extensive history of herpetological exports in Costa Rican territory (Savage, 2002). Nevertheless, the available literature shows that only a few sea snake-collecting expeditions took place on the Pacific coast of Costa Rica during 20th century. These expeditions were carried out mainly in the Northwestern sector of the province of Guanacaste (Tu, 1976) and the North of the province of Puntarenas (Voris, 1983). This last author points out that in September 1970, at the “mouth” of Golfo Dulce, 268 specimens were collected, with 3 % of specimens being completely yellow. There is no evidence of other records or earlier collections in the inner part of this gulf, which eventually explains why this population remained anonymous for so long.
Yellow-colored individuals from the Golfo Dulce population are significantly smaller than the bicolored or tricolored individuals from the oceanic population. Morphological differences in general, and body size in particular, in isolated populations or with restricted gene flow have been extensively reported in different animal groups (Boback, 2003; Case, 1978), and in snakes, this is no exception (Aubret, 2015; Keogh et al., 2005).
Evaluating the factors that mediated the origin and maintenance of morphological and color characters in the Golfo Dulce population is beyond the scope of this article. However, the morphological variation revealed here, and the prevalence of an otherwise rare phenotype in Golfo Dulce could result from adaptive pressure. Coloration modification in snake populations and other ectotherms is often interpreted as adaptive, as natural selection promotes color polymorphism on spatial and temporal scales (Cox & Davis-Rabosky, 2013). Tanaka (2005) found that the heating rate is negatively related to body size in the snake Elaphe quadrivirgata, while the increase in body temperature is significantly faster in the melanistic morph than in the striped morph in that species. Similarly, melanistic morphs of other snake species maintain higher body temperatures than light-colored morphs (Forsman, 1995; Gibson & Falls, 1979). It is tempting to speculate that the Golfo Dulce population’s predominant yellow pattern and smaller body size could also represent a temperature adaptation. Golfo Dulce is a narrow and deep gulf with surface water temperatures higher than those recorded on the external ocean surface (Quesada-Alpízar & Cortés, 2006; Quesada-Alpízar & Morales-Ramírez, 2004; Svendsen et al., 2006). Thus, pelagic ectotherms within the Golfo Dulce could experience higher environmental temperatures than their conspecifics outside, which could relax the pressure to maintain a higher level of melanism and larger size necessary for thermal regulation in colder waters.
Body size in snakes has also been considered a phenotypic trait that varies in response to diet type and size (Boback, 2003; Tanaka & Ota, 2002), especially in gape-limited species or stages where selective pressure can act more strongly (Aubret, 2012; Aubret, 2015; Meik et al., 2010). Thus, the smaller body size and the coloration pattern recorded in individuals from the Golfo Dulce could result independently.
The diet of individuals in this Golfo Dulce population consists exclusively of small fish of several species. However, it is still being determined if there are differences in the relative size of the prey caught by the oceanic population since they also feed on small fish (Kropach, 1975). Likewise, the predatory behavior of the passive stalker that floats on the surface, waiting for fish to approach, is similar to the hunting behavior of oceanic bicolor individuals (Kropach, 1975; Pickwell, 1972). On the other hand, personal observations indicate that specimens fed in captivity generally swallow the offered fish alive and, apparently, only use venom when the prey is captured from the side (Fig. 7B), taking a few seconds longer to ingest it.
A remarkable aspect is the absence of predation on H. platurus; even previous researchers agree that this species lacks significant population-limiting predators (Heatwole, 1975; Kropach, 1975). The few recorded predation events involve bicolored individuals. These instances have generally occurred when snakes are thrown onto the sand of beaches due to seasonal winds that push the strips or waterlines, where snakes are usually found in the surf zone (Kropach, 1975; Solórzano, 2022). Being exposed and with little mobility, they are vulnerable to opportunistic predators such as raptors (Solórzano & Kastiel, 2015; Solórzano & Sasa, 2017). Furthermore, in the Pacific Northwest of Costa Rica, Sheehy III et al. (2011) found individuals with scars on their bodies that suggest the occurrence of sporadic predation attempts, possibly by seabirds. Although no predation records are available for yellow morphs in Golfo Dulce, during our surveys, we recorded anecdotic versions from residents that photographed spotted dolphins (Stenella attenuata) playing and chasing the snakes and sometimes throwing them out of the water but without eating them (Fig. 10), similar to what was reported by Durso et al. (2015) in Puerto Vallarta, Mexico, where a group of dolphins of this species interacted with a sea snake in the same playful way. We were also told of a bicolor specimen of H. platurus found in the stomach of a common fish in the area, a spotted snapper (Lutjanus guttatus), caught in the outer sector of the Golfo Dulce in Punta Matapalo.
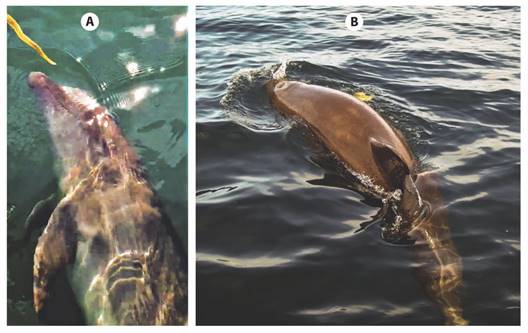
Fig. 10 A.-B. Spotted dolphins (Stenella attenuata) interacting and chasing a yellow Pelagic seasnake (Hydrophis platurus) in Golfo Dulce, Puntarenas province (A. Photo courtesy of Nicuesa Rainforest Lodge. B. Photo from video of Gerson Cedeño).
The physical characteristics of this Gulf and the behavior of the surface currents at its entrance (Quesada-Alpízar & Cortés, 2006; Svendsen et al., 2006) seem to be the events that generate the isolation and development of the yellow-colored population in the interior of Golfo Dulce (Solórzano, 2011). Given that the movements of this pelagic species are determined mainly by surface currents (Kropach, 1975), the current circulation pattern could constitute a natural barrier that restricts the transit of bicolor oceanic sea snakes towards the Golfo Dulce and of the yellow morph towards the outside of it (Fig. 11). Likewise, the physical conditions of the Golfo Dulce explain why snake strandings within the beaches of this basin are extremely rare and occasional. It is likely that the mountains surrounding the gulf function as barriers that cushion and reduce the effect of surface winds (Svendsen et al., 2006), making it more stable.
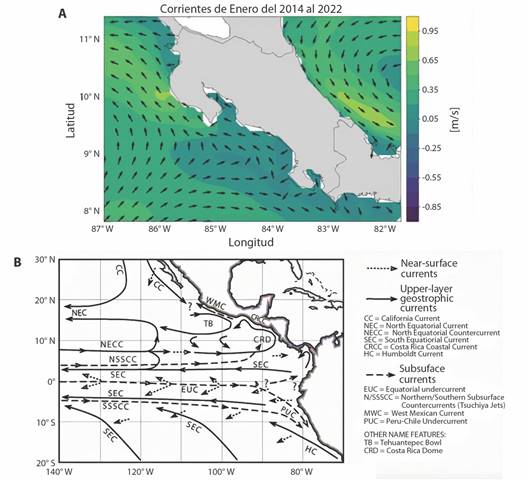
Fig. 11 A. Surface marine currents near the Pacific coast of Costa Rica. Coastal Current of Costa Rica which flows mostly from Southeast to Northwest is noticed. B. Behavior of the surface currents in Costa Rica from January 2014 to 2022 (Courtesy of Omar Lizano, Centro de Investigaciones en Ciencias del Mar y Limnología, CIMAR).
Evidence of genetic structure that could explain the reported phenotypic variation is still pending (see below). The presence of some bicolor or tricolor specimens inside Golfo Dulce (even mating with yellow specimens) suggests that the flow in both directions is not completely interrupted. In any case, the few yellow specimens reported in the Gulf of Panama (Kropach, 1975), Bahía Culebra (Tu, 1976), and other beaches on the Pacific coast (anecdotal reports with photographs) probably dispersed from the Golfo Dulce through the Costa Rican Coastal Current, which flows mostly from Southeast to Northwest (Quesada-Alpízar & Cortés, 2006; Solórzano, 2011).
The taxonomic status of the Golfo Dulce seasnake population has recently attracted the attention of different researchers. Solórzano (2011) emphasized the need to perform additional genetic-molecular and morphometric studies to evaluate the case. Using nuclear and mitochondrial DNA sequences from specimens from the Golfo Dulce and the Northern Pacific of Costa Rica (Gulf of Papagayo, Guanacaste), Sheehy III et al. (2012) found a low level of molecular variation and low population structure, concluding that the local variation at the morphological and color level cannot be explained by isolation. These results, in turn, support the notion that gene flow between populations might not be as restrictive as previously thought. Bessessen & Galbreath (2017) recognized the differences in coloration and morphology, hastening to name the Golfo Dulce population as a subspecies (Hydrophis platurus xanthos). Although some authors argue that the concept of subspecies is a valid taxonomic division that could enrich our understanding of evolution and biogeography by reflecting geographic variation within species (Patten, 2015; Patten & Unitt, 2002), we believe that the proposed trinomial nomenclature could be confusing.
On the one hand, the criteria for defining a subspecies rarely pass the multiple-character test; they are generally defined based on one or a few characters (Wilson & Brown, 1953). In this case, the difference in the color pattern is clear, but further analyses in lepidosis and morphometry are still pending. On the other hand, the low genetic differentiation unraveled by the markers used (Sheehy III et al., 2012) prevents a distinction sustained by molecular characters. Similarly, Lomonte et al. (2014) found no differences in the protein composition of the venoms of yellow individuals of the Golfo Dulce and that of bicolor individuals from the Golfo de Papagayo in the Northwest of the province of Guanacaste. The combined molecular data show a vigorous gene flow between both populations, reinforced by the interbreeding between yellow and bicolor individuals observed in our surveys. Future studies should use nuclear microsatellites or finer-scale markers to investigate the population structure of this species and assess whether this Golfo Dulce population is an incipient state of speciation.
Although the movements of this species and sea snakes, in general, are still poorly understood, the monitoring results suggest, unlike what was suggested by Bessessen et al. (2022), that this population is currently healthy and relatively abundant within the limited perimeter of this basin despite the accelerated growth of the tourism industry and other related activities. Economic activities in the Golfo Dulce (Morales-Ramírez, 2011; Morales-Ramírez et al., 2015) may constitute potential risk factors for the health and stability of this small population of sea snakes. We believe preserving and protecting this small population of yellow sea snakes unique within this species’ wide distribution range is necessary.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgments section. A signed document has been filed in the journal archives.












 uBio
uBio