Introducción
Human activities are causing a drastic transformation of tropical forests due to the proliferation of land dedicated to monocultures, commercial plantations, cattle pasture, and the overexploitation of plants and animals (Dirzo et al., 2014; Gayley & Sridith, 2020). These modifications directly affect the wealth of ecosystem services provided by tropical forests (Brockerhoff et al., 2017). There has been intense debate regarding the role played by biodiversity in reducing or increasing infectious disease risk (Rohr et al., 2020). However, recent studies tend to agree with the idea that biodiversity loss appears to raise the risk of human exposure to zoonotic pathogens as a consequence of increases in the abundance of species that are particularly suitable to be zoonotic hosts (Keesing & Ostfeld, 2021). There is a need for research focused on analyzing the response species sharing zoonotic pathogens with humans have to the most common human activities affecting tropical forests, such as deforestation (Guégan et al., 2020).
Rodents are of great relevance in tropical forests due to their abundance and variety of ecological roles, such as seed dispersers and predators (Vélez & Pérez, 2010). Some rodent species seem to withstand the effects of human perturbations most commonly affecting tropical forests and even, in some instances, benefit from the conditions associated with anthropized landscapes (Mendoza et al., 2020; Morand et al., 2019). In contrast, some species have small distribution ranges and specialized habits that makes them more prone to be negatively impacted by tropical forest perturbation (Mendoza & Horváth, 2013; Rubio et al., 2014). Small rodents interact with an important number of arthropods in the orders Ixodida (ticks), Mesostigmata (facultative parasite mites), Trombidiformes (free-living mites, parasites in larval stage), Sarcoptiformes (commensal and parasite mites) as well as insects in the orders Siphonaptera (fleas), Phthiraptera (lice) and Heteroptera (bugs) (Guzmán et al., 2020; Madinah et al., 2014). Despite the known role of these arthropods as ectoparasites reservoirs and vectors of pathogens (e.g., viruses and bacteria) there is little information on how they are affected by habitat perturbation (Guzmán et al., 2020; Ramalho & Gubler, 2020).
Tropical forest perturbation can affect the prevalence of rodent ectoparasites through different pathways. First, the impacts of forest perturbation on the abundance of rodent populations and the composition of their communities can in turn affect host availability (Ladds, 2015; Ssuuna et al., 2020). Second, habitat perturbation can affect the physiological condition of rodents (e.g., stress levels) making them more prone to be parasitized, or can directly affect the species composition and population abundance of ectoparasites due to the associated changes in abiotic factors such as humidity and temperature (Hammond et al., 2019; Rynkiewicz et al., 2013).
Tropical forests frequently support human populations with high levels of poverty (Fisher & Christopher, 2007). It is, therefore, an issue of prime relevance to document the impacts of tropical deforestation on rodent populations and the corresponding consequences for ectoparasite abundance and diversity to prevent zoonosis affecting people particularly vulnerable. Moreover, research on this issue will help to further our understanding of the ecosystem service tropical forests provide for disease control and how it responds to anthropogenic perturbation (McMurray et al., 2017).
Our goals were: a) evaluating the relationship between forest loss and rodent species richness and abundance, b) assessing the associate effects on the abundance of rodent ectoparasites and their species richness and composition, and c) documenting the presence of ectoparasites of zoonotic relevance such as ticks, fleas, lice, and hematophagous mites. We expect to find a reduction in the overall rodent species diversity as forest extent reduces, due to the increase in the abundance of species adapted to live in transformed habitats. This will be reflected in a greater abundance of the associated ectoparasites. Among the species of ectoparasites increasing their abundance, we expect to find some species identified as vectors of zoonotic diseases.
Materials and methods
Study site: This study was conducted in the Marques de Comillas (MC) region which is part of the Lacandon forest in the state of Chiapas, Southern Mexico. The MC region has an extent of 94 266 km² (INAFED, 2010). It is limited to the West and North by the Lacantún river, to the South by the Guatemala border, and to the East and North by the Salinas river (Fig. 1). The Montes Azules biosphere reserve is located towards the west, covering an extent of 331 200.00 ha (Carabias et al., 2000). The mean annual precipitation in the region is 3 000 mm and the mean temperature is 24 °C (Martínez et al., 2009).
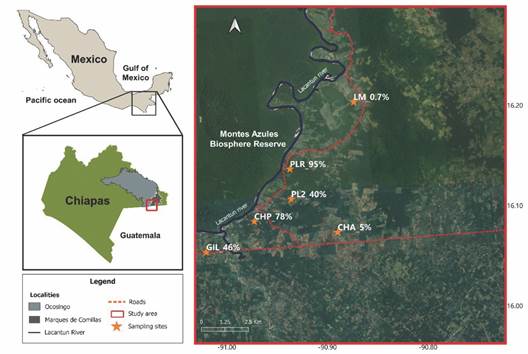
Fig. 1 Location of the study area in the Marques de Comillas region in the state of Chiapas, Southern Mexico. The stars indicate the location of the sampled landscape units.
The Lacandon forest, together with its natural prolongation in Belize and Guatemala, constitutes one of the largest remnants of tropical rainforests in Mesoamerica (Cruz et al., 2004). Unfortunately, over a period no longer than 12 years, the Lacandon forest lost approximately 142 000 ha, accumulating a total loss of nearly 70 % of its original extent (Carabias et al., 2015; de la Torre & Medellín, 2011). Currently, this region supports a mosaic of remnant native forests, secondary forests, cattle pastures, and African palm plantations (Muench & Martínez, 2016).
Selection of sampled landscape units: Wies et al. (2021) estimated the percentage of forest cover in 18 landscape units of 1 km² within the MC, using Sentinel-2 satellite images, with a 10 m spatial resolution, and ground-truthing. Based on this information we selected six sampling sites with different percentages of forest cover (AT1).
Rodent capture: We set 90 Sherman traps in each LU, along two parallel lines separated by 20 m with a distance between consecutive traps of 10 m. These traps remained active for 24 hours during 7 days, were baited daily with a mixture of liquid vanilla and oat flakes, and checked early every morning.
All the captured rodents were euthanized by applying an overdose of pentobarbital sodic in the intraperitoneal zone. This procedure followed the guidelines for the ethical treatment of animals from the American Society of Mammalogists (Gordon & Kirkland, 1998). To limit the number of rodents to be sacrificed we reduced our sample size to the minimum number of six sites as indicated above. The sacrificed individuals belonged to species not listed as threatened at the national or global level.
Ectoparasite sampling: We applied repetitive abrasive combing of the whole body of each one of the sacrificed rodents with the help of a toothbrush (Lareschi et al., 2019). Moreover, we conducted a visual search of ectoparasites attached to the skin of rodents and checked the plastic bags where they were stored. All the collected arthropods were deposited in a solution of Kono's liquid for 7 to 30 days to disintegrate their internal organs and stomach content. The resulting exoskeletons were mounted on a slide with a drop of Hoyer's solution to conduct taxonomic identification with the help of specialized taxonomic keys (Bassols, 1975; Fain & Lukoschus, 1984; Guzmán et al., 2011; Stojanovich & Pratt, 1961).
Data analyses: We conducted a linear regression analysis to assess the relationship between forest cover (%) (mature + secondary forest) and rodent abundance. To compare ectoparasite species richness among the LUs we built accumulation curves using Hill number q = 0. These curves were extrapolated to the largest sample size, using as a sampling unit the corresponding number of trapped rodents. We calculated the 95 % confidence intervals for each curve (Chao et al., 2014) using the iNEXT package (Hsieh et al., 2019).
We generated a dendrogram to explore the relationship among LUs based on their species richness and abundance of ectoparasites. Applying the procedure described in Borcard et al. (2018), we produced a matrix of Euclidean distances among sites based on the standardized abundance (option ''normalized'') of their ectoparasites. We generated four dendrograms using the single linkage, complete linkage, UPGMA, centroid, and Ward's minimum distance algorithms and calculated cophenetic correlations and Gower distances to assess their performance. Gower's distance is a statistic used to assess the comparative performance of data grouping methods. It is calculated as the sum of squared differences between original and cophenetic distances. The grouping method having the lowest Gower value is taken as the one having the best performance (Borcard et al., 2018). To conduct this analysis, we used the package Vegan (Oksanen et al., 2020). We excluded from this analysis the rodent species Ototylomys phyllotis due to the fact it was represented only by one individual and in contrast with the other species recorded is highly arboreal.
To examine the existence of differences in the number of ectoparasites recorded on Sigmodon toltecus among the different trapping sites we applied a Kruskal-Wallis test (Zar, 2010). Afterward, we applied a Dunn test with Bonferroni's adjusted probabilities to identify which pairs of sites were statistically different. The use of this test is recommended in cases in which replication is not balanced among treatment levels (Zar, 2010). To conduct these analyses, we used the package FSA (Ogle et al., 2021). All the above analyses were conducted using the software R ver. 3.6.3 (R Core Team, 2020).
Results
With a sampling effort of 3 780 trap days, we captured a total of 70 rodents (overall 43 males and 27 females) belonging to five species. The most abundant species was Sigmodon toltecus (N= 45), followed by Heteromys desmarestianus (N= 9), Oryzomys couesi (N= 9), Peromyscus mexicanus (N= 6), and Ototylomys phyllotis (N= 1). In the sites where the forest coverage was the lowest, the abundance of S. toltecus and O. couesi was the highest (Fig. 2A). In contrast, the species P. mexicanus and H. desmarestianus occurred only in the sites with greater forest coverage (Fig. 2A). None of the five rodent species occurred in all six LUs.
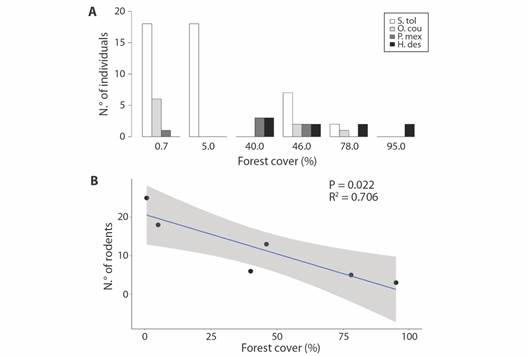
Fig. 2 A. Rodent abundance in six 1 km2 landscape units, with different forest cover extents in the Marques de Comillas region, Southern Mexico. Sigmodon toltecus (S.tol), Oryzomys couesi (O.cou), Peromyscus mexicanus (P.mex), and Heteromys desmarestianus (H.des). B. Relationship between forest coverage and rodent abundance in the six 1 km2 landscape units sampled in the Marques de Comillas region in Southern Mexico. The shaded area corresponds to the 95 % confidence interval for the regression.
The overall abundance of rodents was inversely proportional to the percentage of forest cover (R²= 0.706, df= 5, P= 0.022). Thus, the LUs with less forest cover (LM_0.7 % and CHA_5 %) concentrated the greatest number of rodents (25 and 17, respectively) whereas in the LUs with the greatest forest coverage (CHP_78 % and PLR_95 %) only occurred five and three rodents (Fig. 2B).
We identified 23 different ectoparasites, 15 of them at the species level and eight at the genus level (AT2). The more abundant organisms were mites in the families Listrophoridae (Geomylichus nectomys, Prolistrophorus sp., Prolistrophorus bakery, and Prolistrophorus grassi) and Trombiculidae (Cordiseta mexicana, Pseudoschoengastia brennani, Parasecia kansasensis, Dermadelema mojavense, and Eutrombicula batatas). The less abundant species was Gigantolaelaps boneti (Laelapidae family).
The ectoparasite species accumulation curves showed a trend towards stabilization with the clear exception of site PLR_95 %; however, this site had the smallest number of rodents captured making the extrapolation of limited value. There was a great overlap among the 95 % confidence intervals of accumulation curves but LUs GIL_46 % and CHP_78 % were located over the curves corresponding to LUs LM_0.7 %, CHA_5 %, and PL2_40 % (Fig. 3A).
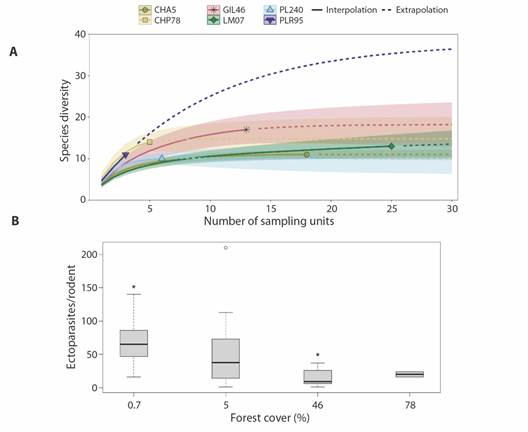
Fig. 3 A. Accumulation curves of ectoparasite species in six sites with different percentages of forest coverage in the Marques de Comillas in Southern Mexico. The shaded areas correspond to 95 % confidence intervals and dashed lines to extrapolations. B. Comparison of the abundance of ectoparasites recorded in individuals of Sigmodon toltecus captured in six 1 km2 landscape units with different levels of forest coverage in the region of Marques de Comillas in the state of Chiapas, Southern Mexico. * Sites statistically different based on Dunn the test (P < 0.05).
There was a significant variation in the number of ectoparasites among individuals of S. toltecus captured (Chi² = 13.2, df = 3, P = 0.004, Fig. 3). Two sites were different LM_0.7 % and GIL_46 % (Dunn test, P = 0.0044). The greatest ectoparasite abundance occurred in the LU with the highest deforestation and the lowest in the LU with 46 % forest coverage (Fig. 3B).
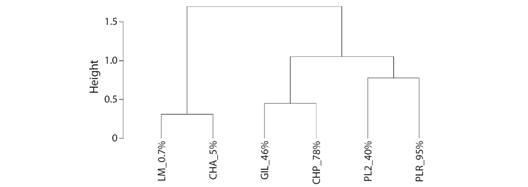
The dendrogram using the UPGMA algorithm had the highest cophenetic correlation (0.846 vs. 0.840, 0.830, 0.782) and the lowest Gower distance (0.401 vs. 1.036, 1.390, 3.037). Sites were segregated into two main clusters. The first one of them was constituted by the sites with the lowest forest coverage. The second main cluster was, in turn, divided into two smaller clusters. The first one of these sub-clusters included sites GIL_46 % and CHP_78 %, which had a relatively high species richness of ectoparasites whereas the second included sites PL2_40 % and PLR_95 % (Fig. 4).
Discussion
In agreement with our predictions, we found that rodent, and ectoparasite abundances increase with forest loss. The rodent species we recorded constitute approximately 50 % of the total number of species recorded in the Lacandon forest (Cruz et al., 2004). It would be highly desirable to include surveys of the canopy fauna in future studies to have a more complete view of the impact forest loss is having on the rodents and their associated ectoparasites. We recorded the greatest species richness of rodents in sites with intermediate levels of forest coverage (GIL_46 % y CHP_78 %). This likely relates to the presence in these sites of greater habitat heterogeneity associated with the mixture of vegetation in different levels of secondary succession, forest fragments, crops, commercial plantations, and cattle pasture (e.g., Ssuuna et al., 2020).
Species such as S. toltecus and O. couesi likely benefit from the extensive transformation of the landscape in our study region due to the fact they are omnivorous and include a variety of grasses and seeds in their diets. This can help them to thrive in areas dominated by cattle pastures and monocultures (Peña et al., 2009). Moreover, these species have reduced gestation periods (no longer than one month) and produce on average eight individuals per litter (Ceballos & Oliva, 2005). In contrast, species such as H. desmarestianus and P. mexicanus are known to have the capacity to move across areas with pasture or crops but their presence tends to be more associated with mature forests (Ceballos & Oliva, 2005).
We obtained some evidence that the ectoparasite load increases with forest loss. A similar effect was reported by Froeschke et al. (2013) in fragmented forests in South Africa. These authors suggest that this effect relates to the fact that the conditions in the transformed habitats offer greater availability of resources such as water and a lower presence of predators. These conditions can in turn favor peridomestic rodents to reach a larger body size and therefore to be able to carry a larger number of ectoparasites.
Studies conducted in Mexico have detected pathogens causing diseases such as leishmaniasis, Chagas, rickettsiosis, and hemorrhagic fever in all the rodent species we recorded in this study (Lorenzo et al., 2017; Panti et al., 2021). Ticks are very dangerous vectors due to their hematophagous diet and need to have several hosts to complete their life cycles (Main & Bull, 2000; Stafford et al., 1995). Moreover, other arthropod species we detected such as Amblyomma sp., O. bacoti, and A. fahrenholzi can be vectors of viruses and bacteria such as Hantavirus, Flavirus, Rickettsia, Borrelia, and Bartonella (Kabeya et al., 2010; Moro et al., 2005). There is evidence that the increased abundance of O. bacoti is associated with disease incidence in domestic animals and humans (Beck & Fölster, 2009). There are no current reports on human infections associated with A. fahrenholzi however, the presence of Rickettsia and Bartonella has been reported in Mexico (Zapata et al., 2017).
We also recorded the presence of parasitic larvae of arthropods in the order Trombidiformes (C. mexicana, P. brennani, P. kansasensis, D. mojavense, and, E. batatas). There are no records of these species being associated with etiological agents however, the larvae of other trombiculid species such as Neotrombicula autumnalis, Microtrombicula sp. and, Leptotrombidium scutellare are known to be vectors of pathogens including Caulobacter yokenella and species in the genera Actinomycetospora, Coxiella, and, Bosea (Masakhwe et al., 2018). The parasitic larvae of these genera cause important affectations on health in both wild mammals and humans by producing severe dermatitis (Santibañez et al., 2014).
We did not detect the presence of fleas in the rodents. Fleas are of particular interest due to the fact they can carry the pest vector Yersinia pestis (Carlson et al., 2022). The continued monitoring of our study area will help to discard the possibility that this lack of detection of fleas is related to seasonal variation in the abundance of these organisms (Herrero et al., 2021).
It is important to highlight the fact that not in all cases arthropods found in the fur of small rodents are true parasites. Some arthropod species take advantage of rodents for transportation (phoresis) (Kim, 1985). These species receive less attention due to the fact they are not associated with disease transmission (Cornejo & Mares, 2021; Guzmán et al., 2020). However, the study of this group of species is needed to gain a deeper understanding of the overall response of biodiversity to anthropic perturbation.
From the different 23 groups of ectoparasites we recorded, we were only able to identify 15 at the species level and the remaining eight at the genus level. In some instances, samples corresponded to individuals in the larval stage (e.g., Amblyomma sp.) complicating their taxonomic determination. Likewise, mites belonging to the genus Echinonyssus (species one to five), Prolistrophorus, and Dermacarus had morphological traits that did not match exactly those of the currently described species, it exists the possibility they constitute new species.
The health, social and economic impacts of zoonotic disease associated with wild fauna have generated great concern among scientists, medical institutions, and people in general (Saba & Balwan, 2021). However, more research is still needed to identify the key factors favoring zoonosis spillovers (Stenvinkel et al., 2021). In the meantime, it looks like one of the best routes to prevent zoonosis is making operative public policies and conservation strategies focused on protecting tropical forests and wildlife.
Ethical statement: the authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio