Introduction
The genus Hechtia Klotzsch is one of the more interesting members of the Mexican Bromeliaceae because it is the only member of the subfamily Hechtioideae and has experience recent discovery of new species (Ramírez et al., 2014). The estimated number of species in Mexico is 65 (Ramírez & Jiménez, 2012) and 94 % of them are endemic (Ramírez et al., 2014). Hechtia plants are terrestrial or lithophile and can grow in volcanic rocks and limestone hills. Bromeliaceae seeds are brown, fusiform, rough, oblong, ellipsoids, one mm wide and three mm long (Espejo et al., 2007). Reyes (2015) suggests that Hechtia can be used in ecological restoration programs because it produces a remarkably high quantity of seeds and the ability of plants to grow under low rainfall, on poor soils, and under contrasting temperatures. However, the knowledge of Hechtia seed biology is limited (Espejo et al., 2007). Information of seed moisture content, dry weight, and seed germination after seed mass maturity of H. perotensis has not been reported. Information about the genus Hechtia includes seed morphological descriptions of H. nuusaviorum (Espejo et al., 2007) and new species descriptions of H. schottii (Escobedo, 2012), H. santanae (Ramírez et al., 2016), H. flexilifolia, H. huamelulaensis, H. nivea (Ramírez et al., 2014).
Seed germination characteristics are fundamental for species conservation (Baskin & Baskin, 1998). In consequence, it is necessary to know the timing of seed harvest as it influences seed quality (physical and physiological) and storage effects (Willan, 1985) to initiate nursery programs and store seeds in germplasm banks. Seed germination is sensitive to environmental conditions (Leck et al., 2008). Sankhla and Chawan (1980) found that seed moisture content is important in regulating seed germination. For example, Phaseolus trilobus seeds had 100 % seed germination at 30 % moisture content and 0 % with moisture content of 2 %. In addition, seed quality continues to improve during development and even after maximum dry weight (Pieta & Ellis, 1991) as seen with seeds of beans (Sanhewe & Ellis, 1996) and cereals (Hay & Probert, 1995). Seed development of H. perotensis has not been reported and this, together with timing of seed harvest is a first requirement to start propagation in nurseries and to store seeds with maximum seed quality in seed banks. The objective of this research was to assess seed moisture content, seed weight and seed germination of H. perotensis during seed development in two sites one in a rocky location and another near a lake. The hypothesis was that after flowering there is a time nearly at the end of seed mass maturity where seeds have low weight due to seed drying, low moisture content and maximum germination. Also, that seed quality differs among three longitudinal sections of the infructescence due to differences in maturity along the stalk.
Material and methods
Sample sites: H. perotensis seeds were collected at site 1 a rocky land, Frijol Colorado, Perote, Veracruz (2 416 m.a.s.l, 19°32´13.95” N & 97°22´98” W) and site 2, Tepeyahualco at the lake of Alchichica, Puebla, México (2 326 m.a.s.l, 19°25’11” N & 97°23’56” W) (geolocation with Garmin eTrex 10 & Google Earth®) (Google Earth, n.d.-a; Google Earth, n.d.-b). The sites were selected to investigate the effect of the lake (site 2) in the relative humidity and on seed mass maturity compared with site 1 plants growing in rocky land. The climate of each region is semiarid with warm summers BS1kw(i´)gw” (García, 1988). The vegetation is typical of a xeric shrublands (Ramírez et al., 2014). Soil was alkaline, not saline, loamy and poor in nutrients in both sites (laboratory analysis).
Plant selection: In a first visit in June 2016 thereafter called day 0, male and female plants in both sites had flowers and insect pollinations. After one month, plants of both sites had green immature capsules and at this moment a white perforated sleeve shape cloth with diameter of 1 mm was inserted along the infructescence in order to keep the seeds inside the cloth in case of dehiscence. Each infructescence length was registered with a measuring tape, divided in three and adjusting a lace in each section to keep the mature seeds separated.
Capsule sampling: Capsules of H. perotensis in both sites were collected in August, September, November 2016 and January 2017. The mean temperature was 15.3 and 17.6 °C, and mean relative humidity for 56.8 and 60 % (data logger Hobo), of site 1 and 2, respectively during the sample months. Temperature and relative humidity were measured to compare the environment characteristics.
Once capsule colour changed from green to brown around 41 d after flowering (DAF) 25 capsules were harvested from each of the base, centre, and apex of the infructescence. Two to four capsule sample dates were made according to the availability of capsules. Samples were made at 41, 87, 152 and 215 DAF in plants named A in site 1 and B in site 2. Plant named C and D of site 1 and 2 respectively were sampled at 41 and 87 DAF due to less availability of capsules.
Seeds extracted from capsules with fine clippers were stored at (25 ± 1 °C) in seal aluminium bags. Seed weight, humidity and germination evaluations were made within one week after each harvest period.
Seed weight: Seed weight was determined in 225 seeds by infructescence in a precision balance (± 0.0001 g; Scientech SA 120) taking 75 seeds from the apex, centre and base of the infructescence this procedure was repeated for each sample date.
Seed moisture content: Seed fresh weight of five replicates each with 15 seeds was determined from the apex, centre, and base of the infructescence this procedure was repeated for each sample date. Seeds were kept at 50 ± 1 °C in an oven until they reach constant weight (5 d). Seed moisture content was calculated using the following expression (International Seed Testing Association, 2010).
Germination: Seeds were placed in petri dishes with filter paper on the bottom and 10 mL of distilled water was added. Five replicates with fifteen seeds (per petri dish of 14 cm) were tested from the apex, centre, and base of the infructescence this procedure was repeated for each sample date. Seeds were superficially disinfested by immersion in sodium hypochlorite (60 g/L of Cl active for 1 min) 1 % (v:v in water). Petri dishes were placed in an incubator (Thermo Scientific. USA: model 846) set at 25 ± 1 °C and photoperiod of 12 h darkness x 12 h light (12 μmol m-2 s-1 of irradiation). A seed was considered germinated when 1 mm of radicle was exposed using a digital caliper Vernier. Evaluations were made at 8 and 15 d from placement in the incubator (International Seed Testing Association, 2010).
Seeds in development: Seeds were observed with a stereo microscope (Leica EZ4 HD) to identify embryo development this procedure was repeated for each sample date. Seed testa was dissected near the embryo and the embryo was extracted with tweezers (International Seed Testing Association, 2010).
Experimental design: A repeated measures design (RMD) was used to analyze the effects of time (day 41, 87, 152 and 215) and infructescence section on seed weight, moisture content, seed germination and seed development at each site. Each evaluation time comprised five replicates each of 15 seeds as an experimental unit from the apex, centre, and base of the infructescence. Multiple comparisons were made with the general lineal model (α = 0.05).
Results
Plant A site 1 Frijol Colorado, Perote, Veracruz: Seeds of the three sections of the stalk (base, medium and apex) were similar on germination, moisture content and seed fresh weight (Fig. 1A, Fig. 1B, Fig. 1C) in each date sampled (P > 0.05). However, there was statistical difference over time; at 41 DAF seed fresh weight average at three sections was 0.0360 g, moisture content of 76 % and seed germination of 20 %. Whereas seeds sampled at 215 DAF had seed fresh weight average at the three sections of 0.0139 g, seed moisture content of 6 % and 94 % of seed germination (P ≤ 0.05). Seeds in development were similar in the three sections of the stalk at 41 DAF with an average of 80 % of seeds. At 87, 152 and 215 DAF seeds in development were on average 6 % without differences in the sections of the stalk (Fig. 1D).
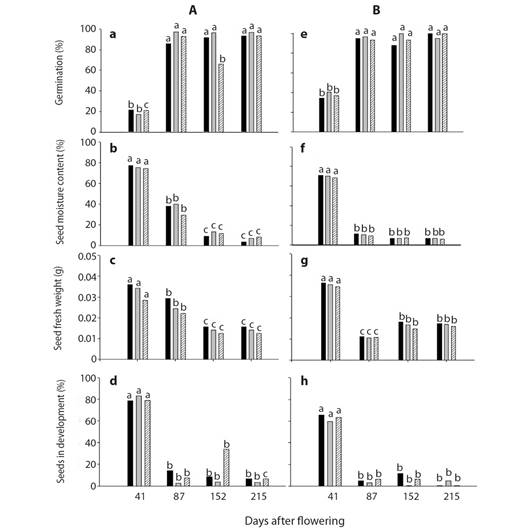
Fig. 1 A. B. C. and D. Germination, seed moisture content, seed fresh weight and seeds in development from the plant A site 1 (Frijol Colorado, Perote, Veracruz) and E. F. G. and H of plant B, from site 2 (Tepeyahualco at the lake of Alchichica, Puebla). Each column represents the average of 5 replicates of 15 seeds each. The apex ( ), centre (
), centre ( ) and base (
) and base ( ) of the stalk is represented by three columns. The letters on the columns represent the effects of the time in each section of the stalk by date of days after flowering.
) of the stalk is represented by three columns. The letters on the columns represent the effects of the time in each section of the stalk by date of days after flowering.
Plant B site 2 Tepeyahualco at the lake of Alchichica, Puebla: Seed germination, moisture content and seed fresh weight (Fig. 1E, Fig. 1F, Fig. 1G) was similar in the three sections of the stalk in each date sampled, however seed germination, moisture content and seed fresh weight showed evident differences with time. On average, seed germination at 215 DAF (98 %) was 2.6 times higher than that at 41 DAF (37 %) (Fig. 1E). Conversely, seed moisture content diminished from 69 to 6 %, nearly ten times in the same period (Fig. 1F).
Seed fresh weight (Fig. 1G) was different (P ≤ 0.05) in the three sections of the stalk over time. The average seed fresh weight was more than double at 41 DAF (0.036 g) compared with 215 DAF (0.016 g) this decrease of seed moisture was due to seed drying.
Seeds in development were similar in the three sections of the stalk at 41 DAF with an average of 60 % of seeds. At 87, 152 and 215 DAF seeds in development were on average 4 % without differences in the sections of the stalk (Fig. 1H).
Plant C site 1 Frijol Colorado, Perote, Veracruz: Seeds of the three sections of the stalk did not differ in germination, moisture content and seed fresh weigh at 41 and 87 DAF (Fig. 2A, Fig. 2B, Fig. 2C) (P > 0.05). However, the average seed germination at 41 and 87 DAF was 23 and 95 % respectively (Fig. 2A). Seed moisture content and seed fresh weight of the three sections on average was 72 % and 0.035 g at 41 DAF. At 87 DAF seed moisture content diminished to 29 % and seed fresh weight was 0.017 g (Fig. 2B, Fig. 2C).
Seeds in development were similar in the three sections of the stalk at 41 DAF with an average of 76 % of seeds. At 87 DAF seeds in development were on average 4 % without differences between the sections of the stalk (Fig. 2D).
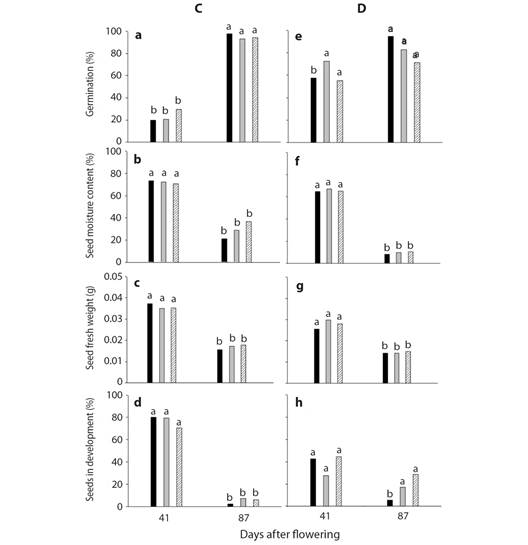
Fig. 2 A. B. C. and D. Germination, seed moisture content, seed fresh weight and seeds in development from the plant C, site 1 (Frijol Colorado, Perote, Veracruz) and E. F. G. and H of plant D from site 2 (Tepeyahualco at the lake of Alchichica, Puebla). Each column represents the average of 5 replicates of 15 seeds each. The apex ( ), centre (
), centre ( ) and base (
) and base ( ) of the stalk is represented by three columns. The letters on the columns represent the effects of the time in each section of the stalk by date of days after flowering.
) of the stalk is represented by three columns. The letters on the columns represent the effects of the time in each section of the stalk by date of days after flowering.
Plant D site 2 Tepeyahualco at the lake of Alchichica, Puebla: Seed germination, moisture content and seed fresh weight (Fig. 2E, Fig. 2F, Fig. 2G) was similar in both dates sampled. Also, seed moisture content and seed fresh weight differed over time (P ≤ 0.05), but not between stalk sections. The average seed germination of the stalk was 83 % at 87 DAF (Fig. 2E). Seed moisture content and fresh weight (Fig. 2F, Fig. 2G) decreased to 9 % and 0.0144 g respectively averaged across, the three sections of the stalk at 87 DAF.
Seeds in development were similar in the three sections of the stalk at 41 DAF with an average of 35 % of seeds. At 87 DAF seeds in development were on average 15 % differing between the apex and centre or base of the stalk (Fig. 2H).
Discussion
Our research showed homogeneity and high seed germination (87 %) of H. perotensis at 87 DAF at the two sites of seed collection and across three sections of the infructescence. In previous research Elizalde et al., (2017) showed variation of seed germination of H. perotensis from two different harvest dates (20 % in 2012 and 92 % in 2015), and as a consequence, it was necessary to establish the time at which H. perotensis reaches high seed quality.
Gutterman (1980) confirmed that seed position in the fruit or infructescence is important for seed germination. The perennial shrub Mesembryanthemum nodiflorum had different seed germination according to the position of the infructescence in which the seed grows. For example, in the apex seed germination was of 61 %, in the centre 5.5 % and in the base 1 %. M. nodiflorum grows in the desert and the difference in germination of the fruit is related with the cycle life of this plant. As a consequence, undetermined factors could have influenced seed quality along an infructescence, such as environmental conditions during the development of the mother plant, flower aperture, and occurrence of pollination insects (Gray & Thomas, 1982).
In contrast, Gesch et al., (2016) did not find differences in seed germination between different seed positions in plants of Thlaspi arvense L. Similarly, in our research seeds of H. perotensis did not show such locational differences in germination.
Willson and Price (1977) found that production of more flowers in one plant attracts more pollinating insects and as a result plant produce more seeds. McKinney et al., (2012) showed that flowering timing coincides with activity of pollinating insects to improve homogenous flower pollination (Hegland et al., 2009). H. perotensis was flowering with pollination insects and we assumed that flower pollination was efficient. In addition, the capsule formation was homogenous in the three sections of the stalk mainly in seed germination.
Seed moisture content diminished gradually during seed development: the reserves are accumulated (sugars) and solutes (proline) in the vacuole and there is water displacement to protect cell membranes. After, physiological seed maturity (mass maturity) orthodox seeds increase the process of seed drying to reach the end of seed maturation (Bewley et al., 2013). We found that seeds of H. perotensis improved seed germination by about 80 % when seed moisture content decrease to 20 % at the last sample analyzed. H. perotensis seeds drying in an environment with low relative humidity (60 %) and a medium temperature 16 °C, reaching a low moisture content in about 45 days.
Bhawna et al., (2011) indicated that seed maturity of Prunus cerasoides was at seed moisture content of 31.96 % ± 1.42 %. According to Mai-Hong et al., (2003) 100 % seed germination of Peltophorum pterocarpum (DC) K. Heyne is reach when seed moisture content was 56 % with maximum seed dry weight of 0.5 g. The seed moisture content has been used as a reliable indicator of seed maturity in several studies e.g., by Shah et al., (2010). In our study we found that seed moisture is an indicator of seed maturity in H. perotensis. Harvest seeds at 41 DAF were in development with an average moisture content of 70 %. Seeds reached embryo maturity and increased germination at 87 DAF with only 10 % of seeds in development. At this stage, maturation is approaching and maturation drying is the event for orthodox seeds to enter in a metabolic quiescent state. Seeds can remain in this state for days to years with high viability (Bewley et al., 2013).
Shah et al., (2006) showed that seed moisture content of 23.4 to 36.1 % can be associated with an optimum seed germination of Pyracantha crenulata. Bewley et al., (2013) reported that seed physiological maturity (vigour and seed germination) is reached when seed dry weight is greatest, and after this moment seeds start to decrease in quality. For example, in soya seeds maximum dry weight was obtained 45 d after flowering. For seeds of Caesalpinia lutea and C. wrightii physiological maturity was reached around 18 to 21 d after flowering (Kaliangile & Grabe, 1988). Our results agree with the above authors and demonstrated that H. perotensis reach minimum seed fresh weight (low seed moisture content) with coincidence of maximum seed germination at the end of the samples periods (87 DAF).
Maturity of seeds also has been associated with seed moisture content, for example, Ricinus communis L. seed maturity was reported to occur at 22 % seed moisture content (Vallejos et al., 2011). Seeds of Aesculus indica Colebr, Albizzia lebbeck and Celtis australis had physiological maturity at 58, 52 and 32 % seed moisture content respectively (Majeed et al., 2010; Bhardwaj et al., 2002). Our research showed that seeds of H. Perotensis reached physiological maturity with fresh seed weight of 0.010 to 0.015 g, moisture content lower than 20 % and seed germination of 85 %. Similar results of seed fresh weight were reported by Elizalde et al., (2017), however they mainly focused on methods to improve seed germination.
Sankhla and Chawan (1980) claim that seeds with high moisture content could produce low seed germination. The authors explained that a seed before drying is in development with immature embryos and in the process of storage of nutritive reserves. Therefore, seed drying is necessary to reach high germination percentages and seed maturity. After seed drying the events of seed development stop and with seed water uptake the seed germination metabolism starts (Bewley et al., 2013).
We conclude that H. perotensis reached seed mass maturity 87 d after flowering with 20 % moisture content. With this knowledge the point of harvest of seeds can be used to store seeds with high percentage of germination in seed banks and to start a plant nursery and use H. perotensis for ecological restoration in their origin place and has the potential to use this plant in other regions with similar environments. Both sites studied reached high seed germination at the same time.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 



