Introduction
Aedes aegypti (Linnaues, 1762) is the main vector of arboviruses such as dengue, chikungunya fever, Zika virus and yellow fever (Fofana, Beugré, Yao-Acapovi, & Lendzele, 2019). The combat to disseminate this mosquito is a challenge in tropical countries, mainly in South America, and a problem to health due to the high morbidity and mortality rates (Pavela, 2015; Benelli & Mehlhorn, 2016; González et al., 2019). In Brazil, in 2019, there were 1 544 987 recorded cases of dengue with 782 deaths, besides 132 205 cases of chikungunya with 92 deaths, and 10 768 cases of Zika virus with three deaths (Ministério da Saúde, 2020).
Synthetic chemical insecticides from organophosphate class are utilized against A. aegypti, mainly temephos, an organothiophosphate insecticide (Benelli et al., 2017). However, organothiophosphates are reported to have genotoxic action on human hepatic cells (Benitez-Trinidad et al., 2015) and neural damages in rats (Laurentino et al., 2019). Besides, the broad action spectrum of this insecticide causes non-specific deaths of insects (Braga & Valle, 2007; Čolović & Krstić, 2013; Mrdaković et al., 2016), bees, butterflies, and other pollinating insects (Morais, Bautista, & Viana, 2000). Moreover, the increased concentration of this insecticide in Brazilian cities indicates that there is a resistance process to this chemical to control A. aegypti (Jonny, Silva, Fantinatti, & Silva, 2015). An alternative to organophosphates for the control of A. aegypti are the non-cumulative synthetic pyrethroids which are, however, toxic to aquatic arthropods, fish, and bees, eliminating essential insects that act on the biological control of insects, besides causing adverse effect to human health due to long exposure to it (Camargo et al., 1998; Bellinato et al., 2016).
Another alternative to organophosphates would be phytoinsecticides such as essential oils that in general have a quicker degradation and/or volatilization, causing smaller environmental damage, mainly to pollinating insects. For instance, to control Varroa destructor that parasites bees, essential oils of neem (Azadirachta indica), lemon (Citrus limon), and eucalyptus (Eucalyptus citriodora, current name Corymbia citriodora) are utilized without killing the beehive (Bakar et al., 2017). However, some phytoinsecticides can have a similar action to organophosphates such as the garlic essential oil (Allium tuberosum and Allium sativum) used to control larvae and/or repel mosquitoes such as Aedes spp., Anopheles spp., and Culex spp. (Denloye, Makanjuola, & Babalola, 2003; Trongtokit, Rongsriyam, Komalamisra, & Apiwathnasorn, 2005; Raimundo et al., 2018).
Gallesia integrifolia (Spreng.) Harms, Phytolacaceae family, popular names pau d’alho, garlic tree, and guararema in Brazil, is known due to its strong alliaceous odor because of the presence of sulfur molecules in the plant, despite not belonging to the Alliaceae family (Barbosa, Teixeira, & Demuner, 1997; Sambuichi, Mielke, & Pereira, 2009). It has several synonyms such as Gallesia gorazema, Gallesia integrifolia var. ovata, Gallesia ovata, Gallesia scorododendrum, and Thouinia integrifolia (Hassler, 2018). This plant has been reported since 1821 for common treatments of orchitis, verminosis, and rheumatism (Corrêa-Filho, 1984; Akisue, Akisue, & Oliveira, 1986), and the essential oil has been reported to act against bovine tick (Rhipicephalus microplus) (Raimundo et al., 2017) and to have antifungal activity (Raimundo et al., 2018).
The aqueous crude extract of G. integrifolia leaves are used in organic agriculture to control Botrytis cinerea during pre- and post-harvest of grapes in viniculture (Silva et al., 2017). Hydroalcoholic extracts of G. integrifolia leaves were effective to control eggs and larvae of bovine ticks (Rhipicephalus microplus) (Dias, Tanure, & Bertonceli, 2018). The hydroethanolic extract of G. integrifolia inner bark had bacteriostatic activity and no toxicity to epithelial cells of Chinese hamster ovary (Arunachalam et al., 2016). The dichloromethane and methanolic extracts of G. integrifolia bark had antifungal activity (Freixa et al., 1998). Moreover, dichloromethane extract of G. integrifolia roots has antinociceptive and anti-inflammatory activities in vivo with mice and antiviral activity against herpes simplex virus (Silva et al., 2013). In addition, riverside dwellers usually burn G. integrifolia dried leaves to repel mosquitoes from the genus Anopheles, vector of malaria, showing its potential biocide activity (Pérez, 2002). However, despite these reports, there are no studies on the extract from G. integrifolia leaves to control insect, mainly A. aegypti. Thus, this study aimed to evaluate the biological activity of the ethanolic crude extracts from G. integrifolia leaves, flowers, and fruits to control A. aegypti third-stage larvae and pupae to develop an alternative for the control of this insect.
Materials and methods
Plant material: fruits, leaves, and flowers of G. integrifolia were harvested in Umuarama at the coordinates (23º46’16” S & 53º19’38” WO) and altitude of 442 m. Leaves and flowers were harvested in the morning in December 2017 and fruits were harvested in the morning in May 2017. The plant was identified and a sample was deposited in the Herbarium of Western Paraná State University, Cascavel Campus, Center of Biological Sciences and Health, under the number 1 716. This species was registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen, acronym in Portuguese) under the registration number A0A10E1.
Crude extract preparation: leaves, flowers, and fruits of G. integrifolia (500 g) were pulverized to 850 μm with a knife mill (Willye TE-650) and three extracts were obtained after dynamic maceration with solvent (ethanol 96 ºGL) depletion. The extracts were filtered and the solvent was removed in a rotating evaporator (Tecnal TE-210) at 40 °C. The yield of each crude extract (dry basis) was calculated in triplicate by the mass of the obtained crude extract (g) divided by the mass of the vegetal matter (leaves, flowers, or fruits) (g), multiplied by 100 and expressed in percentage (Ministério da Saúde, 2010).
Chemical characterization of the crude extract: for the chemical identification of the compounds, the crude extracts from G. integrifolia leaves, flowers, and fruits (100 mg) were analyzed by gas chromatography (Agilent 19091S-433) coupled to a mass spectrophotometer (Agilent 19091J-433) (CG-MS). An HP-5MS UI 5 % analytical column (30 m × 0.25 mm × 0.25 μm) was utilized with an initial temperature of 60 °C, and kept for 3 min. Then, a ramp of 5 °C/min and the temperature was increased to 300 °C and kept for 10 min and, finally, to 310 °C with a ramp of 10 °C/min for 10 min. Helium was utilized as the carrier gas at the linear speed of 1 mL/min until 300 °C and pressure release of 56 kPa. The injector temperature was 300 °C; the injection volume was 2 μL; the injection was in split mode (20:1). The transfer line was kept at 285 °C and the ionization source and quadrupole at 230 °C and 150 °C, respectively. The MS detection system was utilized in scan mode, in the range of mass/load ratio (m/z) of 40-550 with 3 min solvent delay. The compounds were identified by comparing their mass spectra with the ones from Wiley 275 libraries, and comparing their retention indices (RI) obtained by a homologous series of n-alkane standards (C7-C40) (Adams, 2017).
Insecticidal activity: Aedes aegypti third-stage larvae and pupae were obtained from the Department of Sanitary Surveillance of Umuarama, Paraná, Brazil. The extracts were diluted from 0.001 to 25 000 mg/mL in an aqueous solution containing 20 mL/L polysorbate-80. Ten A. aegypti larvae were placed in 25 mL flasks with 1.0 mL extract solution at different concentrations and kept in the dark at room temperature for 24 h (Costa et al., 2005; Fernandes, Souza Freitas, da Costa, & da Silva, 2005; Bonato, Cavalca, & Lolis, 2010). Larvae without movement and response to stimuli were considered dead (Bonato et al., 2010). The same procedure was used for A. aegypti pupae. The negative control was 20 mL/L polysorbate-80 aqueous solution and the positive control was the organothiophosphate temephos at the concentration ranging from 0.009 to 500 mg/mL (Macoris, Camargo, Silva, Takaku, & Andrighetti, 1995; Camargo et al., 1998). Lethal concentrations (LC50 and LC99.9) were calculated by the probit analysis.
Anticholinesterase activity: the anticholinesterase activity was determined by the bioautographic method according to Marston, Kissling, & Hostettmann (2002) with modifications (Yang et al., 2009). The methanolic solutions of the leaf, flower, and fruit extracts, and the positive control temephos were tested at concentrations varying from 50 to 0.000095 mg/mL (in methanol). The samples were plotted in aluminum TLC (thin-layer chromatography) plates (10 × 10 cm; 0.2 mm-thick 60 F254 silica gel), dried and sprayed with an acetylcholinesterase enzyme (500 U/mL) solution in a buffer solution of tris (hydroxymethyl) aminomethane hydrochloridrate (0.05 M, pH 7.8), sprayed with an α-naphthyl acetate solution (0.15 %) and kept at 37 °C for 20 min. After that, the chromo plates were sprayed with fast blue B salt colorimetric reagent (0.05 %) resulting in a purple color surface. The anticholinesterase activity of each G. integrifolia crude extract was determined by the emergence of white stains after 10 min, showing the inhibitory action of the evaluated concentration on the enzyme activity by contrasting with the purple color of the colorimetric reagent. Temephos was used at the same concentrations of the crude extracts as a positive control.
Statistical analysis: the experimental design was completely randomized and all assays carried out in triplicate. Data were submitted to analysis of variance (ANOVA) and compared utilizing SPSS statistics 22 program by Duncan test (P ≤ 0.05). Lethal concentrations (LC50 and LC99.9) and confidence intervals were calculated by probit analysis.
Results
The yield of the crude extracts from G. integrifolia flowers, fruits, and leaves was 8.2, 9.1, and 17.3 %, respectively, with the greatest yield for the leaf extract. The chemical identification of the extracts showed 20 chemical compounds in the flowers, 22 in the fruits, and 17 in the leaves, considering only the compounds in the relative area greater than 0.5 % (Table 1). The major compounds of the ethanolic extract from G. integrifolia flowers were vitamin E (18.0 %) and disulfide, ethyl iso-allocholate (10.6 %), from fruits were vitamin E (20.9 %), linolenic acid methyl ester (14.0 %), disulfide, bis(2-sulfhydryl ethyl) (11.9 %), and phytol (10.2 %), whereas from leaves were phytol (30.9 %), linolenic acid methyl ester (30.5 %), and methyl palmitate (10.9 %).
Table 1 Chemical composition obtained by gas chromatography-mass spectrometry of the extracts from Gallesia integrifolia leaf, flower, and fruit
| Peak | aCompound | aRIcalc | Relative area (%) | m/z | Structure | cMS | ||
| Leaves | Flowers | Fruits | ||||||
| 1 | 2,4-dithiapentane | 808 | - | 1.06 | 2.12 | 108.01 | C3H8S2 | a,b,c |
| 2 | 1,2,4-trithiolane | 810 | 0.86 | 1.10 | 1.76 | 124.23 | C2H4S3 | a,b,c |
| 3 | R-limonene | 847 | - | 0.91 | - | 139.98 | C10H16O3 | a,b,c |
| 4 | 2,3,5-trithiahexane | 885 | - | 6.17 | 4.67 | 140.27 | C3H8S3 | a,b,c |
| 5 | Disulfide, bis(2-sulfhydryl ethyl) | 935 | - | 11.91 | - | 185.96 | C4H10S4 | a,b,c |
| 6 | Cedr-8-en-13-ol | 936 | - | 0.93 | - | 220.18 | C15H24O | a,b,c |
| 7 | n-hexadecanoic acid | 937 | - | 5.57 | 1.04 | 256.24 | C16H32O2 | a,b,c |
| 8 | Palmitoleic acid, methyl ester | 938 | 1.57 | - | - | 268.24 | C17H32O2 | a,b,c |
| 9 | Methyl palmitate | 949 | 10.91 | 4.69 | 5.05 | 270.26 | C17H34O2 | a,b,c |
| 10 | Linoleic acid | 973 | 5.99 | - | - | 280.45 | C18H32O2 | a,b,c |
| 11 | Ethyl palmitate | 1 099 | 1.43 | - | 2.11 | 284.48 | C18H36O2 | a,b,c |
| 12 | Stearic acid | 1 100 | 1.05 | - | - | 284.28 | C18H36O2 | a,b,c |
| 13 | Linolenic acid methyl ester | 1 101 | 30.53 | - | 4.54 | 292.24 | C19H32O2 | a,b,c |
| 14 | 10,13-octadecadienoic acid, Methyl ester | 1 103 | - | 6.07 | 5.04 | 294.26 | C19H36O2 | a,b,c |
| 15 | Methyl linoleate | 1 107 | - | 5.17 | - | 294.47 | C19H34O2 | a,b,c |
| 18 | Phytol | 1 137 | 30.92 | 10.24 | 6.50 | 296.31 | C20H40O | a,b,c |
| 19 | Linoleic acid ethyl ester | 1 171 | 3.87 | 14.00 | 2.28 | 308.27 | C20H36O2 | a,b,c |
| 20 | Ethyl octadecanoate | 1 174 | - | 0.55 | - | 312.30 | C20H40O2 | a,b,c |
| 22 | α-monoolein | 1 262 | - | - | 2.61 | 354.61 | C21H40O4 | a,b,c |
| 24 | Isohumulone | 1 367 | 4.95 | - | - | 356.29 | C21H30O5 | a,b,c |
| 25 | n.i. | 1 430 | 0.60 | 0.74 | 0.30 | - | - | a,b,c |
| 26 | Stigmasterol | 1 478 | 1.57 | - | 7.38 | 412.70 | C29H48O | a,b,c |
| 27 | β-sitosterol | 1 479 | 0.64 | - | 5.59 | 414.38 | C29H50O | a,b,c |
| 28 | γ-sitosterol | 1 506 | 2.61 | - | 2.14 | 414.38 | C29H50O | a,b,c |
| 29 | β-tocopherol | 1 559 | 0.56 | - | - | 416.36 | C28H48O2 | a,b,c |
| 30 | γ-tocopherol | 1 618 | 0.73 | - | - | 416.36 | C28H48O2 | a,b,c |
| 32 | Lupeol | 1 671 | - | - | 6.84 | 426.38 | C30H50O | a,b,c |
| 33 | α-amyrin | 1 716 | - | - | 1.97 | 426.38 | C30H50O | a,b,c |
| 35 | Vitamin E | 1 761 | - | 20.86 | 18.04 | 430.38 | C29H50O2 | a,b,c |
| 36 | Ethyl iso-allocholate | 1 780 | - | - | 10.60 | 436.63 | C26H44O5 | a,b,c |
| 37 | Betulin | 1 782 | 1.11 | - | 1.10 | 442.38 | C30H50O2 | a,b,c |
| 38 | Inotodiol | 1 792 | - | 2.63 | - | 442.38 | C30H50O2 | a,b,c |
| 40 | Lupeol acetate | 1 847 | - | - | 2.60 | 468.39 | C32H52O2 | a,b,c |
| 41 | Cycloartenol acetate | 1 914 | - | 0.99 | 4.88 | 468.76 | C32H52O2 | a,b,c |
| 42 | 13,14-epoxyoleanan-3-ol, acetate | 1 916 | - | 1.18 | - | 470.37 | C31H50O3 | a,b,c |
| 44 | Barringtogenol C | 2 174 | - | 3.53 | - | 490.36 | C30H50O5 | a,b,c |
| 45 | 3-O-Acetyl-6-methoxy-cycloartenol | 2 194 | - | 1.68 | 0.77 | 498.40 | C33H54O3 | a,b,c |
| Total identified | 99.30 | 99.24 | 99.63 | |||||
| Fatty acids | 7.04 | 5.57 | 1.04 | |||||
| Sulfur compounds | 0.86 | 20.24 | 8.55 | |||||
| Monoterpene hydrocarbons | - | 0.91 | - | |||||
| Oxygenated sesquiterpene | - | 0.93 | - | |||||
| Oxygenated diterpene | 30.92 | 10.24 | 6.50 | |||||
| Triterpenes | 1.11 | 6.16 | 12.52 | |||||
| Fatty acid esters | 48.31 | 29.93 | 19.02 | |||||
| Vitamin precursor | 1.29 | - | - | |||||
| Phytoesterol | 4.82 | 0.99 | 19.99 | |||||
| Vitamins | - | 20.86 | 18.04 | |||||
| Alpha acids | 4.95 | - | - | |||||
| Steroidal compound | - | - | 10.60 | |||||
| Others | - | 3.41 | 3.37 | |||||
aCompounds listed in order of elution in column HP-5MS; b RI = Identification based on retention index (RI) using a homologous series of n-alkane C7-C40 on Agilent HP-5MS column; c MS = identification based on comparison of mass spectra using Wiley 275 libraries; Relative area (%) = percentage of the area occupied by the compounds in the chromatogram; m/z = mass values; n.i. = not identified; (-) = absent.
The projection of the main classes of compounds by principal component analysis indicates that factor 1 represents 68 % of the variability of the classes found in the extracts of the leaves, flowers, and fruits of G. integrifolia (Fig. 1). In factor 2, the extracts of leaves and flowers showed a positive correlation, this can be explained because both presented as majority class fatty acid esters (48.2 and 29.9 %, respectively) (Table 1, Fig. 1). However, the extracts of leaves and fruits presented negative correlation in factor 2, the fruit extract showed high concentrations of phytosterols (18.0 %) and vitamins (20.0 %), while in the leaves extract was identified lower concentrations of phytosterols (4.8 %) and did not identified vitamins (Table 1, Fig. 1). In general, the class projection indicates variation between the chemical composition of extracts, mainly those related to the esters of fatty acids, oxygenated diterpenes, vitamins, phytosterols, and sulfur compounds (Fig. 1).
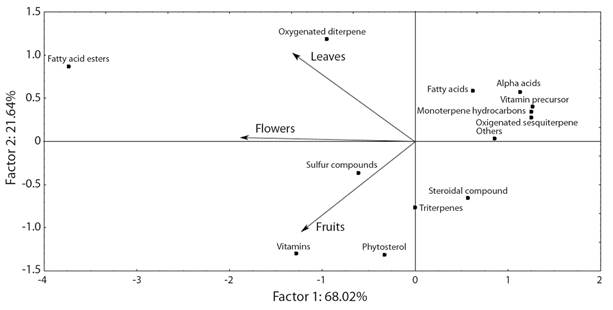
Fig. 1 Biplot of principal component analysis scores and loadings for the gas chromatography and mass spectrometry representing the projection of chemical classes of the crude extract from leaves, flowers, and fruits of Gallesia integrifolia.
A class of predominant compounds was the esters of fatty acids in flowers (29.9 %), fruits, (19.0 %) and leaves (48.3 %). The vitamins were observed in the flowers (20.9 %) and fruits (18.0 %). The sulfur compounds of the characteristic alliaceous odor of this plant were predominant in the flowers (20.2 %), followed by fruits (8.6 %) and a smaller concentration in the leaves (0.9 %) (Table 1, Fig. 1). The major compounds in the crude extract in the flowers were ethyl ester of linoleic acid (14 %) (Fig. 2) and disulfide, bis (2-sulfhydryl ethyl) (11.9 %) (Fig. 3); in the fruits, they were ethyl iso-allocholate (10.6 %) and stigmasterol (7.4 %); and in the leaves, they were phytol (30.9 %) (Fig. 4), linoleic acid methyl ester (30.5 %) and methyl palmitate (10.9 %). Some major compounds were found only in one part of the plant such as linoleic acid (6.0 %) and isohumulone (5.0 %) just in the leaves; disulfide, bis(2-sulfhydryl ethyl) (11.9 %) and methyl linoleate (5.2 %) were found only in the flowers; and lupeol (6.8 %) and ethyl iso-allocholate (10.6 %) were present just in the fruits (Table 1).
Fig. 2 Mass spectrum of linoleic acid ethyl ester of the extract from Gallesia integrifolia flower (14.0 %) and fruit (2.3 %) obtained by gas chromatography-mass spectrometry.
Fig. 3 Mass spectrum of disulfide, bis(2-sulfhydryl ethyl) of the extract from Gallesia integrifolia flower (11.9 %) obtained by gas chromatography-mass spectrometry.
Fig. 4 Mass spectrum of phytol of extract from Gallesia integrifolia leaves (30.9 %) obtained by gas chromatography-mass spectrometry.
The main biomolecules of organosulfate class present in the extract from G. integrifolia flower were disulfide, bis(2-sulfhydryl ethyl) (C4H10S4) (Fig. 2, Table 1), methyl (methylsulfinyl) methyl sulfide (C3H8S3), 1,2,4-trithiolane (C2H4S3), and 2,4-dithiapentane (C3H8S2) (Table 1) with compounds with four, three, and two sulfur atoms, respectively.
Besides the chemical composition, the biological potential of three G. integrifolia extracts was evaluated against A. aegypti third-stage larvae and pupae. Overall, all the tested samples were more efficient for the larvae as well as for the pupae than the positive control. The best lethal concentrations were for the larvae when compared to pupae, as seen in the extract of G. integrifolia flowers where the LC99.9 for larvae was 0.032 mg/mL, and for pupae was LC99.9 of 0.969 mg/mL, which is 3 % more effective than in larvae when compared to pupae. Followed by the raw fruit extract the LC99.9 for larvae was 0.124 mg/mL, and for pupae was LC99.9 of 6.086 mg/mL. However, the extract from G. integrifolia leaves was 5 % more effective for larvae when compared to pupae (Table 2).
Table 2 Lethal concentration (LC50 and LC99.9) and confidence interval (CI) of Gallesia integrifolia extracts from flowers, fruits, and leaves against Aedes aegypti larvae and pupae by probit analysis
| G. integrifolia extract of | Larvae | Pupae | ||
| LC50 (mg/mL) [CI] | LC99.9 (mg/mL) [CI] | LC50 (mg/mL) [CI] | LC99.9 (mg/mL) [CI] | |
| Leaves | 0.094 ± 0.001b [0.091-0.093] | 0.278 ± 0.001c [0.279-0.282] | 2.177 ± 0.177a [2.000-2.354] | 14.040 ± 0.581a [13.459-14.622] |
| Flowers | 0.006 ± 0.001a [0.006-0.006] | 0.032 ± 0.001a [0.030-0.032] | 0.036 ± 0.004a [0.035-0.037] | 0.969 ± 0.026a [0.968-0.970] |
| Fruits | 0.025 ± 0.002a [0.023-0.027] | 0.124 ± 0.003b [0.120-0.126] | 1.037 ± 0.080a [0.840-1.152] | 6.086 ± 0.039a [5.704-6.307] |
| Temephos (control) | 0.398 ± 0.050c [0.348-0.448] | 1.140 ± 0.060d [1.080-1.200] | 234.370 ± 22.090b [212.280-256.460] | 443.640 ± 14.870b [428.770-458.510] |
LC50 = lethal concentration that kills 50 % of A. aegypti larvae and pupae populations; LC99.9 = lethal concentration that kills 99.9 % of A. aegypti larvae and pupae populations; CI = confidence interval; Positive control = commercial organophosphate temephos; Equal letters in the same column indicate that there is no significant difference among treatments by Duncan’s test (P ≤ 0.05).
To help elucidate the action mechanism of these crude extracts, acetylcholinesterase enzyme (AChE) was evaluated using the crude extracts from G. integrifolia flowers, fruits, and leaves and temephos (commercial organophosphate) as a positive control (Table 3).
Table 3 Inhibiting activity of acetylcholinesterase enzyme at different concentrations of extracts from Gallesia integrifolia flowers, fruits, and leaves by bioautographic method
| Concentration (mg/mL) | Inhibition of acetylcholinesterase enzyme | ||||||||
| Leaf | Flower | Fruit | PC | Concentration (mg/mL) | Leaf | Flower | Fruit | PC | |
| 50 | +++ | +++ | +++ | +++ | 0.0488 | - | + | + | ++ |
| 25 | ++ | +++ | +++ | +++ | 0.0244 | - | + | + | + |
| 12.5 | + | ++ | ++ | +++ | 0.0122 | - | + | - | + |
| 6.25 | + | ++ | ++ | +++ | 0.0061 | - | + | - | + |
| 3.125 | + | + | ++ | +++ | 0.0030 | - | + | - | + |
| 1.5625 | + | + | ++ | +++ | 0.0015 | - | + | - | + |
| 0.7812 | + | + | + | ++ | 0.00076 | - | + | - | + |
| 0.3906 | + | + | + | ++ | 0.00038 | - | + | - | + |
| 0.1953 | - | + | + | ++ | 0.00019 | - | + | - | - |
| 0.0976 | - | + | + | ++ | 0.00009 | - | - | - | - |
Concentration = mg/mL; PC = positive control (commercial organophosphate temephos); (+++) = strong inhibition of acetylcholinesterase enzyme; (++) = moderate inhibition; (+) = weak inhibition; (-) = absence of inhibition.
For the inhibiting activity test of acetylcholinesterase enzyme, the crude flower extract presented inhibitory effect at the concentration of 0.00019 mg/mL, followed by the positive control at 0.00038 mg/mL, by the fruit extract (0.0244 mg/mL) and the leaf extract (0.3906 mg/mL). When compared to the ex situ test (LC99.9) with A. aegypti larvae, the extracts from flowers, fruits, and leaves were 16.8, 0.5, and 0.07 %, respectively, less effective than in the bioautographic test in TLC.
Discussion
The chemical identification of crude extracts of G. integrifolia flowers, fruits, and leaves was carried out by CG-MS and it was verified the presence of esters of fatty acids as the predominant class in the three extracts: leaf extract (48.3 %), flower extract (29.9 %), and fruit extract (19 %). The amount and distribution of fatty acids varied in the several species of the plants, besides being affected by seasonal influences (Kozlowski & Pallardy, 1996). The fatty acids found in the leaves are responsible for controlling water loss to the environment during gas exchanges, and protect the plant against the nutrient loss due to the high incidence of ultraviolet rays and intense rainfall (Kozlowski & Pallardy, 1996); in the flowers, as well as in the leaves, the fatty acids are part of the surface impermeabilization, avoiding water loss, and part of the pollination process as they are in the chemical composition of flower nectar (Levin, McCue, & Davidowitz, 2017) whereas, in the fruits and seeds, these acids act as a barrier to moist diffusion, as a form of protection, because it is a reserve organ to the embryo (Esau, 1986; Kunst & Samuels, 2009). In general, fatty acids act on the cellular structure of vegetables, growth, nutrition, senescence, and protection against phytopathogens and the environment (Meï et al., 2015; Li, Xu, Li-Beisson, & Philippar, 2016; Silva et al., 2016).
The linoleic acid ethyl ester found in the extract from flowers and fruits (Fig. 2) is a long-chain polar compound with antibacterial and anti-inflammatory activities utilized in the cosmetic industry (Jelenko, Wheeler, Anderson, Callaway, & McKinley, 1975; Park et al., 2014). The linoleic acid ethyl ester as well as the linolenic acid methyl ester are present in the dark green leaves because they are part of the apolar lipid fraction of plants (Simopoulos, 2002). There are no reports on the activity of these molecules against A. aegypti, but fatty acid methyl ester had LC99.9 of 0.17 mg/mL in Culex quinquefasciatus larvae (Silva et al., 2016), indicating insecticide effect against larvae of this insect.
Ethyl iso-allocholate found in the fruits (10.6 %) does not present cytotoxicity in zebrafish and can induce apoptosis through caspases signaling pathway, causing morphological alterations in the cell membranes (Thakur & Ahirwar, 2019). According to Cooper, Thi, Chamberlain, Pio, & Lowenberger (2007), this signaling is also possibly responsible for A. aegypti mortality because it acts as a biochemical cascade, triggering a proteolytic effect. Another compound with biological potential found in G. integrifolia is methyl palmitate (leaves 10.9, flowers 4.7, and fruits 5.0 %), an antagonist for muscarinic receptors with broad toxicity against phytophagous mites such as Tetranychus cinnabarinus (Boisduval) at 10 mg/mL (Wang et al., 2009).
Moreover, phytol found at high concentration in the extract from G. integrifolia leaves (Fig. 4) chemically corresponds to a branched long-chain aliphatic alcohol that gives hydrophobic characteristic to chlorophyll molecules; however, when it is broken by chlorophyllase, phytol is converted into phytanic acid with an important biological effect on thermogenic activities and inhibitor of teratogenic effects of retinol (Marquez, 2003). Phytol also has anti-inflammatory activity by releasing histamine (26.9 %), serotine, and bradykinin (49.9 %), and prostaglandin (68 %) compared to control (diclofenac 5 mg/kg) (Phatangare, Deshmukh, Murade, Hase, & Gaje, 2017). Phytol has been reported as an inhibitor of proteins and enzymes from bacteria (Ghaneian, Ehrampoush, Jebali, Hekmatimoghaddam, & Mahmoudi, 2015). The compound stigmasterol (Table 1) obtained from G. integrifolia fruit extract has been related to the inhibition of acetylcholinesterase enzyme in human embryonic kidney cells (HEK293), that is the same mechanism of action of organophosphate insecticides (Alout et al., 2012; Gade et al., 2017).
Vitamin E (Fig. 5) was identified at a high concentration in the extracts of G. integrifolia flower (20.9 %) and fruit (18.0 %). Vitamin E, which consists of tocopherols and tocotrienols, is found in plants giving them photoprotective and antioxidant characteristics (Havaux, Eymery, Porfirova, Rey, & Dormann, 2005). Vitamin E was not detected in the extract from G. integrifolia leaves but the presence of its precursors, β-tocopherol and γ-tocopherol, was identified.
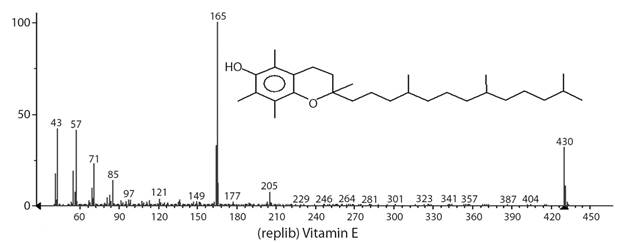
Fig. 5 Mass spectrum of vitamin E identified in the extract from Gallesia integrifolia flower (20.9 %) and fruit (18.0 %) obtained by gas chromatography-mass spectrometry.
Sulfur compounds were predominant in G. integrifolia flower extract (20.2 %), followed by fruits (8.6 %) and in a small concentration in the leaf extract (0.9 %) (Table 1). The major chemical compounds were disulfide, bis(2-sulfhydryl ethyl) (flowers 11.9 %) (Fig. 3). This biomolecule presents antioxidant potential due to its high potential to eliminate free radicals (Murugesan, Pandiyan, Saravanakumar, Moodley, & Mackraj, 2019). Other present compounds were 2,3,5-trithiahexane (flowers 6.2 and fruits 4.7 %), 1,2,4-trithiolane (flowers 1.1, fruits 1.8, and leaves 0.9 %) and 2,4-dithiapentane (flowers 1.1 and fruits 2.1 %). All these biomolecules can be related to the characteristic alliaceous odor of the plant, mainly during flowering to attract specific pollinators (Kishimoto, Maeda, Haketa, & Okubo, 2014).
When the insecticidal activity against A. aegypti was analyzed, the values of LC99.9 for the control of A. aegypti was 0.032 mg/mL for larvae and 0.969 mg/mL for pupae, using the extract from G. integrifolia flower (Table 2), whereas in the positive control (temephos) at LC99.9 was 1.140 mg/mL for larvae and 443.64 mg/mL for pupae. However, the extract from G. integrifolia flowers was 3.6 % more effective against A. aegypti larvae than the positive control. This indicates that this crude extract may be an alternative to be used in the control of this insect. Nevertheless, these values are much over the values of 0.0000031 mg/mL for temephos against Rockefeller strain larvae cited by Jonny et al. (2015). According to Cheng, Chang, Chang, Tsai, & Chen (2003), compounds that presented LC50 < 0.100 mg/mL are considered active as larvicides on A. aegypti, and highly active with LC50 < 0.05 mg/mL. The crude extract of G. integrifolia leaves showed active (LC50 = 0.094 mg/mL) on A. aegypti larvae and the crude extracts of flowers (LC50 = 0.006 mg/mL) and fruits (LC50 = 0.025 mg/mL) of G. integrifolia demonstrating highly effective in controlling the larvae of A. aegypti (Kiran, Bhavani, Devi, Rao, & Reddy, 2006).
The lethal concentrations found for larvae were lower than the ones for pupae, as it occurred in the extract from G. integrifolia fruit in which the LC99.9 for larvae was 0.124 mg/mL, and LC99.9 of 6.086 mg/mL for pupae, which was 4.9 % more effective for A. aegypti larvae than pupae. Moreover, the extract from G. integrifolia leaves was 5 % more effective for larvae when compared to pupae (Table 2). The intoxication pathways of the product are distinct for larvae and pupae because in the pupal stage, there is no food intake and, therefore, there is no compound intake. However, in this stage, the compounds can act on the cutaneous surface of the pupae and cause death by protein denaturation, enzymatic inhibition, and/or disintegration of the cell membrane (Consoli & Oliveira, 1994; Regnault, 1997; Carvalho et al., 2003; Cavalca, Lolis, Reis, & Nonato, 2010). In the larval stage, the culicid is more susceptible, because, besides the contact by the external membranes, when ingesting food, it can intake compounds found in the water, increasing its potential of action (Procópio et al., 2015).
Extracts of G. integrifolia presented insecticide activity for the holometabolic phase of A. aegypti when compared to the positive control (commercial organophosphate temephos). When analyzing LC99.9 of A. aegypti larvae, it was verified that the best results were for the extract from G. integrifolia flower, followed by the extract from fruits, and then from leaves, that was 35.6-, 9.2-, and 4.1-fold more effective, respectively, than the positive control (Table 2). The greatest insecticide activity (P ≤ 0.05) was for the extract from G. integrifolia flower with LC99.9 of 0.032 mg/mL for larvae and 0.969 mg/mL for pupae, which is 3.9-fold more effective than the extract from G. integrifolia fruit and 8.7-fold more effective than the extract from G. integrifolia leaves for larvae.
LC99.9 of the crude ethanolic extract from G. integrifolia flowers is 3.2-fold more effective to control A. aegypti larvae than Lippia alba essential oil, 3.7-fold more effective than Ocimum gratissimum essential oil, 4.3-fold more than Cymbopogon citratus essential oil, and 9-fold more effective than Eucalyptus citriodora (current name Corymbia citriodora) essential oil (Cavalcanti, Morais, Lima, & Santana, 2004; Vera et al., 2014). This indicates the larvicide potential of the extract from G. integrifolia flowers as a phytoinsecticide. In addition, the extract from G. integrifolia flowers has 20.2 % of sulfur compounds that can be related to this greater insecticide activity efficiency and the sulfur compounds were also verified in the extract from G. integrifolia fruits (8.5 %) and leaves (0.9 %) (Table 1).
The major biomolecules of the organosulfurates class found in the extract from G. integrifolia flower were disulfide, bis(2-sulfhydryl ethyl) (C4H10S4) (Fig. 3), methyl (methylsulfinyl) methyl sulfide (C3H8S3), 1,2,4-trithiolane (C2H4S3), and 2,4-dithiapentane (C3H8S2) (Table 1) with compounds containing four, three, or two sulfur atoms. Garlic essential oil (Allium tuberosum and Allium sativum) has been utilized to control insect larvae and/or as a repellent of mosquitoes such as Aedes spp., Anopheles spp., and Culex spp. due to the presence of sulfur atoms in their composition (Denloye et al., 2003; Trongtokit et al., 2005). Liu, Liu, Zhou, & Liu (2014) observed that Allium macrostemon essential oil with 98.1 % of sulfur compounds presented LC99.9 of 0.139 mg/mL and the methyl propyl disulfide isolates had LC99.9 of 0.151 mg/mL, whereas dimethyl trisulfide presented LC99.9 of 0.058 mg/mL against Aedes albopictus. The concentrations of the extract from G. integrifolia flowers were 1.5-fold more efficient than A. tuberosum essential oil and 2-fold more efficient than allyl methyl trisulfide (C4H8S3) (Liu, Liu, Chen, Zhou, & Liu, 2015).
Another plant with sulfur compounds in the essential oil is Petiveria alliacea that has 35.3 % of dibenzyl disulfide (C14H14S2) (Zoghbi, Andrade, & Maia, 2002; Kerdudo et al., 2015) and insecticide activity against A. aegypti larvae with LC99.9 of 0.023 mg/mL (Hartmann, Silva, Walter, & Jeremias, 2018), similarly to what was found in the extract from G. integrifolia flowers (LC99.9 of 0.036 mg/mL). Pseudocalymma alliaceum (current name Mansoa alliacea) presents, in the composition of leaf essential oil, sulfur molecules such as 11.8 % diallyl sulphide (C6H10S), 50 % diallyl disulphide (C6H10S2), and 10.4 % trisulfide, di-2-propenyl (C6H10S3), and insecticide activity with LC50 of 0.267 mg/mL and LC99.9 of 0.547 mg/mL against C. quinquefasciatus pupae (Echegoyen et al., 2014). This suggests that the insecticide activity may be related to the sulfur molecules in the chemical composition of the essential oil from these plants.
The biological activity of sulfur compounds against A. aegypti is still not well understood. However, sulfur compounds can form disulfide bridges (covalent bonds) with amino acids and proteins that are important to keep the protein structure and the catalytic functions of enzymes; however, exogenous sulfur compounds can cause the destabilization of protein quaternary structure and enzymatic inactivation (Belitz, Grosch, & Schieberle, 2009; Berkmen, 2012). The greater the number of sulfur atoms in a molecule, then the greater the number of polysulfide bridges (-Sn-) are present in the chemical structures of the compound. Consequently, the water solubility will be smaller, increasing the chemical affinity of the molecule with the structure of the cell wall and membranes, mainly consisting of ergosterol and chitin which in microbial cells promote the membrane rupture and cell imbalance (Cahagnier, 1988; Peacock & Goosey, 1989; Levinson, 2016). This suggests that these results can be related to the cell wall permeability, physical, and chemical characteristics of solubility, and molecular absorption in lipophilic and hydrophilic media, inherent to the test in A. aegypti larvae and pupae (Benson, 2005; Brain, Green, & Apia, 2007). In addition, Kumar (2015) reported that sulfur compounds in A. sativum such as allicin (C6H10S2O) act by inhibiting acetylcholinesterase enzyme up to the concentration of 0.05 mg/mL because they present a greater chemical affinity by anion sites of cholinesterase (Mahfouz, Metcalf, & Fukuto, 1969). These data corroborate our studies because the presence of sulfur atoms in the flower (20.2 %) and fruit (8.6 %) extract of G. integrifolia can be related to the best results of the acetylcholinesterase enzyme inhibition in relation to the leaf extract (0.9 %). This indicates that the extracts from G. integrifolia strongly inhibit the acetylcholinesterase enzyme, mainly when correlated to the presence of sulfur compounds. However, more studies are necessary to identify the active compounds with this biological activity.
The yield of crude extract from G. integrifolia flowers, fruits, and leaves was 8.2, 9.1, and 17.3 %, respectively. The major compounds of the ethanolic crude extract from G. integrifolia leaves are phytol (30.9 %), linolenic acid methyl ester (30.5 %), and methyl palmitate (10.9 %), from flowers are vitamin E (20.9 %), linolenic acid methyl ester (14 %), disulfide, bis(2-sulfhydryl ethyl) (11.9 %), and phytol (10.2 %), and from fruits are vitamin E (18 %) and ethyl iso-allocholate (10.6 %). Only extracts from fruits and flowers present a high concentration of vitamin E whereas only flower extract presents a high concentration of disulfide, bis(2-sulfhydryl ethyl). There are organosulfurates compounds such as 1,2,4-trithiolane in flowers (1.1), fruits (1.8), and leaves (0.9 %), 2,3,5-trithiahexane in flowers (6.2 %) and fruits (4.7 %), 2,4-dithiapentane in flowers (1.1 %) and fruits (2.1 %), and disulfide, bis (2-sulfidril ethyl) in flowers (11.9 %). The flower extract has greater larvicidal activity on A. aegypti LC99.9 of 0.032 mg/mL and the smallest concentration for acetylcholinesterase enzyme, which is of 0.00019 mg/mL. The flower extract is 35.6-fold more efficient for A. aegypti larvae than the positive control, and 12.8 % more efficient for the anticholinesterase activity test. The crude extracts from G. integrifolia fruits, leaves and, mainly flowers are a potential alternative bioinsecticides to control A. aegypti larvae and pupae.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 

