Introduction
One of the main issues of ecological studies is to understand how biotic and abiotic drivers shape plant community assemblages along environmental gradients (Götzenberger et al., 2012). Two major factors determine plant community assemblages, environmental filters (e.g., elevation, growth substrates) and limiting similarity (e.g., biotic interactions). Limiting similarity implies that low niche overlap between two species will allow their coexistence (Götzenberger et al., 2012). Under environmental filtering, habitat restrictions result in the establishment of species with similar environmental requirements (e.g., Mudrák et al., 2016). When habitat filtering is the major driver, some species with similar environmental requirements may limit the presence of one another in the community (Götzenberger et al., 2012). Most plant community assemblage studies in the Brazilian Atlantic Forest have evaluated the effect of environmental drivers (e.g. elevation, soil properties) on species richness and community composition, mainly in vascular plant communities (e.g., Neri et al., 2016; Rodrigues, Villa, & Viana, 2019; Silva et al., 2019). Some studies recognize altitude, temperature, substrate availability and microhabitat as important filters of tropical bryophyte diversity (Amorim, Carvalho, Santos, & Luizi-Ponzo, 2017; Peñaloza-Bojacá et al., 2018; Silva, Sfair, Santos, & Pôrto, 2018), while there is little knowledge about the biotic interactions and mechanisms that affect the structure of bryophyte communities (Zamfir & Goldberg, 2000; Mulder, Uliassi, & Doak, 2001; Benavides, 2007).
The composition and structure of bryophyte communities have been considered the result of environmental conditions, and interactions with other organisms, such as the effect of vascular plants on competition and facilitation (Mulder et al., 2001; Benavides, 2007). Studies indicate a positive relationship between rupicolous and courticolus bryophytes with some ferns (Dubuisson, Hennequin, Rakotondrainibe, & Schneider, 2003; Ellyson & Sillett, 2003). For the Atlantic Forests of Brazil, Sylvestre (2001) mentions the development of Asplenium auritum, on bryophyte-covered rocks and trunks located near streams.
The Atlantic Forest is recognized as a biodiversity hotspot with a high percentage of endemic species (Stehmann et al., 2009). Unfortunately, it is under intensive process of deforestation and destruction, and only ca. 10 % of the original area persists as isolated fragments in different stages of succession (Costa & Lima, 2005). Still, the Atlantic Forest fragments are important maintainers of biodiversity, with a positive relationship between biodiversity and ecosystem functioning (Rodrigues et al., 2019).
Bryophytes represent one of the important functional groups in the maintenance of the diversity and structure in the Atlantic Forest (Costa & Gomes, 2003). Studies in this phytogeographic domain in Southeastern Brazil shown the influence of local environmental filters (physical and chemical characteristics of substrates, environment temperature and water availability) on the cover and composition of bryophyte species in these communities (Amorim et al., 2017; Batista & Santos, 2016). However, there are limited studies that analyze how biotic relationships filters shape communities of bryophytes in the Atlantic Forest.
In this study we evaluate whether the influence of fern Asplenium auritum cover determine changes in species richness and composition of bryophyte communities, in a semideciduous remnant forest in the Parque Estadual da Serra do Brigadeiro (State of Minas Gerais, Brazil). We addressed the following questions: (1) what is species richness and community composition pattern of bryophytes associated with A. auritum? (2) What is the species cover distribution of bryophyte communities associated with A. auritum? (3) Does fern cover affect bryophyte species richness? This is the first study of biotic interactions between the bryophyte communities and a fern species in a remnant of the Atlantic Forest in Southeastern Brazil.
Materials and methods
Study area: Our study was developed in the Parque Estadual da Serra do Brigadeiro (PESB), a protected area under state administration, located in the state of Minas Gerais, Brazil (20°43’57.1” S & 42°28’09.8” W; Fig. 1). The PESB covers an area of 14 985 ha and is inserted in the Atlantic Forest phytogeographic domain, and has two types of plant formations, according with IBGE (2012): (1) Semideciduous forest from the altomontana formation, and (2) high-altitude fields (campos de altitude). The climate is mesothermal (CWb) in Koeppen’s (1948) classification, with an average temperature of 18 °C and a minimum of less than 0 °C in the highest areas, and an annual average rainfall of 1 500 mm/year, with a dry period from June to August (PESB, 2007).
We worked in the semideciduous forest (Oliveira-Filho & Ratter, 1995) along six sites distributed in the middle and Southern sectors of the PESB (Fig. 1). These areas correspond to vegetation cover located mainly on the mountain slopes, between 1 000 and 1 360 m of elevation, and the predominant vegetation is characterized by secondary forests in various stages of succession (Caiafa & Silva, 2005).
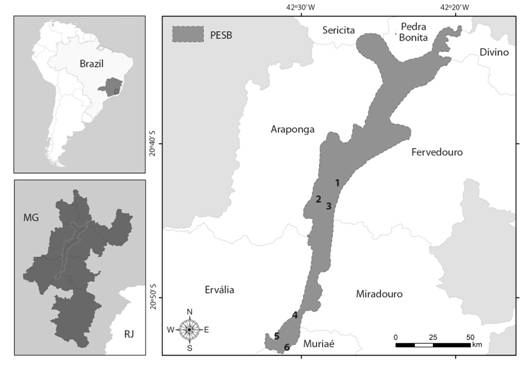
Fig. 1 Location of the study sites within Parque Estadual da Serra do Brigadeiro (PESB), Minas Gerais, Brazil: (1) Trilha do Carvão, (2) Trilha do Pico do Grama, (3), Trilha da Pedra do Pato (4) Trilha do Cruzeiro do Careço, (5) Trilha do Itajurú e (6) Trilha do Avião. South America and Brazilian map with the Minas Gerais State (gray map) and the boundaries of PESB. Map generated using ArcGIS®.
Vegetation sampling: Plant measurements and collections were carried out during four expeditions between May 2017 and April 2018. We selected one transect of 300 m in each of six areas where Asplenium auritum was present. In all transect we established 39 10 × 10 cm plots randomly distributed, divided into 25 subplots 2 × 2 cm. Each plot was located in the middle of each substrate occupied by the fern for estimated plant cover (Garcia-Cancel, Melédez-Ackerman, Olaya-Arenas, Flores, & Tremblay, 2013) and identification of bryophyte species. From each plot, all bryophytes were identified to the species level. We recorded A. auritum cover and represented in four fern cover levels (25, 50, 75 and 100 %). All bryophytes were collected for later re-identification in the VIC Herbarium.
Bryophyte identification: Species identification included the preparation of slides and observation in stereo- and optical microscope, using keys and specialized literature (Sharp, Crum, & Eckel, 1994; Gradstein, Churchill, & Salazar-Allen, 2001; Gradstein &Costa, 2003; Costa, Almeida, Santos, Gradstein, & Churchill, 2010; Yano & Peralta, 2011; Bordin & Yano, 2013). All specimens are deposited in the herbarium VIC (Acronym follows Index Herbariorum,Thiers, 2019). Our classification follows Söderström et al. (2016) for Marchantiophyta and Goffinet, Buck, and Shaw (2009) and Carvalho-Silva et al. (2017) for Bryophyta. Eight types of bryophyte life-forms were recognized (Mägdefrau, 1982): cushion, fan, dendroid, mat, pendant, turf, thalloid and weft.
Data analysis: To comparebryophyte species richness between four fern cover levels and between mosses and liverworts, we used sample-based data to estimate rarefaction and extrapolation curves, using the first Hill number (Chao et al., 2014). Extrapolations were made based on presence/absence of species in the plot data, using Hill’s number of order 0 (Colwell et al., 2012). These estimates were obtained using the “iNEXT” package (Hsieh, Ma, & Chao, 2016).
Non-metric multidimensional scaling (NMDS) and permutational multivariate analysis of variance (PERMANOVA, 9999 permutations) were performed using ‘Vegan’ package (Oksanen et al., 2017) to determine the differences in species composition between four fern cover levels.
The species rank curves were based on plant cover. To obtain species rank curves, all species were ranked from the most to the least dominant ones based in cover (e.g. Villa et al., 2018). Finally, we tested the main effects of bryophyte cover, and fern cover level on bryophyte species richness, using a covariance analysis (ANCOVA). Variables were grouped into three categories, viz. bryophyte species richness (continuous response variable) at community level (overall species); and fern and bryophyte cover (continuous explanatory variable).
Results
Floristic composition and life-forms: We found a total of 60 species (30 liverworts and 30 mosses), grouped in 35 genera and 23 families (Table 1). Among liverworts, families Lejeuneaceae and Plagiochilaceae stood out as the richest, with 15 and five species, respectively. The richest moss families were Fissidentaceae and Pilotrichaceae, with four species each. The most representative genera in the liverworts were Lejeunea, with eight species, and Plagiochila with five; for mosses, Fissidens, with four species was the most speciose genus.
TABLE 1 List of bryophytes species in the Parque Estadual da Serra do Brigadeiro, Minas Gerais, Brazil, frequency in sample plots and life-forms
| Species | Frequency | Life-form |
| Mosses | ||
| Brittonodoxa subpinnata (Brid.) W.R. Buck, P.E.A.S.Câmara & Carv.-Silva | 1 | Mat |
| Campylopus arctocarpus (Hornsch.) Mitt. | 2 | Turf |
| Chryso-hypnum diminutivum (Hampe) W.R.Buck | 5 | Weft |
| Chryso-hypnum elegantulum (Hook.) Hampe | 1 | Weft |
| Cyclodictyon albicans (Hedw.) Kuntze | 1 | Weft |
| Eulacophyllum cultelliforme (Sull.) W.R.Buck & Ireland | 1 | Weft |
| Fissidens dissitifolius Sull. | 1 | Fan |
| Fissidens hornschuchii Mont. | 7 | Fan |
| Fissidens pellucidus Hornsch. | 1 | Fan |
| Fissidens zollingeri Mont. | 1 | Fan |
| Isopterygium subbrevisetum (Hampe) Broth. | 1 | Mat |
| Isopterygium tenereum (Sw.) Mitt. | 1 | Mat |
| Isopterygium tenerifolium Mitt. | 1 | Mat |
| Leiomela bartramioides (Hook.) Paris | 2 | Turf |
| Lepidopilum muelleri (Hampe) Mitt. | 1 | Mat |
| Lepidopilum scabrisetum (Schwägr.) Steere | 1 | Mat |
| Leucobrym crispumMüll. Hal. | 1 | Cushion |
| Pelekium schistocalyx (Müll.Hal.) A. Touw | 1 | Weft |
| Plagiomnium rhynchophorum (Hook.) T.J.Kop. | 7 | Mat |
| Porotrichum longirostre (Hook.) Mitt. | 1 | Dendroide |
| Pyrrhobryum spiniforme (Hedw.) Mitt. | 1 | Turf |
| Racopilum tomentosum (Hedw.) Brid. | 8 | Mat |
| Rhynchostegium serrulatum (Hedw.) A.Jaeger | 3 | Mat |
| Syrrhopodon prolifer Schwägr. | 1 | Turf |
| Thamniopsis langsdorffii (Hook.) W.R. Buck | 3 | Mat |
| Thuidium tomentosum Schimp. | 4 | Mat |
| Tortella humilis (Hedw.) Jenn. | 1 | Turf |
| Vitalia cuspidifera (Mitt.) P.E.A.S. Câmara, Carv.-Silva & W.R.Buck | 1 | Mat |
| Vitalia galipensis (Müll. Hal.) P.E.A.S. Câmara, Carv.-Silva & W.R.Buck | 1 | Mat |
| Zelometeorium patulum (Hedw.) Manuel | 1 | Pendant |
| Liverworts | ||
| Bryopteris filiciana (Sw.) Nees | 1 | Pendant |
| Cheilolejeunea clausa (Nees & Mont.) R.M.Schust. | 2 | Weft |
| Cheilolejeunea rigidula (Nees ex Mont.) R.M. Schust. | 1 | Weft |
| Cryptolophocolea martiana (Nees) L. Söderstr., Crand.-Stotler & Stotler | 5 | Weft |
| Drepanolejeunea orthophylla (Nees & Mont.) Bischl. | 1 | Weft |
| Lejeunea bermudiana (A.Evans) R.M.Schust. | 3 | Weft |
| Lejeunea caulicalyx (Steph.) E.Reiner & Goda | 1 | Weft |
| Lejeunea cristulata (Steph.) E.Reiner & Goda | 3 | Weft |
| Lejeunea flava (Sw.) Nees | 3 | Weft |
| Lejeunea laetevirens Nees & Mont. | 2 | Weft |
| Lejeunea oligoclada Spruce | 1 | Weft |
| Lejeunea pterigona (Lehm. & Lindenb.) Mont. | 2 | Weft |
| Lejeunea sp.1 | 1 | Weft |
| Lejeunea sp.2 | 1 | Weft |
| Lophocolea bidentata (L.) Dumort | 5 | Weft |
| Lophocolea muricata (Lehm.) Nees | 1 | Weft |
| Metzgeria albinea Spruce | 1 | Thalloid |
| Metzgeria conjugata Lindb. | 4 | Thalloid |
| Metzgeria fruticola Spruce | 1 | Thalloid |
| Neurolejeunea breutelii (Gottsche) A.Evans | 2 | Weft |
| Plagiochila corrugata (Nees) Nees & Mont. | 1 | Pendant |
| Plagiochila patentissima Lindenb. | 1 | Weft |
| Plagiochila patula (Sw.) Lindenb. | 1 | Weft |
| Plagiochila rutilans Lindenb. | 10 | Weft |
| Plagiochila subplana Lindenb. | 1 | Weft |
| Porella reflexa (Lehm. & Lindenb.) Trevis. | 1 | Mat |
| Radula javanica Gottsche | 1 | Fan |
| Radula nudicaulis Steph. | 5 | Weft |
| Telaranea diacantha (Mont.) Engel & Merr. | 1 | Weft |
Among the eight life-forms recorded, weft had the highest species richness, with 28 (47 %), followed by mat, with 14 (23 %), turf and fan with five each (8 %), whereas only one species was found as cushion and another as dendroid (Table 1). The more frequent species in the samples were, Plagiochila rutilans, found in ten samples, Racopilum tomentosum, in eight, and Plagiomnium rhynchophorum and Fissidens hornschuchii, found in seven samples each (Table 1).
Species richness and community composition: The accumulated richness in relation to the sample units presented similar trends between different cover levels of Asplenium auritum, with significant differences (P < 0.05). The species accumulation curve tended to stabilize the asymptote approximately from the 50th sample units under different cover levels of A. auritum (Fig. 2). The NMDS did not show differences in floristic composition among the bryophyte communities with different cover levels of A. auritum (Stress-Euclidean = 0.16; F 3.33 = 0.97; P > 0.51) (Fig. 3). When analyzing the floristic grouping of plots by fern cover levels, high levels of similarity (Fig. 4).
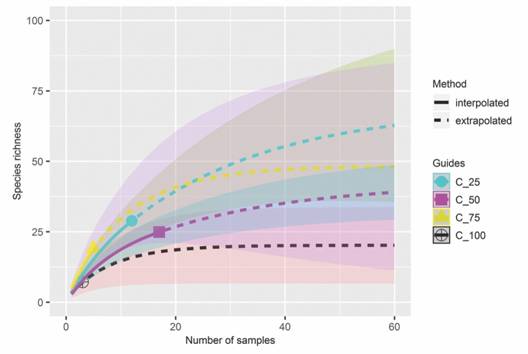
Fig. 2 Rarefaction curve of bryophytes at the Parque Estadual da Serra do Brigadeiro, Minas Gerais, Brazil, based on number of sample units with different A. auritum levels cover.
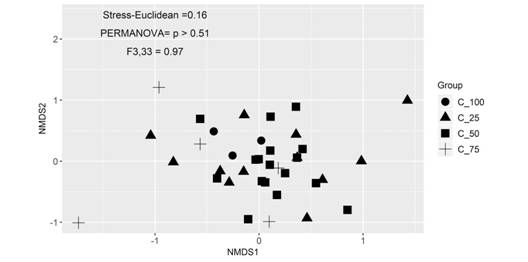
Fig. 3 Non-parametric multidimensional scaling (NMDS) representing species composition among samples of bryophytes evaluated under different A. auritum levels cover at the Parque Estadual da Serra do Brigadeiro, Minas Gerais, Brazil.
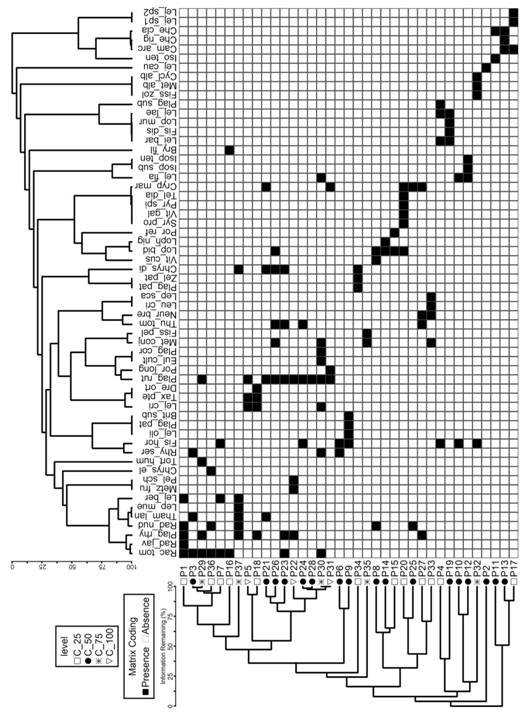
Fig. 4 Dendrogram of floristic similarity between bryophyte plots distributed in different fern cover levels based on presence-absence matrix (Euclidean distance).
Effects of cover on species richness: Fern cover (Ancova, F1,70 = 0.50; P > 0.92) did not have significant effects on species richness.
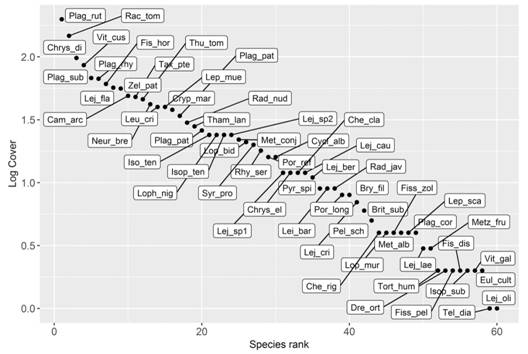
Species cover distribution: The distribution of the bryophyte species cover through the dominance-diversity curve showed Plagiochila rutilans as the dominant species in the study area (12 % cover), followed by Racopilum tomentosum (9 %) and Chrysohypnum diminutivum (6 %), which accumulated the highest cover values in the samples evaluated. Telaranea diacantha and Lejeunea oligoclada were the species with the lowest cover values in the samples, with only 0.06 % each (Fig. 5).
Discussion
The Atlantic Forest is the region with the greatest diversity of bryophytes in Brazil, with 71 % of the taxa recognized for the country (Costa, 2009); thus the high species richness (60) recorded in this study, in a total sampled area of less than 50 m2, comes as no surprise. This high diversity has been explained by different ecological processes in the Atlantic Domain, with a long geological history, a favorable climate, a higher source of native species (see, e.g., Frahm, 2003; Stehmann et al., 2009).
The genera and families found in this study have been commonly recorded in other Atlantic Forest surveys (Gradstein &Costa, 2003; Costa, 2009; Costa & Peralta, 2015). Lejeuneaceae (25 % of all species) was the most representative family in a number of species in the sampled areas, as expected since it is the most diverse family in Brazil (Costa& Peralta, 2015).
Bryophyte life-forms are related to the availability of light and moisture in the environment (Mägdefrau, 1982). The high representativeness of weft species (47 %) in the studied community can be explained in relation to their capacity to retain considerable amounts of water for long periods of time -capillarity-, which favors their proliferation in areas with high luminosity, high air humidity, and dry periods, such as those of the microhabitats sampled. Santos and Costa (2008) found a high number of weft and turf species occurring as corticolous and rupicolous in forest and stream border microhabitats, as is the case of the areas sampled in this study.
The accumulation curve showed that our sampling provided a reasonable representation of the richness for the area evaluated. This result was consistent with the stabilization trend of the asymptote (N = 50), close to the number of samples evaluated (N = 39). Our results also suggest that the accumulated richness in relation to sample units is not affected by the different cover levels of Asplenium auritum. In addition, the NMDS showed that floristic composition did not change significantly having high similarity between plots, which was probably due to biotic relationships or limitation by dispersion, and not by environmental filter influence.
These results suggest that the cover and richness of the bryophyte species could favor the establishment and growth of A. auritum. Facilitative interaction, like the maintenance of higher humidity by the bryophyte species, as a result of their life-forms (weft and mat), and the full contact with the substrate (Silva et al., 2018), would favor the development of adventitious buds along the fern stolons, and consequently promote the formation of shoots in different stages of development. According to Sylvestre (2001), A. auritum propagates vegetatively through stolons, forming continuous populations in carpets, with sporophytes of different sizes, in some cases in the first stages of development.
Other authors also found an association between bryophytes and ferns. Pócs (1982) mentioned bryophytes, both rupicolous and corticolous, growing frequently associated with Hymenophyllaceae ferns, whereas Dubuisson et al. (2003) and Ellyson and Sillett (2003) reported fern gametophyte and adult seedling development in the midst of bryophyte cover, and suggested such cover was a potential facilitator of spore germination and establishment of gametophytes for some pteridophytes.
The bryophyte species cover had a positive relation with species richness. Sanaei, Ali, Chahouki and Jafari (2018) explained how high vegetation density produces niche differentiation, promotes species coexistence and niche facilitation processes between rare and abundant species (Carrión, Gastauer, Mota, & Meira-Neto, 2017). We observed bryophyte communities dominated by Plagiochila rutilans, Racopilum tomentosum and Chrysohypnum diminutivum with high cover values (Fig. 5), and morpho-ecological characters related to an increase of the water absorption surface and resistance to desiccation (Glime, 2006). This, in turn, favors the colonization of more exposed habitats and survival during periods of drought (Parolly & Küschner, 2005). It would probably generate a microhabitat that favors the coexistence of a high number of species with lower cover values, like Telaranea diacantha and Lejeunea oligoclada, more sensitive to environmental changes and requiring a close association with the above-mentioned species, in order to develop.
Bryophytes are poikilohydric -quickly absorb water over the entire surface and lose it in the same way-, so density (cover) is relevant because this should favor shoot growth and reduce evapotranspiration (Proctor, 1982). This suggests that facilitative effects are predominant (Zamfir & Goldberg, 2000). Consequently, bryophyte cover could determine variations in species richness of the community and presumably could drive the species assembage. Thus, vegetation cover is a mechanism linking high species diversity with a high production of biomass in the ecosystems as a whole, as suggested by Sanaei et al. (2018).
In conclusion, our results suggest that, contrary to our initial assumption, in the PESB, A. auritum cover does not affect species richness and composition or cover of the bryophyte communities. Bryophyte cover could possibly improve fern development and promote the coexistence of dissimilar bryophyte species, although this should be confirmed by further research.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio