Introduction
The scarcity, small size and lack of identification keys have contributed to the little knowledge that we currently have of Neotropical land snails, and the few studies cover a limited part of the region, mostly Mexico, Brazil, Argentina, Costa Rica and Cuba (Correa-Sandoval, Strenth, Rodríguez Castro, & Horta Vega, 2009; Barrientos, 2010, 2016; Nunes & Santos, 2012; Suárez Torres & Fernández-Velázquez, 2012; Miranda, Fontenelle & Pecora, 2015). Five genera of euconulids have been reported from the Neotropics: Habroconus Fischer & Crosse, Velifera Binney, Euconulus Reinhardt, Tikoconus Barrientos and Ovachlamys (Barrientos, 1998, 2000, 2019; Schileyko, 2002; Thompson, 2011). Only two species have been studied regarding their ecology and life history: the Brazilian native Habroconus semenlini (Moricand, 1845) and the introduced Japanese species Ovachlamys fulgens (Gude, 1900). The population density of H. semenlini was negatively affected by drought and temperature in a semi-deciduous seasonal forest (Silva, 2007). Similarly, in a forest with 5 to 6 dry months, O. fulgens population density followed, with some lag, the precipitation curve (Barrientos, 2000). The abundance of living O. fulgens snails and eggs increased with leaf litter abundance and depth, leaf litter and soil moisture, and early morning temperature; but decreased with temperature around noon (Barrientos, 2000). Until the present study, the native neotropical euconulids had not been studied in low montane and premontane forests.
As explained by Barrientos (2012) there are three major trends on forest restoration techniques applied in Costa Rica and little is known about their impact on native fauna. In this paper, I analyse the demography of T. (T.) costaricanus Barrientos, an endemic terrestrial snail, in three habitats with different restoration techniques in tropical low montane and premontane regions. I also present basic information on its biology.
Materials and methods
Research area: The study took place in the Reserva Forestal Río Macho, Orosi Valley, Costa Rica. This reserve limits with Tapantí -Macizo Cerro de la Muerte-National Park. The Reserva Forestal Río Macho has mean temperatures between 14 and 26 °C and a mean annual precipitation of 2 416 mm. The dry season lasts from December through April with precipitations between 78 and 190 mm per month; while the rainy season lasts from May through November with precipitations between 269 and 376 mm (Instituto Metereológico Nacional, station El Llano dam, years 2009-2010, only complete monthly data were considered).
I selected three habitats (Fig. 1): a mature forest; a restored forest with natural ecological succession, known as unassisted restoration or secondary forest; and a Cupressus lusitanica plantation, a commercially important introduced species, its understory with unassisted restoration. The mature forest is located near El Llano dam (9º45'56" N & 83º51'47" W, 1 640 masl), in a tropical lower montane wet forest area. The restored forest (9º45'29" N & 83º51'23" W, 1 684 masl) has been under natural succession for about 15 years, and the understory is composed mainly of native species; the place borders a mature forest and is inside a tropical lower montane wet forest area. The Cupressus lusitanica plantation (09º47’52 N & 83º51’51 W, 1 309 masl) is within a tropical humid premontane forest. This plantation has been abandoned for nearly 40 years and an understory dominated by hardwood species has developed, but is still sparse. The three places have slopes of over 40°.
Sampling: I collected terrestrial molluscs every three months from April 2009 to April 2010. Due to logistic problems the C. lusitanica plantation was sampled in April 2010 instead of April 2009. I did a total of four sampling trips in each habitat; two during the dry season (January 2010, April 2009 and April 2010) and two during the rainy season (July and October 2009).
In each sampling occasion, I set a 200 m line at least 10 m away from any trail to avoid any border effect (Pérez, 1994). With a random number generator, I chose 20 sampling plots along each line. Most ecological land snail studies use one square meter plots (Correa-Sandoval et. al., 2009; Suárez Torres, Hernández, & Fernández, 2012; Cuasapaz-Sarabia & Salas, 2019). However, depending on field characteristics, other plot sizes give better results (Schilthuizen, 2011; Miranda et al., 2015). In this case, I used a larger plot (3 x 3 m2) to compensate for low specimen abundance and shrub differences among habitats. I set the plots with aluminum rods and plastic ropes. In each plot, I used several collecting methods (details below) for a more reliable representation of the snails at the site, because each method has its own bias (Durkan, Yeung, Meyer, Hyes, & Cowie, 2013).
In each plot, I collected arboreal snails with a 50 x 50 cm entomological beat sheet (http://mississippientomologicalmuseum.org.msstate.edu//collecting.preparation.methods/Beating.sheet.htm) for about 7 min (Barrientos, 2003a). I slightly beat branches, shrubs and seedlings between 0 and 200 cm above ground; tree trunks were slightly scrubbed with a gardening glove. I also combined this method with visual survey: if I saw a snail, I placed it in a collecting jar and continued with the beating-sheet method. In the laboratory, I examined the litter, first with a magnifying glass (10X) and after that, with a stereoscopic microscope. I counted only specimens collected alive, because empty shells can mislead abundance surveys (Barrientos, 2000). I measured shell diameters with a Zeiss microesteroscopic scale 0.15 mm at 16.25X.
In each plot, I randomly selected a 50 x 50 cm subplot for additional measurements: leaf litter depth, quantity and humidity, and snail number. I measured leaf litter depth with a standard millimetric ruler in five independent places of each subplot and used the average as depth (Barrientos, 2000). I assessed leaf litter quantity by the number of hardwood tree leaves that could be threaded with an ice pick, again using the average from five samples (Barrientos, 2012). In each subplot, I collected litter, including small branches less than 5 mm in diameter, fragmented litter, humus and topsoil. The size of the litter sample was 439 g in average (SD = 188, min = 89, max = 1 470). I kept litter samples in a plant oven for several days until constant dry weight was reached to apply the formula: Humidity percentage = (wet weight - dry weight)/wet weight * 100. I searched for specimens before and after drying the leaf litter sample first with magnifier glasses (10X) and after that with a stereoscopic microscope.
Natural history: I followed the methodology explained by Barrientos (1998). I collected T. (T.) costaricanus specimens in the mature forest and kept them in terraria with soil, leaves (mainly from Piperaceae and Melastomataceae), moss, small orchids and other plants that naturally grow on shrubs and tree stems. I kept other specimens in terraria just with fresh leaves collected in the field. I changed the leaves periodically. I kept all terraria at room temperature and checked them daily. I took away the eggs as soon as found and placed them in a new terrarium. I took note of number of eggs, size, development time, newborn shell diameter, growth rate, places where eggs were laid and parts of the terraria where snails where seen more often.
Analysis: I studied the snail quantity in relation with habitat, microhabitat, sampling date and leaf litter humidity, depth and quantity. I also analyzed specimen size in relation with habitat and sampling date. I detailed leaf litter humidity, quantity and depth dynamics in a previous paper (Barrientos, 2012).
The following symbols or abbreviations are used: Ø = mean, n = size sample, p = probability, SD = standard deviation, NS = non-significant, * = statistically significant, ** = strongly statistically significant, min = minimum, max = maximum.
Voucher specimens: I deposited voucher specimens in the Zoology Museum of the University of Costa Rica. Catalogue number MZUCR-242-01 (9 specimens). Costa Rica, Cartago, Orosi, Reserva Forestal Río Macho, at “El llano” water dam. 1 640 masl. 9º45'56" N & 83º51'47" W. collected on 5 March 2012 by Zaidett Barrientos, Maribel Zúñiga & Andrea Induni (T.: ZB-251).
Results
Demography in vegetation: I found T. (T.) costaricanus in mature and secondary forest and in the C. lusitanica plantation. In all sampling seasons, specimens were far more abundant in mature forest (April 2009 and April 2010; Kruskall-Wallis = 17.77, P = 0.0001, **), (July 2009; Kruskall-Wallis = 13.71, P = 0.0011, **), (October 2009; Kruskall-Wallis = 7.85, P = 0.0197, *), (January 2010; Kruskall-Wallis, df = 22.9092, P = 0.00001, **). Mean density was higher in mature forest, followed by secondary forest and C. lusitanica plantations (Table 1).
TABLE 1 Yearly average density for Tikoconus (Tikoconus) costarricanus occurring in three habitats in Orosi Valley, Costa Rica
| Mature forest | Restored forest | C. lusitanica plantation |
| Ø= 0.174ind/m2 | Ø= 0.047ind/m2 | Ø= 0.018ind/m2 |
| n= 78 | n= 75 | n= 80 |
| SD= 1.748 | SD= 0.661 | SD= 0.489 |
| min= 0ind/m2 | min= 0ind/m2 | min= 0ind/m2 |
| max= 0.89ind/m2 | max= 0.33ind/m2 | max= 0.33ind/m2 |
Ø= mean density, n= sample size, SD= Standard deviation, min= minimum, max= maximum).
In each habitat the number of specimens throughout the year was the same independently of the season (Kruskall-Wallis = 2.0118, P = 0.57, NS) but the size distribution shows a higher quantity of small specimens during October (Kruskall-Wallis = 17.3061, P = 0.00061, **) (Fig. 2). Specimen size was similar in all habitats (Kruskall-Wallis = 4.08, P = 0.13, NS) (Fig. 3).
There were more specimens where litter was moister (Spearman correlation, r = 0.3524, n = 232, P < 0.001, **), more abundant (Spearman correlation, r = 0.3922, n = 232, P < 0.001, **) and deeper (Spearman correlation, r = 0.2543, n = 232, P < 0.001, **).
Demography in leaf litter: I did not find T. (T.) costaricanus in the litter of the C. lusitanica plantation. I found specimens in the mature forest litter only during the dry season, especially in April (Mann-Whitney (Wilcoxon), W = 152, P = 0.004, **). In April, the mature forest`s litter had a snail density of 0.35 ind/m2 (min = 0 ind/m2, max = 8 ind/m2, SD = 0.5871, n = 20) and the correlation between litter humidity and specimen quantity was positively significant (Spearman correlation, r = 0.4951, n = 20, P = 0.0309,*). I found only two specimens in January in the mature forest and one specimen in April in the secondary forest, therefore no statistical analysis was conducted.
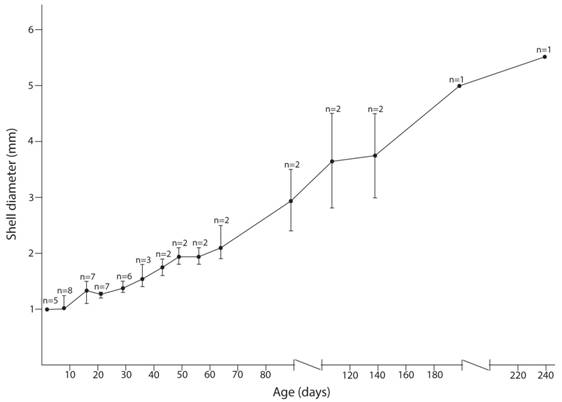
Life history: This species lays egg masses with an average of 3.78 eggs per mass (min= 1, max= 10, SD= 2.73, n= 9 egg masses) in moss and in soil. When moss or soil was not added to the terrarium no eggs were laid (n= 20). During oviposition snails withdraw tentacles (all four) with the head stretched between very wet soil clots (n= 3), one specimen laid eggs on a leaf. Eggs are round and well hydrous. Egg diameter was 1 mm (min= 1, max= 1, n= 5), egg shell is translucent and looks like made of bubbles (Fig. 4A). Egg eclosion took 20 days (n= 11) and shell diameter two days after eclosion was 1 mm (min= 1, max = 1, n= 5). Recently hatched specimens were transparent with a poorly defined pigmentation pattern, the species typical pigmentation appeared only after they were 64 days old (Fig. 4B, Fig 4D). Eight days after hatching specimens’ shells were about 1 mm wide (diameter) and moved among moss and leaves but spent most time on moss. They only spent most time on leaves after 21 days from hatching, when their shell diameter was 1.27 mm (Fig. 5). They appear to grow during their whole life (Fig. 5). Only one terrarium-born specimen survived to the adult stage; it began laying eggs 5 months after hatching and died when it was 9 months old.
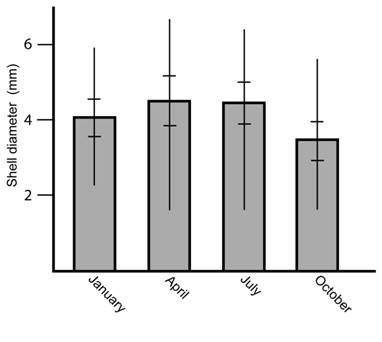
Fig. 2 Shell diameter of the snail T. (T.) costaricanus under natural conditions according to month. Vertical lines represent maximum and minimum values. Horizontal lines represent standard deviation.
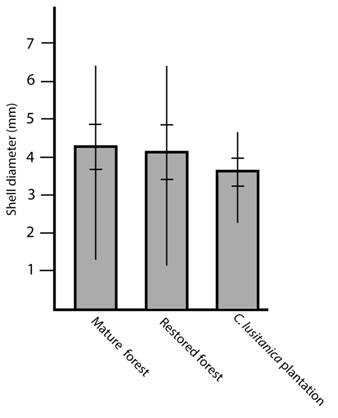
Fig. 3 Shell diameter of T. (T.) costaricanus in mature forest, secondary forest and a C. lusitanica plantation. Vertical lines represent maximum and minimum values. Horizontal lines represent standard deviation.

Fig. 4 Egg and pigmentation patterns of T. (T.) costaricanus according to age. A) Egg. B) Two days old specimen. C) 36 days old specimen. D) 64 days old specimen.
DISCUSSION
The land snail T. (T.) costaricanus occurs in the three kinds of habitat analysed in this study. Nevertheless, it was considerably more abundant in mature forest suggesting that density depends greatly on some habitat features. This is in agreement with findings from leaf litter land snail studies, in which the number of specimens is favoured by: denser canopy and understory, higher litter humidity, thicker litter composed of many layers, higher diversity of canopy and understory composition, older and bigger trees and presence of rotten logs (Boag, 1985; Barrientos, 2000; Müller, Strätz, & Hothorn, 2005). However, why abundance of species that spend most of their time on above ground vegetation is so related to soil litter characteristics? Probably because litter helps keeping environmental moisture. Humidity is of key importance in tropical ecosystems (Barrientos, 2012) and T. (T.) costaricanus is adapted to forests and microhabitats with high humidity levels. Although the three habitats of this study have similar precipitations patterns, the comparison between habitats show different humidity levels (Barrientos, 2012) and the snail density varies according to their moisture.
Tropical dry habitats tend to have smaller land snail populations during the dry season (Barrientos, 2000). On the contrary, in this study, in a wetter forest the population size has no change around the year within a given habitat. However, in October, when the area has the highest litter humidity and rainfall level (Barrientos, 2012), the mean size of shells was smaller. One possible explanation is that the snail quantity was underestimated due to the presence of juveniles that hide among moss growing on trunks and stems. It is also reasonable to think that the hatching season takes place in the middle of the rainy season. A sympatric native species, Drymaeus tripictus, also synchronized hatchling with rainy season (Barrientos, 2016).
There are few studies on native neotropical snail density. The density of T. (T.) costaricanus in mature forest is similar to that of wild Liguus fasciatus in Cuba (Fernández-Velázquez & Berovides-Alvarez, 2000). Nevertheless, their shell size differs considerably as L. fasciatus is around 10 times bigger than T. (T.) costaricanus. However, its density is low when compared with agriculturally important species like Succinea costaricana (28 ind/m2), Subulina octona (1063 ind/ m2) and O. fulgens (13ind/m2) (Villalobos, Monge-Nájera, Barrientos, & Franco, 1995; Monge-Nájera, 1997; Barrientos, 2000). Soil acidity, natural CO3 concentration and artificial field liming could be contributing with this difference because of their effect on shell development (Gärdenfors, 1992; Müller et al., 2005). With some notable exceptions like Cuba and Jamaica (Rosenberg & Muratov, 2006), neotropical wildland soils are acid and poor in limestone, therefore snail abundance is low, while species diversity could be high (Emberton, 1995; Emberton, Pearce, & Randalana, 1996; Winter & Gittenberger, 1998; Barrientos, 2003b, 2010).
As T. (T.) costaricanus can be found on leaves eating algae that grow on them the year around (Barrientos, 2019), it can be considered an understory dweller. Nevertheless, the understory litter also plays an important role, not only keeping environmental moisture as explained before, but also in its life history. At the end of the dry season specimens may migrate vertically between vegetation and leaf litter, at least during the sunny hours, while in the rest of the year their presence in the litter is only casual probably because litter is too wet and compacted (Barrientos, 2012). Seasonal and daily vertical migration is not unusual in other invertebrates, however it is poorly studied in land snails (Barrientos, 2000; Naranjo-García, 2003; Doblas, 2007; Cutz-Pool, Castaño-Meneses, Palacios-Vargas, & Cano-Santana, 2010).
Litter provides protection against drought and creates structurally complex microhabitats where molluscs and other invertebrates can hide from predators and unsuitable weather (Fournier & Herrera de Fournier, 1978; Barrientos, 2000, 2012; Sabo, Soykan, & Keller, 2005; Palacios-Vargas et al., 2007; Bonilla, Roncall, Jimeno, & García, 2008; Sabu, Vineesh, & Vinod, 2008; Sampaio, Rodríguez-González, Varandas, Cortes, & Ferreira, 2008). However, if the litter is too dry and structurally simple, as it is in a cypress plantation (Barrientos, 2012), snails may avoid being there.
As shown in the laboratory, T. (T.) costaricanus is probably laying eggs on soil and moss and spending the first 2 or 3 weeks of their life on moss that grows on stems. This behaviour is also reported in zonitids (Heller, 2001) and helps avoiding the problems caused by the compaction effect of heavy rain in litter during rainy season (Barrientos, 2012). Small moisture-dependent species that live in a tropical cloud forest, where soil and litter can get easily saturated, find ecological and evolutionary advantages in this behavior. Moss probably provides more stable moisture and temperature than decaying leaves, as excess water can drain more easily in the moss than in the leaf litter. In this way, recently hatched specimens (shell diameter less than 1.3 mm) avoid dehydration and problems caused by the cohesion force of water. Migrations between exposed and unexposed habitats have been also reported for Theba pisana (Johnson, 1981). However, more studies are need to establish if small horizontal migration is constant along their life or related only with seasons or even only with oviposition and hatching.
The places choose by T. (T.) costaricanus to lay eggs, its shell structure and its hydration level indicates that this is a species that evolve in a very humid environment with constant and high moisture. Other euconulids have more complex or derivate physiology to adapt them self to humidity. For example, O. fulgens can lay eggs which are not fully hydrated (Barrientos, 1998), this can be an adaptation to save corporal liquids while taking advantage of the presence of wet litter. Further research is needed to fully understand the evolutionary meaning of this difference.
Small specimens were underestimated with the method applied in this study. Future studies should collect all the moss that grows on stems and, due to the specimen’s small size and lack of colour, sort them in the laboratory. It is important to learn more about the microhabitat that stem mosses provide for invertebrates and their role in forest moisture in tropical habitats, as has been done for temperate ecosystems (Cutz-Pool et al., 2010; García-Gómez, Castaño-Meneses, & Palacios-Vargas, 2011). For example, it has been shown that moss moisture is important for some acari (Hoffmann & Riberón, 1992). However, I do not know of any paper dealing with the impact of moss moisture changes and the survival of recently hatched or small arboreal neotropical snails. I would expect an important relation, because treefall gaps can affect snail density, probably due to their effect on water loss rates (Alvarez & Willing, 1993).
Incubation time of T. (T.) costaricanus is a little long compared to other neotropical species: Polymita muscarum (10 days) (Bidart, Fernández, & Iglesias, 1998), Polymita venusta (8 days) (Bidart et al., 1998), O. fulgens (14 days) (Barrientos, 1998) and Succinea costaricana (11 days) (Villalobos et al., 1995). This can be a specific species characteristic or a result of different environmental temperatures (Montresor, Teixeira, Paglia, & Vidiga, 2012): T. (T.) costaricanus’ habitat is colder than the habitats of the other species for which there are reports.
The pigmentation of T. (T.) costaricanus varies considerably with age, and the reason may be camouflage. The small snails lack of pigmentation fits the moss where they start their life. Later, their pigmentation matches the epiphytes (lichen, moss and algae) where they live as adults. Another species, Succinea costarricana, also lacks pigmentation during the first seven weeks; the explanation for this is also camouflage and microhabitat changes during their life span (Villalobos et al., 1995). On the contrary, O. fulgens hatchlings and adults have the same pigmentation pattern throughout their life (Barrientos unpublished data). The explanation may also be camouflage: O. fulgens hatch on the soil and leaf litter, where they spend the rest of their life. Therefore, having the same colour pattern their entire life helps camouflage.
Although the sample size is small, data collected point out to a relatively short life cycle of around 9 months. Similar results were obtained for other euconulids like O. fulgens (Barrientos, 1998) and Habroconus semenlini, which lives only 4 months (Silva, Meireles, Vargas, Junqueira, & Bessa, 2009). Nevertheless, both O. fulgens and H. semenlini started laying eggs much sooner, around 40 days after hatching (Barrientos, 1998, Silva et al., 2009). A late reproduction and the production of few eggs that need a long time for development, is worrisome from the point of view of conservation. More research is needed to fully understand its ecology and the population risk with habitat and climate change.
Although T. (T.) costaricanus is more common on mature forest, colonization of restored habitats is possible. Restoration techniques can chiefly affect environmental and biological features (Morrison & Lindell, 2011; Barrientos, 2012). However, unlike the general pattern found by Morrison & Lindell (2011) for birds, restoration strategies do affect molluscan communities. Cypress plantations are drier, have litter with less layers and shallower leaf litter than mature forest (Barrientos, 2012). Therefore, these plantations are unsuitable for animals like molluscs that depend deeply on environmental moisture (Müller et al., 2005). These restoration techniques should be reconsidered and improved in order to achieve the environmental conditions and dynamics needed by these snails and by other native species. Improvements should include plants that produce more litter and shade.
The unassisted restoration (ecological succession) represents a better restoration technique for the conservation of native snail fauna. The litter dynamic is similar to the dynamic of a mature forest and even though its environmental characteristics are more variable, it can also reach its humidity levels (Barrientos, 2012), therefore being more friendly to moisture dependent organisms. This restoration technique could be improved with some fast growing native trees that provide a denser canopy. Future comparison with other native species and, biology and behaviour analysis with bigger samples should show if T. (T.) costaricanus is significantly susceptible to climate and land use change












 uBio
uBio