Introduction
The State of Oaxaca is considered among the top three of the states with highest biodiversity in Mexico (López-Pérez et al., 2012; Llorrente-Bousquets & Ocegueda, 2008); nevertheless, richness, distribution and conservation status of many species, including polychaetes, remain poorly known (López-Pérez et al., 2012). The knowledge of the Polychaeta from Oaxaca has been compiled by Bastida-Zavala et al. (2013); they made an historical review of all the records of polychaetes from the coast of Oaxaca, from 1919 to 2011, listing 222 species, however, many of these records (at least 62.92 %) are questionable.
Nowadays, there are new records of polychaetes, and the polychaeta-fauna from Oaxaca is represented by 241 species (i.e. Tovar-Hernández & Carrera-Parra, 2011; Salazar-Vallejo, 2012; Bastida-Zavala et al., 2013; Granja-Fernández, Hernández-Moreno & Bastida-Zavala, 2013; Salazar-Silva, 2013; Salazar-Silva & Carrera-Parra, 2014; Bastida-Zavala, Rodríguez Buelna, de León-González, Camacho-Cruz & Carmona, 2016; Cruz-Gómez & Bastida-Zavala, 2018). These records constitute only 1.6 % of the polychaetes known worldwide (~15 000 species) and 16.2 % of the polychaetes recorded for Mexican waters (1 500 species, fideTovar-Hernández, Salazar-Silva, de León-González, Carrera-Parra & Salazar-Vallejo, 2014).
Even though, many of these works are monographic studies including many sites along the coast of Oaxaca, only seven include poorly studied locations, with varying ecosystems, such as: Chacahua Lagoon (coastal lagoon), Agua Blanca (intertidal rocky shore) and San Agustín (coral reef) (Hartman, 1944; Hartman, 1950; Hartman, 1961; Ruiz-Cancino, Carrera-Parra & Bastida-Zavala, 2011; Salazar-Vallejo, 2012; Bastida-Zavala et al., 2016; Cruz-Gómez & Bastida-Zavala, 2018). Furthermore, some of the studies of these locations have focused on three families only, Eunicidae (Ruiz-Cancino et al., 2011), Serpulidae (Bastida-Zavala et al., 2016) and Chrysopetalidae (Cruz-Gómez & Bastida-Zavala, 2018), giving a total of 20 species recorded. Thus, a study of the polychaetes in these locations is necessary to improve our knowledge of the group.
The aim of this study was to identify the polychaetes from three different marine ecosystems from Oaxaca, Mexico. A checklist of polychaetes species recorded from Chacahua Lagoon, Agua Blanca and San Agustín, is included.
Materials and methods
Specimens analyzed came from the Sección de poliquetos de la Colección Científica (OAX-CC-249-11) at the Laboratorio de Sistemática de Invertebrados Marinos (LABSIM), Universidad del Mar (Puerto Ángel, Oaxaca, Mexico). This material was collected between 2007-2017 from three locations in Oaxaca, Mexican Pacific. Chacahua Lagoon (15°57’57” N - 97°40’38” W), a coastal lagoon belonging to Lagunas de Chacahua National Park; Agua Blanca (15°43’58” N - 96°48’50” W), an intertidal rocky shore; and San Agustín (15°41’09” N - 96°14’05” W), a bay with a wide area of coral reef.
Specimens were fixed in 10 % formalin solution and preserved in 70 % ethanol solution. In contrast, the material collected in 2017 (obtained through snorkeling in the intertidal zone to 3 m depth), was fixed and preserved in 96 % ethanol solution. The new specimens collected and all of the material examined was separated in glass vessels and labeled, and deposited in the Sección de poliquetos of LABSIM.
The identification of the polychaetes was made using the keys of de León-González et al. (2009); it was also corroborated with specialized literature for each family. The checklist is shown in alphabetical order.
Family and species richness by location was obtained using the accumulation function of linear dependence model (Moreno, 2001). The expected richness was estimated as the number of families or species, considering the sampling effort as number of sampling event for locality. The analyses were performed with EstimateS 9 (Colwell, 2013) and Statistica 8.0 (StatSoft, 2007) software.
To avoid an overestimation of the values of richness, damaged polychaetes, juveniles or specimens with a non-defined taxonomic status were removed prior to the analysis.
Results
A total 100 lots and 271 specimens were revised. Seventy one taxa belonging to 47 genera and 21 families were recorded (Table 1). Among these records, 20 are confirmed species (28 %), 19 are close to nominal species due to present morphological peculiarities or type locality distant from Oaxaca (27 %), 14 have unclear systematic status since are incomplete, damaged or juvenile specimens (20 %), and 18 are possible new species (25 %) which will be formally described by the authors in upcoming papers. One family (Chaetopteridae), 18 genera and 37 species are new records for the coast of Oaxaca. Twenty-six species are new records for the Mexican Pacific.
TABLE 1 Checklist of the polychaetes species and ecological features from three locations of Oaxaca coast, Mexico
| Species | Agua Blanca (rocky shore) | Chacahua Lagoon (coast lagoon) | San Agustín (coral reef bay) | Depth (m) | Substrata | Record |
| Amphinomidae Savigny in Lamarck, 1818 | ||||||
| Eurythoe cf. complanata (Pallas, 1766) | ● (1) | ND | coralline | This study | ||
| Notopygos ornata Grube, 1856 | ● (1) | ND | rocks | This study | ||
| Capitellidae Grube, 1862 | ||||||
| Capitellidae sp. | ● (1) | ND | rocks | This study | ||
| Chaetopteridae Audouin & Milne-Edwars, 1833 | ||||||
| Spiochaetopterus sp. | ● (1) | 0.5 | sand | This study | ||
| Chrysopetalidae Ehlers, 1864 | ||||||
| Bhawania cf. goodei Webster, 1884 | ● | 0.5 | SABI | Cruz-Gómez & Bastida-Zavala (2018) | ||
| Chrysopetalum elegantoides Aguado, Capa & San Martín, 2003 | ● | ● | intertidal/ 3.3-6.4 | rocks/ Pocillopora damicornis | Cruz-Gómez & Bastida-Zavala (2018) | |
| C. occidentale Johnson, 1897 | ●(5) | ● (1) | 0.5/ND | SABI and rocks/ coralline | Cruz-Gómez & Bastida-Zavala (2018); this study | |
| Chrysopetalum sp. | ● (1) | ND | coralline | This study | ||
| Paleaequor psamathe Watson Russell, 1986 | ● (1) | ND | rocks | Cruz-Gómez & Bastida-Zavala (2018); this study | ||
| Paleanotus bellis (Johnson, 1897) | ● | ND | ND | Cruz-Gómez & Bastida-Zavala (2018) | ||
| Paleanotus sp. | ● (1) | ● (2) | ND/24 | rocks/coralline and Mayrakenna sp. | This study | |
| Cirratulidae Ryckholt, 1851 | ||||||
| Cirratulus cf. cirratus (O.F. Müller, 1776) | ● (1) | ND | sand | This study | ||
| Cirratulus cf. megalus Chamberlin, 1919 | ● (1) | ND | dead coral | This study | ||
| Dorvilleidae Chamberlin, 1919 | ||||||
| Dorvillea vittata (Grube, 1856) | ● (1) | ND | P. damicornis | This study | ||
| Dorvillea cf. cerasina (Ehlers, 1901) | ● (1) | ● (3) | ND/6.4 | algae/P. damicornis and dead coral | This study | |
| Eunicidae Berthold, 1827 | ||||||
| Eunice chicasi de León- González, Rivera & Romero, 2004 | ● | ● | ND | ND | Ruiz-Cancino et al. (2011) | |
| E. vittatopsis Fauchald, 1970 | Q | ND | ND | Ruiz-Cancino et al. (2011) | ||
| Eunice sp. 1 | ● (1) | ND | dead coral | This study | ||
| Eunice sp. 2 | ● (2) | ND | dead coral | This study | ||
| Lysidice cf. unicornis (Grube, 1840) | ● (2) | ND | dead coral | This study | ||
| Marphysa sp. Ruiz-Cancino et al., 2011 | ● (2) | ND | coralline | This study | ||
| Flabelligeridae de Saint-Joseph, 1894 | ||||||
| Piromis gracilisHartman, 1961 | ● | 40-45 | mud | Hartman (1961) | ||
| Semiodera inflata (Treadwell, 1914) | ● (5) | ND | Porites sp. | This study | ||
| Trophoniella bastidaiSalazar-Vallejo, 2012 | ● | 40-45 | mud | Salazar-Vallejo (2012) | ||
| Hesionidae Grube, 1850 | ||||||
| Leocrates sp. | ● (1) | ND | rocks | This study | ||
| Oxydromus minutus (Hartmann-Schröder, 1959) | ● (3) | ND | rocks | This study | ||
| Lumbrineridae Schmarda, 1861 | ||||||
| Lumbrineris cf. inflata Moore, 1911 | ● (1) | ND | Padina sp. | This study | ||
| Nephtyidae Grube, 1850 | ||||||
| Nephtys caecoides Hartman, 1938 | Q | 10-15 | mud | Hartman (1950) | ||
| Nereididae Blainville, 1818 | ||||||
| Ceratonereis sp. | ● (1) | intertidal | Padina sp. | This study | ||
| Nereis cf. eugeniae (Kinberg, 1866) | ● (2) | ND | ND | This study | ||
| N. cf. lamellosa Ehlers, 1868 | ● (1) | ND | rocks | This study | ||
| N. (Pelagica) cf. occidentalis Hartman, 1945 | ● (1) | intertidal | Padina sp. | This study | ||
| Nereis sp. | ● (1) | ND | rocks | This study | ||
| Perinereis elenacasoi Rioja, 1947 | ● (5) | intertidal | Padina sp. | This study | ||
| Pseudonereis cf. gallapagensis Kinberg, 1865 | ● (1) | ND | algae | This study | ||
| Oenonidae Kinberg, 1865 | ||||||
| Arabella cf. iricolor (Montagu, 1804) | ● (5) | ND | ND | This study | ||
| Onuphidae Kinberg, 1865 | ||||||
| Onuphis nebulosa Moore, 1911 | Q | 40-45 | mud | Hartman (1994) | ||
| O. vexillaria Moore, 1911 | Q | 40-45 | mud | Hartman (1994) | ||
| O. eremita Audouin & Milne Edwards, 1833 | Q | ND | coral | Hartman (1944) | ||
| Orbiniidae Hartman, 1942 | ||||||
| Naineris cf. setosa (Verril, 1900) | ● (15) | ND | mangrove | This study | ||
| Protoaricia sp. | ● (5) | ND | mangrove | This study | ||
| Phyllodocidae Örsted, 1843 | ||||||
| Eteone cf. californica Hartman, 1936 | ● (1) | ND | rocks | This study | ||
| Eteone sp. | ● (1) | ND | on wood, sifted | This study | ||
| Eulalia gracilior (Chamberlin, 1919) | ● (2) | ND | ND | This study | ||
| E. cf. magalaensis Kinberg, 1866 | ● (1) | ND | ND | This study | ||
| E. cf. mexicana Fauchald, 1972 | ● (2) | ND | mangrove | This study | ||
| Eulalia sp. | ● (4) | ● (8) | intertidal | SABI/ on wood, sifted and inside of bivalve | This study | |
| Eumida cf. punctifera (Grube, 1860) | ● (2) | ND | ND | This study | ||
| Phyllodoce cf. nicoyensis Treadwell, 1928 | ● (1) | ND | ND | This study | ||
| P. cf. schmardaei Day, 1963 | ● (1) | ND | ND | This study | ||
| Phyllodoce sp. | ● (1) | ND | coralline | This study | ||
| Pterocirrus sp. | ● (1) | ND | on wood, sifted | This study | ||
| Phyllodocidae sp. epitocus stage | ● (2) | ND | coralline | This study | ||
| Polynoidae Kinberg, 1856 | ||||||
| Halosydna cf. olgaeSalazar-Silva, 2013 | ● (2) | ND | rock and algae | This study | ||
| Halosydna sp. | ● (1) | ND | mangrove | This study | ||
| Lepidonopsis barnichaeSalazar-Silva & Carrera-Parra, 2014 | ● (7) | ND | mangrove/ rocks | This study | ||
| Lepidonotopodium cf. riftense Pettibone, 1984 | ● (1) | 5.8 | P. damicornis | This study | ||
| Polynoinae sp. | ● (2) | 15.3 | Porites sp. | This study | ||
| Sabellariidae Johnston, 1865 | ||||||
| Idanthyrsus cretus Chamberlin, 1919 | ● (1) | 0.4 | rocks | This study | ||
| Phragmatopoma sp. 1 | ●(43) | 0.2 | sand/rocks | This study | ||
| Phragmatopoma sp. 2 | ●(13) | ND | sand | This study | ||
| Sabellaria nanella Chamberlin, 1919 | ● (1) | ND | Phragmatopoma sp. | This study | ||
| Sabellidae Latreille, 1825 | ||||||
| Acromegalomma sp. | ● (1) | ND | sand | This study | ||
| Serpulidae Rafinesque, 1815 | ||||||
| Hydroides brachyacantha Rioja, 1941 | ● | ● | ● (2) | intertidal/ ND/15.3 | rocks/ND/ Porites sp. | Bastida-Zavala et al. (2016); this study |
| H. crucigera Mörch, 1863 | ● | intertidal | rocks | Bastida-Zavala et al. (2016) | ||
| H. inermis Monro, 1933 | ● | intertidal | rocks | Bastida-Zavala et al. (2016) | ||
| H. ochotereana Rioja, 1941 | ● | intertidal | rocks | Bastida-Zavala et al. (2016) | ||
| H. panamensis Bastida-Zavala & ten Hove, 2003 | ● | intertidal | rocks | Bastida-Zavala et al. (2016) | ||
| Pomatostegus kroyeri Mörch, 1863 | ● | 5.8 | P. damicornis | Bastida-Zavala et al. (2016) | ||
| Spirobranchus cf. corniculatus (Grube, 1862) | ● (2) | 1.5 | rocks | This study | ||
| S. incrassatus Krøyer in Mörch, 1863 | ●(20) | 1.5 | rocks | This study | ||
| S. minutus (Rioja, 1941) | ● | high tide | SABI | Bastida-Zavala et al. (2016) | ||
| Spionidae Grube, 1850 | ||||||
| Dipolydora cf. socialis (Schmarda, 1861) | ● (7) | 0.1 | mangrove | This study | ||
| Dipolydora sp. | ●(41) | ND | mangrove | This study | ||
| Malacoceros sp. | ● (1) | 0.1-0.2 | mangrove | This study | ||
| Marenzelleria cf. bastropi Bick, 2005 | ● (1) | ND | ND | This study | ||
| Polydora heterochaeta Rioja, 1939 | ● (1) | intertidal | rocks | This study | ||
| Pygospio sp. | ● (3) | intertidal | rocks | This study | ||
| Syllidae Grube, 1850 | ||||||
| Inermosyllis mexicana (Góngora-Garza & de León-González, 1993) | ● (2) | ND | mangrove/ rocks | This study | ||
| Opisthosyllis arboricola Hartmann-Schröder, 1959 | ● (2) | 0-2 | Padina sp./sand | This study | ||
| O. cf. corallicola Hartmann-Schröder, 1965 | ● (1) | intertidal | rocks | This study | ||
| O. cf. longidentata San Martín, 1991 | ● (1) | intertidal | rocks | This study | ||
| Streptosyllis sp. | ● (1) | 0-2 | sand | This study | ||
| Trypanedenta gemmipara (Johnson, 1901) | ● (1) | 15.3 | Porites sp. | This study | ||
| Trypanosyllis cf. vittigera Ehlers, 1887 | ● (2) | ND | sifted | This study | ||
| T. cf. zebra (Grube, 1960) | ● (1) | ND | rocks | This study | ||
| Terebellidae Johnston, 1846 | ||||||
| Lanicola cf. guillermoi Capa & Hutchings, 2006 | ● (1) | ● (1) | ND | rocks/ dead coral | This study | |
| Terebellinae sp. | ● (4) | ● (3) | ND | mangrove /SABI | This study | |
| Species / specimens numbers | 27/92 | 50/149 | 21/32 |
Depth in m = meters, SABI = Sabellariidae aggregation, Q = questionable record, ND = no data. Symbols: = possible new species, ● = record. The number of specimens is indicated between parentheses.
In general, Sabellaridae presented the highest abundance with 58 specimens (Fig. 1A, Fig. 2E-F), perhaps due to the fact that its members are gregarious polychaetes. Phyllodocidae was the richest family with 12 species, followed by Syllidae with eight, Nereididae with seven and Spionidae with six species.
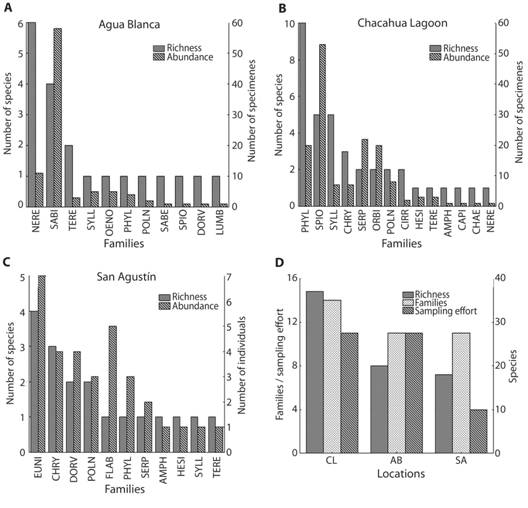
Fig. 1 Abundance and richness of polychaetes from (A) Agua Blanca; (B) Chacahua Lagoon; (C) San Agustín. (D) Comparison between locations. The short names of the Polychaeta families are following Salazar-Vallejo, de León-González & Saláices-Polanco (1988).
As results for each site, Chacahua Lagoon had the highest richness and abundance with 37 taxa and 149 specimens. In Agua Blanca 21 taxa and 92 specimens were identified, and from San Agustín, 19 taxa and 32 specimens. Nereididae was the family with highest richness (six species) in Agua Blanca, whereas Sabellariidae presented the highest relative abundance (58 specimens) (Fig. 1A). In Chacahua Lagoon, Phyllodocidae was the richest family (10 species) and Spionidae had the highest abundance (53 specimens) (Fig. 1B). In San Agustín, Eunicidae had the highest richness and abundance (four species, seven specimens) (Fig. 1C). Lumbrineridae, Chaetopteridae, Capitellidae and Sabellidae had the lowest abundance in these locations, with only one specimen each one.
To avoid an overestimation of the values of the richness, the only specimens removed were Terebellinae sp. in Agua Blanca and Phyllodocidae sp. epitocus stage in San Agustín, since these could be duplicate data (Lanicola cf. carus and Phyllodoce sp., respectively). With the accumulation function, the expected richness was 14 families (R = 0.999, R2 = 99.998 %, asymptote = 13.92, b = 0.141) and 44 species for Agua Blanca (R = 1.000, R2 = 100 %, asymptote = 43.71, b = 0.055), 18 families (R = 0.999, R2 = 99.964 %, asymptote = 17.93, b = 0.239) and 70 species for San Agustín (R = 1.000, R2 = 100 %, asymptote = 70.32, b = 0.073), and 23 families (R = 0.999, R2 = 99.926 %, asymptote = 22.71, b = 0.0.085) and 165 species for Chacahua Lagoon (R = 1.000, R2 = 100 %, asymptote = 164.50, b = 0.023).
Discussion
In the Mexican Pacific, some studies has incremented the polychaetes knowledge from particular ecosystems such as rocky and sandy zone in La Paz, Baja California Sur (Bastida-Zavala, 1993), coralline zone in Cabo Pulmo, Baja California Sur (Bastida-Zavala, 1995), buoys zone in Mazatlán, Sinaloa ( Villalobos-Guerrero & Tovar-Hernández, 2014), and coralline and rocky zone in Huatulco-Puerto Angel, Oaxaca (Gómez, Mercado, Mitchell, & Salazar-Vallejo, 1997).
In Oaxaca, before this study, there were only 20 recorded species of polychaetes from Chacahua Lagoon, Agua Blanca, and San Agustín (Hartman, 1944; Hartman, 1950; Hartman, 1961; Ruiz-Cancino et al., 2011; Salazar-Vallejo, 2012; Cruz-Gómez & Bastida-Zavala, 2018). With this work, this has increased to 71 species, and 75 new records were added: 21 in Agua Blanca, 19 in San Agustín and 35 in Chacahua Lagoon.
Particularly, the study by Gómez et al. (1997) found the highest values of richness (36 species) in coralline zone of La Entrega, Oaxaca. In contrast, among the three locations of study, the highest values of abundance (149 specimens) and richness (37 species) were found in Chacahua Lagoon; this might be due to the diversity of microhabitats studied in this locality: rocky zone, mangrove, sand and muddy zone. On the other hand, the coralline zone of San Agustín showed the lowest abundance (32 specimens) and richness (19 species); however, this location also presented the lowest sampling effort (Fig. 1D).
The Polychaeta-fauna in Chacahua Lagoon, Agua Blanca and San Agustín only match in 9 % of their composition, which are six families and eight taxa (Chrysopetalum elegantoides, C. occidentale, Paleaonotus sp., Dorvillea cf. cerasina, Eunice chicasi, Eulalia sp., Hydroides brachyacantha, and Lanicola cf. guillermoi). This indicates that these three locations present particular environmental conditions with different types of marine biota. On the other hand, the results of this work coincide with Gómez et al. (1997) in the presence of C. occidentale, Idanthyrsus cretus, H. brachyacantha, H. crucigera, and Spirobranchus incrassatus in the Oaxaca coast. The results also coincides with Bastida-Zavala (1993) in the record of Dorvillea vittata and with Bastida-Zavala (1995) in the record of Perinereis elenecasoi, but also these studies recorded many questionable species, to avoid questionable records here were referred this kind of species as confer (e.g. Bhawania cf. godei). The study by Villalobos-Guerrero and Tovar-Hernández (2014) coincides with our study in the presence of Dorvillea vittata, Eunice sp. 2, Marphysa sp., Perinereis elenacasoi and Eulalia gracicolor.
Considering the results obtained, to date the Polychaeta-fauna from Oaxaca is represented by 304 species, belonging to 154 genera and 42 families. Comparatively, the Polychaeta from the coast of Oaxaca are better known than other states, such as Chiapas (five species) and Michoacán (57 species) (Bastida-Zavala & García-Madrigal, 1998; Bastida-Zavala & García-Madrigal, 2012; Bastida-Zavala & Guevara-Cruz, 2012); however, the species richness of polychaetes from Oaxaca still less known than other states as Sinaloa with 464 species recorded (Villalobos-Guerrero & Molina-Acevedo, 2014). The number of species and records of the polychaetes in the Mexican south Pacific could increase if intensify and explore new locations and other habitats (e.g. sandy beaches, deep reefs, submarine canyons, mixohaline lagoons) (Fig. 2).

Fig. 2 Examples of the species identified (A) Eulalia gracicolor (B) Eulalia sp. (C) Lanicola cf. guillermoi (D) Semiodera inflata (E) Idanthyrsus cretus (F) Sabellaria nanella (G) Lepidonopsis barnichae (H) Halosydna cf. olgae (I) Lepidonotopodium cf. riftense (J) Streptosyllis sp. (K) Chrysopetalum occidentale (L) Dorvillea vittata.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio 

