Introduction
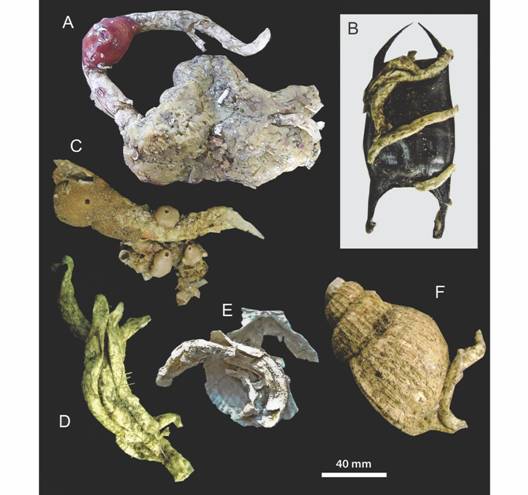
The shelf-break frontal area in the Argentine Sea is one of the most productive ecosystems in the southwest (SW) Atlantic Ocean (Acha, Mianzan, Guerrero, Favero, & Bava, 2004). It is characterized by the presence of extensive beds of the Patagonian scallop Zygochlamys patagonica (King, 1832), a pectinid species exploited by scallopers since 1996 and distributed between 37° S - 47° S along the 100m isobath (Lasta & Bremec, 1998; Schejter, Bremec, Escolar & Giberto, 2017). This commercial resource and the invertebrate by-catch are yearly monitored as part of the scallop management plan. Early research on composition and structure of the by-catch in the spatial and temporal scales (Bremec & Lasta, 2002; Bremec et al., 2008; Escolar, Diez, Hernandez, Campodónico, & Bremec, 2009; Escolar, Hernández, & Bremec, 2011; Mauna, Acha, Lasta, & Iribarne, 2011; Schejter et al., 2012; Schejter, Bremec, Escolar & Giberto, 2017), production and trophic interactions (Bremec, Brey, Lasta, Valero, & Lucifora, 2000; Schejter, Bremec, Akselman, Hernandez, & Spivak, 2002; Botto et al., 2006; Souto, 2009) showed the high frequency and abundance of the parchment worm Chaetopterus antarcticus Kinberg, 1866 (see Moore, Nishi, & Rouse, 2017) between 37º S and 40º S, associated to the commercial scallop. This polychaete was usually collected within its free U tubes, typical of infaunal habit (see Rouse, 2001; Nishi, Hickman, & Bailey-Brock, 2009) (Fig. 1).

Fig. 1 Free Chaetopterus antarcticus currently collected as associated species in the benthic samples during the period 1995-2006 in scallop beds between 37° S and 40° S (A). Typical U tube (B).
However, the yearly assessment of invertebrate by-catch of the scallop fishery conducted during November 2007 monitoring cruises (Bremec, Marecos, Schejter, Escolar, & Souto, 2008) showed notorious settlement of parchment worms on Z. patagonica. Previous works on Z. patagonica epibionts (Diehl, 1977; Walossek, 1991; Rosso & Sanfilippo, 1991; Sanfilippo, 1994; Bremec & Lasta, 2002; Bremec, Marecos, Schejter, & Lasta, 2003) mentioned organisms like calcareous algae, poriferans, hydrozoans, anthozoans, polychaetes, molluscs, cirripeds, foraminiferans, ascidians and bryozoans, without records of Chaetopterus as one of the worms. In general, during the period 1995-2006, 10 years since the start of the fishery (Bremec & Lasta, 2002; Bremec, Schejter, & Marecos, 2006), yearly sampling showed that the polychaetes that heavily encrusted scallops in all the fishing grounds were tube worms of the families Sabellariidae, Sabellidae and Serpulidae (Idantyrsus macropaleus (Schmarda, 1861), Potamilla antarctica (Kinberg, 1866) and Serpula narconensis Baird, 1865 respectively) (Bremec et al., 2006), in agreement with the research about polychaetes in the Argentinean shelf from extensive samplings during R/V Shinkai Maru cruises (Hartmann-Schroeder, 1983; Bremec, Souto, & Genzano, 2010). Posteriorly, after extensive benthic sampling carried out in 2001 to study richness of taxa encrusting Z. patagonica, 4 % of the specimens analyzed resulted basibionts of C. antarcticus (Schejter & Bremec, 2007). In consequence of the surprising settlement of epibiotic worms during 2007, the presence of C. antarcticus on scallops and the spatial distribution of infaunal worms in the study area were analyzed through time, during the period 2007 - 2015. The objectives of this paper are to report variability of life habits of C. antarcticus in scallop grounds distributed between 37º S and 40º S in Argentinean waters.
Materials and methods
The study area extends between 37° S and 40° S along the 100m isobath in Argentinean waters (Fig. 2). It includes two management units (MU) of the Patagonian scallop (Zygochlamys patagonica) fishery: MU A (7 500 km2, 37° S - 38° S) and MU B (16.186 km2, 38º S - 39º 52’ S) (Campodónico & Mauna, 2014).

Fig. 2 Location of the samples collected in scallop beds between 2007 and 2015 in the study area (○). Location of samples used to study epibiosis of polychaetes on Patagonian scallops (♦).
The presence of the parchment tube worms on Z. patagonica was analyzed in 892 scallops coming from three samples collected in September (previously obtained for other purposes) and November 2007 and April 2008, between 99-106 m depth in MU B, on board F/V Atlantic Surf I (Figure 2). Polychaetes were identified and quantified on both valves.
In order to explore the dynamics of this behavior in the study area, between 37º S and 40º S, both epibiont and infaunal worms were registered through time. The settlement of Chaetopterus antarcticus on scallops was monitored during the period 2007-2015 and the presence of free tubes, both empty and inhabited by worms, were registered during the period 2008-2015. The number of sites sampled during the study period, with and without living worms, is shown in Table 1. Information includes the general composition of epifaunal benthos in the area: relative wet weight of invertebrate by-catch (i.e. without scallops) and chaetopterid tubes and the relative abundance of inhabited tubes (Nº individuals/100 m2). It was obtained in the context of the Z. patagonica stock assessment and by-catch monitoring program developed by INIDEP, from a total of 374 representative subsamples (10 l) collected with trawling tools (Figure 2), on board R/V Cap. Cánepa (INIDEP) during 2007 and 2009, F/V Erin Bruce during 2008 and 2012 and F/V Atlantic Surf I during 2010, 2013, 2014 and 2015 (Schejter & Escolar, 2015; Schejter, Webb, Marecos, & Escolar, 2015; Schejter, Escolar, Marecos, & Bastida, 2016).
Table 1 Number of sampling stations and general composition of the benthic samples collected during the study period 2007-2015 between 37° S - 40° S, Argentine Sea. The relative percentages (wet weight) of associated invertebrates (not scallops) in the total sampling, Chaetopterus antarcticus total tubes (inhabited+empty) related with invertebrates and C. antarcticus empty tubes related with total tubes are shown. During 2007 sampling inhabited and empty tubes were not computed
| 2007 | 2008 | 2009 | 2010 | 2012 | 2013 | 2014 | 2015 | |
| % invertebrates | 62,17 | 56,99 | 65,52 | 53,23 | 56,20 | 55,25 | 59,21 | 52,25 |
| % total tubes | 24,90 | 30,90 | 17,95 | 9,32 | 9,11 | 23,37 | 25,86 | 9,74 |
| % empty tubes | - | 39,95 | 0,04 | 3,25 | 1,35 | 4,07 | 25,52 | 55,93 |
| Nº st. sampled | 88 | 92 | 34 | 34 | 24 | 35 | 30 | 37 |
| N° st. inhabited tubes | - | 31 | 29 | 22 | 11 | 13 | 12 | 12 |
| Nº st. empty tubes | - | 46 | 4 | 3 | 5 | 13 | 5 | 14 |
| N° st. epibiotic C. antarcticus | 25 | 57 | 10 | 9 | 4 | 0 | 0 | 0 |
Results
The detection of epibiotic Chaetopterus antarcticus occurred after analysis of benthic samples obtained during November 2007 in Patagonian scallop beds. This settlement behavior was registered throughout the study area during 2007 and 2008 (Fig. 3). During 2008 monitoring, the species encrusted 28 % of 4 351 commercial scallops (> 55 mm) observed throughout the study area. Sampling programs developed during the following years, showed the decrease of the epibiotic behavior of C. antarcticus in 2009, 2010 and 2012 and its total absence on scallops collected in 2013, 2014 and 2015 (Fig. 3).

Fig. 3 Location of sampling stations (○) and spatial distribution of samples (*) with epibiotic Chaetopterus antarcticus between 2007-2012 in the study area (37° S - 40° S), SW Atlantic.
The analysis of 892 scallops collected in 2007 and 2008 to study epibiotic worms (Table 2), showed that 468 (> 50 %) scallops were encrusted; in 66 % (311 specimens) of them C. antarcticus was settled. We found 352 worms on upper and 83 worms on lower valves. The position adopted by the worms on lower valves was on the margins, with the chimneys free of attachment. Up to seven worms coexisted on the same upper valve (Fig. 4). Other conspicuous colonizers were 81 sabellariid worms Idanthyrsus macropaleus, which built sandy tubes on the upper valve. The serpulid Serpula narconensis encrusted auricles and margins, 42 and 13 specimens were found on lower and upper valves respectively. A few individuals of the sabellid Potamilla antarctica, 15 in total, encrusted both valves of scallops (Fig. 4). C. antarcticus was always found on scallops higher than 55mm, commercial size abundant in the grounds.
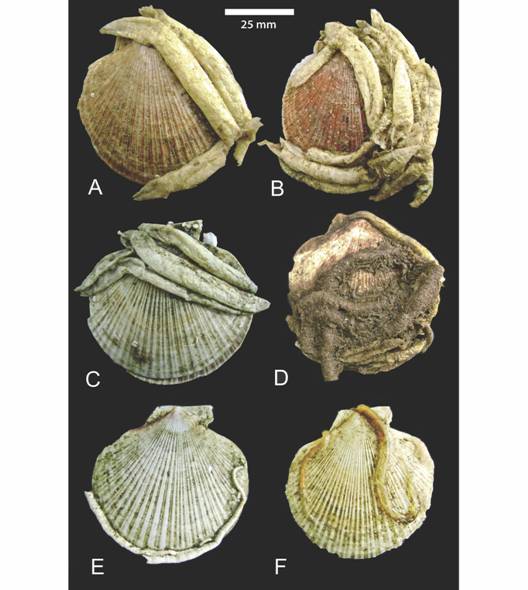
Fig. 4 Tubes of polychaetes settled on Patagonian scallops in the study area (37° S - 40° S), SW Atlantic. Chaetopterus antarcticus (A, B, C), Idanthyrsus macropaleus (D), Serpula narconensis (E) and Potamilla antarctica (F).
Table 2 Abundance (number of individuals) of polychaete species encrusting scallops on Upper and Lower valves of Zygochlamys patagonica. Period 2007-2008, 38° 37’ S - 39° 09’ S, SW Atlantic
| C. antarcticus | I. macropaleus | S. narconensis | P. antarctica | ||||||
| Sampling | U | L | U | L | U | L | U | L | |
| Sept. 2007 | 269 | 53 | 24 | 1 | 8 | 22 | 2 | 3 | |
| Nov. 2007 | 34 | 10 | 10 | - | 2 | 1 | 3 | - | |
| April 2008 | 49 | 20 | 47 | - | 3 | 19 | 2 | 5 | |
The spatial distribution of infaunal worms inside U tubes and empty tubes was assessed during the period 2008 - 2015 (Fig. 5). Infaunal worms were registered throughout the area (37° S - 40° S) during the period 2008-2009. The presence of empty tubes in 50% of the locations was conspicuous in 2008, as well as a high percentage (wet weight) of empty tubes related with the total tubes (inhabited + empty) (Table 1). Living infaunal worms were collected in part of the locations monitored during the following years, between 38° S - 40° S; empty tubes in more locations and with a relative high proportion during the period 2013 - 2015. Occasional settlement of worms was observed, not quantified, on chondrichthyes capsules during sampling 2008 and on scallop shells during sampling 2012, while empty Chaetopterus tubes were found to be available substrate for a variety of other invertebrates (Fig. 6) (observations of the authors).
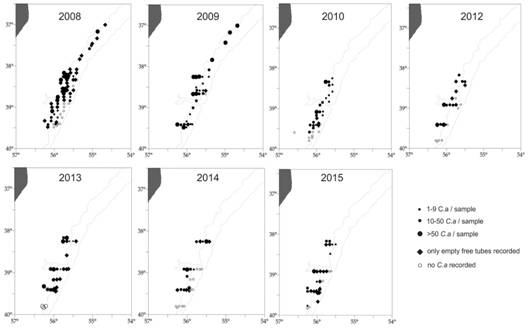
Fig. 5 Location of sampling stations (○) and spatial distribution of empty (♦) and inhabited (●) tubes of Chaetopterus antarcticus between 2008-2015 in the study area (37° S - 40° S), SW Atlantic.
Discussion
An extraordinary settlement of Chaetopterus antarcticus on the Patagonian scallop Zygochlamys patagonica is herein reported. This event was observed during 2007/2008 for the first time, after a decade of sampling in the study area. Previous yearly research on the faunal benthic assemblage where C. antarcticus is an associated species, conducted between 1995 and 2006, did not register any massive settlement of the worm on hard substrates (Schejter & Bremec, 2007; Romero, Schejter, & Bremec, 2017). Moreover, although Chaetopterus tubes were also recorded in a submarine canyon close to the scallop beds (Bremec & Schejter, 2010), also in this deeper and near environment the studied scallops lack this worm in its epibiotic habit (Schejter, López Gappa & Bremec, 2014). This annelid species was conspicuous, together with the sea star Labidiaster radiosus Lütken, 1871 and the ophiuroid Gorgonocephalus chilensis (Philippi, 1858), and characterized the Patagonian scallop assemblage between 37° S - 39° S, along the 100m isobath (Bremec et al., 2008; Schejter, Bremec, Escolar & Giberto, 2017). After extensive sampling in Argentinean shelf waters developed decades before, it was always registered inhabiting soft bottoms (Hartmann-Schroeder, 1983). Posterior studies about most common epibionts on Z. patagonica based on samples taken in scallop beds (36°20’ S - 39°00’ S), San Jorge Gulf (45°08’ S) and Beagle Channel (55°09’ S) showed that from a total of 256 specimens, 4 % of scallops coming only from Reclutas bed (39°00’ S, MU B) was encrusted by Chaetopterus (Schejter & Bremec, 2007). Conversely, the present results show predominance of C. antarcticus settled on scallops, together with the already reported tube worms I. macropaleus, S. narconensis and P. antarctica in shelf areas (see Hartmann-Schroeder, 1983; Sanfilippo, 1994; Schejter & Bremec, 2007; Romero, Schejter & Bremec, 2017) and deeper waters of a submarine canyon located at 43°13.5’ S - 59°13.3’ W, 325 m depth (Schejter, López Gappa, & Bremec, 2014). The majority of individuals were encrusting the upper (left) valves of Z. patagonica, sedentary species with limited swimming capacity, in accordance to its morphology (Stanley, 1970). It is presumably developed as escape response in presence of predators, as seen in other pectinid species (see Wilkens, 1991). The most frequent living position adopted by the scallop seems to be with the lower (right) valve in contact with the substrate, as shown by the differential morphology of the valves, being the right ones less concave. The preference of the upper valve, as seen in other epibiotic species, may be related with competitive interactions among epibionts, adaptations to the physical abrasion effects, and/or feeding strategies (Sanfilippo, 1994; Schejter & Bremec, 2007).
The referred massive epibiotic behavior of the parchment worm was registered in coincidence with huge proportion of empty chaetopterid tubes on the bottom, in half the locations sampled in 2008. During the following years, both infaunal worms in U tubes and empty tubes were collected in variable proportions, while it is clear that the epibioic behavior of C. antarcticus decreased dramatically during the study period. Other studies already showed the increment of empty polychaete tubes in areas subjected to trawling activities (Bremec, Giberto, Escolar, Schejter, & Souto, 2012; Bremec, Schejter, & Giberto, 2015). Polychaete tubes biomass showed an increase from 1998 to 2002 in fishing grounds located in the present study area, whereas in the reserve zone located within it they were not registered at all in the same period (Schejter, Bremec, & Hernández, 2008). These polychaete tubes corresponded to the species Chaetopterus antarcticus and Idanthyrsus macropaleus and the increase in their biomass was suggested a consequence of fishing disturbance; Chaetopterus tubes could be removed from the bottom with the nets and could be transported because of the fishery fleet activities or the bottom currents, as well as the detached sandy tubes of Idanthyrsus from the scallop valves. In the case of Chaetopterus tubes, the complex structure-property relations observed indicate that the worm produces such a structure to endure mechanical stresses from specific planes/axes (Shah, Vollrath, Porter, Stires, & Deheyn, 2014), favoring accumulation of empty resistant tubes on the bottom.
As the extractive activity between 1998 and 2009 was intensive in Patagonian scallop beds, with an increase in the fishing effort in part of the study area in 2007 and 2009 (Escolar, Campodónico, Marecos, & Schejter, 2015), the chronic trawling could produce removal both of worms inside the tubes and soft sediments, consequently unavailable for infaunal settlement of juvenile worms. In the case of the related species C. variopedatus, larvae are planktonic, drifting and feeding in the water column before settling and building a permanent pergamentaceous U-shaped tube buried into the soft bottoms (Fauchald, & Jumars, 1979; Rouse, 2001). C. antarcticus showed to be successful colonizer of hard substrata, as during 2007-2008, scallops supplied the most important settlement substrate in the study area, although it is characterized by sandy soft-bottoms, like more than 90% of the Argentinean continental shelf (Parker, Paterlini, & Violante, 1997). The sediment type was characterized by fine sands in this area from samples collected between 38° - 38° 30’ S (Lasta, 2013) and the absence of biogenic substrates was shown by means of multibeam sonar (Madirolas, Isla, Tripode, Alvarez Colombo, & Cabreira, 2005).
Regarding the presence of free worms during the study period, these results show great variations in the spatial distribution both of inhabited and empty tubes among years. In general, the whole study area was subjected to closures to scallop fishery that covered different areas during different periods of time throughout 2008-2014, ranging between ~8.2 % of the management unit during 4 months in 2009 and ~83.3 % during 12 months in 2012 (Campodónico & Mauna, 2014). Life cycles and population dynamics of the majority of the invertebrate species living in the benthic community dominated by the Patagonian scallop are unknown (Bremec & Echeverría, 2005), and hence, basic knowledge lacks to differentiate between natural and anthropogenic variations. The life span of a related Chaetopterus species in lower Chesapeake Bay showed that few individuals lived > 1 yr during two years period, 1994-1995 (Thompson & Schaffner, 2001). It is well known that populations of benthic species with planktonic larvae are influenced by a multitude of factors, such as larval transport processes or unpredictable food supply, however, assuming an annual life cycle of C. antarcticus, these results are more than expectable and showed a trend already demonstrated worldwide. Trawling fisheries affect marine bottoms and ecosystems in many different, but interrelated, ways, as they produce resuspension of sediments and remove a great number of individuals depending on the gear and type of sediment (Hall, 1999; Collie, Hall, Kaiser, & Poiner, 2000; Kaiser, Collie, Hall, Jennings, & Poiner 2002; Hiddink, Jennings, & Kaiser, 2007; Hinz, Prieto, & Kaiser, 2009; Clark et al., 2016). Although the aptitude of some chaetopterids to attach tubes to hard substrates was already reported (Bailey-Brock, 1976; Rouse, 2001), the described behavior of C. antarcticus was not commonly registered previously in the study area and constitutes an extraordinary event that, in the present case, could be the result of intensive sediment disturbance, and shows the capacity of adaptation of the species to environmental changes.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. All financial sources are fully and clearly stated in the acknowledgements section. A signed document has been filed in the journal archives.












 uBio
uBio