Introduction
Based on the experimental results obtained by Kloepper and Schroth (1978) on radishes, the term plant growth-promoting rhizobacteria (PGPR) refers to the beneficial soil bacteria that colonize plant roots and confer beneficial effects, such as increased plant growth and decreased susceptibility to diseases. The potential of PGPR in agriculture has been significant during the last ten years as they offer an ecofriendly way to reduce the use of fertilizers, pesticides, and other chemical supplements (Bhattacharyya & Jha, 2012). Some publications have suggested that PGPR strains operate through a wide variety of metabolic mechanisms such as synthesis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase, nitrogen fixation, nutrient solubilization and mineralization (e.g. P, K, Zn), production of plant growth regulators (e.g. indoles, cytokinins and gibberellic acid), and exhibition of antagonistic activity against phytopathogenic microorganisms by siderophores and antibiotics (Timmusk, Behers, Muthoni, Muraya, & Aronsson, 2017). Recently, numerous studies have shown positive effects on growth, nutritional value, and productivity parameters on fruits, cereals, and vegetables using PGPR inoculation (Pii et al., 2015; Bhardwaj, Ansari, Sahoo, & Tuteja, 2014). However, most of these reports have been developed under fully controlled in vitro conditions that do not demonstrate whether beneficial bacterial effects also occurs under greenhouse or field conditions (Bashan, Alexander, & de-Bashan, 2013).
Although there are many studies on plant growth promotion carried out using bacteria on various crops, there is still limited information about the effects of PGPR on the Kikuyu grass growth (Pennisetum clandestinum Hochst. ex Chiov). P. clandestinum is the most widely cultivated grass in Colombia, and it is used as a forage food source for livestock in intensive dual purpose milk and meat production systems (Mejía-Taborda, Ochoa-Ochoa, & Medina-Sierra, 2014). One of the departments with the highest production of P. clandestinum is César, where its economy depends mainly on livestock farming. This grass has nutritional and physiological characteristics that milk and meat producers search for, such as moderate tolerance to water stress, elevated levels of protein, good palatability, and adaptability (Sidari, Panuccio, & Muscolo, 2004). According to Hungria, Nogueira and Silva-Araujo (2016), several factors affect pasture degradation, including the following: (i) low planting density before grazing is initiated, (ii) forage species unsuitable for local conditions, and in particular (iii) decrease in soil fertility due to chemical fertilizer overuse. In Colombia, grazing soils are poor in essential nutrients and they have moderate concentrations of exchangeable aluminum; moreover, these also have deficiencies in phosphorus, calcium, and magnesium (Muscolo, Panuccio, & Eshel, 2013). Therefore, the maintenance of pasture growth becomes a pivotal challenge to the sustainable management of Colombian tropical soils under livestock production. Considering the above mentioned, the overall aim of this study was to characterize in vitro plant growth promotion (PGP) traits of native strains of rhizobacteria from the Colombian high-altitude tropics and to study the beneficial effects of their inoculation on the growth of Kikuyu grass under glasshouse conditions.
Materials & Methods
Microorganisms and culture: Four rhizobacterial strains 28P, 35P, 37L, and E37 isolated from soils of a silvopastoral system composed of Leucaena leucocephala, Eucalyptus tereticornis, and Megathyrsus maximus located in Codazzi, department of Cesar, Colombia, were used. These microorganisms were provided by the Soil Microbiology Laboratory of AGROSAVIA, Colombia. Each bacterium was grown in Luria-Bertani medium (10 g tryptone, 10 g NaCl, 5 g yeast extract and 17 g agar in 1 L of distilled water) at 30 °C for 24 h. To produce bacterial inoculum strains these were cultivated in Moreno-Bonilla-Rojas (MBR) broth (Moreno, Rojas-Tapias, & Bonilla, 2011) for 24 h at 30 °C and stirred at 120 rpm. In all in vitro experiments, the bacterial suspensions used were comprised by cells that were previously washed twice in 0.85 % w/v of saline solution. On the contrary, in other plant studies, unwashed bacterial cultures were inoculated into Pennisetum clandestinum stolons.
16S rRNA gene sequencing and data analysis: Strains were identified by amplifying and sequencing the 16S rRNA gene. Bacterial DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Germany) according to the manufacturer's instructions. Genomic DNA extracted was diluted in sterile Milli-Q water before conducting PCR analysis under the following conditions: a 25 μL PCR mixture contained 1× Taq DNA polymerase buffer (Invitrogen, USA), 2.5 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate, 25 pmol of each forward and reverse primers, 1 U of DNA polymerase (Invitrogen, USA), and 50 ng of genomic DNA as template (Rojas-Tapias et al., 2012). Amplification of 16S-rRNA gene was carried out by PCR with an iCycler thermocycler (BioRad, USA) using the forward primer 27F 5'-AGAGTTTGATCCTGGCTCAG-3' and the reverse primer 1492R 5'-GGTTACCTTGTTACGACTT-3'. The PCR conditions including a pre-heating step for 2 min at 95 °C, denaturation in 35 cycles of 30 sec at 95 °C, annealing for 30 sec at 57 °C, elongation for 2 min at 72 °C and extension step for 10 min at 72 °C. PCR products were purified using Wizard® SV Gel and PCR Clean-Up System kit (Promega, USA). Finally, the products were run in the BigDye terminator cycle sequencing kit and the ABI 310 DNA Sequencer (Applied Biosystems, Foster City, CA). Partial sequences obtained were analyzed using the BLAST algorithm and compared with registered sequences found in the GenBank database of NCBI where they were later deposited.
Synthesis of ACC deaminase and indoleacetic acid: To evaluate whether our microorganisms exhibit two mechanisms related to plant growth promotion, biochemical tests under in vitro conditions were performed. All strains were evaluated for their capacity to metabolize ACC as a nitrogen (N) source for bacterial growth as described by Habib, Kausar and Saud (2016). Three types of minimal salt mediums (MSM) were used: (1) without N source and replaced with MgSO4 (0.1 M); (2) with (NH4)2SO4 (0.1 M); and (3) with ACC (3 mM). The optical density (OD 600 nm) was measured in a 96-well plate for 6 d at 30 ºC and 150 rpm. To determine if our strains were able to consume ACC, we statistically compared the OD values of the three broths mentioned above. Additionally, indolic compounds were estimated using the colorimetric assay based on the modified Salkowski reagent (Glickmann & Dessaux, 1995) and standard tryptic soy broth (TSB) medium supplemented with 100 mM of tryptophan. Cells were incubated for 72 h at 150 rpm. Supernatants were mixed with the Salkowski reagent in a 4:1 ratio for 20 min under dark conditions. Indoleacetic acids (IAA) were spectrophotometrically determined at 535 nm. Each measurement was performed by triplicate.
Plant inoculation experiment: To study plant growth promotion in Pennisetum clandestinum mediated by individual inoculation with 28P, 37L, 35P, and E37 strains, we used a completely randomized design with three replicates under glasshouse conditions at the C.I. Tibaitatá (CIT), AGROSAVIA in Mosquera, Cundinamarca, Colombia. Grass stolons of P. clandestinum with a length of 9 cm were collected and surface-sterilized with 5 % sodium hypochlorite (10 min), followed by 70 % ethanol (1 min), and finally rinsed three times (5 min) in distilled-sterilized water (Ryu et al., 2003). Two stolons were grown separately in 2 kg polyethylene pots, each pot filled with soil from the P. clandestinum crops at CIT. A distance of 20 cm was used between each experimental unit (pot). The soil was air-dried at 37 °C for three days, and then, it was sieved with a 2.0 mm mesh. Soil characteristics were established by the Soil Chemistry Laboratory of AGROSAVIA. Phosphorous was quantified using the Bray II method, and sulfur was measured by extraction with Ca(H2PO4)2 and turbidimetric quantification with BaCl2 (pH 6.5, percentage of organic matter (11.6 %), effective cation exchange capacity (3.29 cmol kg-1), exchange capacity (1.13ds m-1), P (4.08 mg kg-1), S (13.25 mg kg-1), Ca (1.29 mg kg-1), K (0.77 cmol kg-1), Na (0.11 cmol kg-1), and Mg (0.63 cmol kg-1). To inoculate according to treatments, stolons were submerged in the appropriate bacterial inoculum (OD 600 nm = 0.5) for 30 min and then planted. MBR liquid medium (Moreno et al., 2011) was used as a control. Moreover, 5 mL of inoculum or MBR broth were applied manually around the planted stolon in each pot. Grass stolons were thinned to one plant per pot 15 days after planting. These were cultivated in a glasshouse at 15-25 °C and at a 16/8 h day/night light regime. After 30 days, shoot and primary root length were recorded. Then, these vegetative tissues were oven-dried separately at 60 °C for 48 h to measure their dry weights.
Statistical analysis: Data on different plant parameters measured in the pot experiment were statistically analyzed with an analysis of variance (ANOVA) and using the software package SPSS version 17.0. Treatment means were compared by applying the HSD Tukey test (LSD) at 5 % probability.
Results
Identification of bacterial strains: Through 16S rDNA sequence analysis, we found that strain 28P and strain 35P have homology with bacteria belonging to the genus Klebsiella, strain 37L with bacteria belonging to the Beijerinckia genus, and strain E37 with the Achromobacter genus. All sequences were submitted to the NCBI/GenBank database (Table 1).
TABLE 1: Plant growth promoting traits of the strains assessed
| - | - | - | - | Synthesis | - |
| Strain | Accession number | Nearest phylogenetic neighbor | Identity (%) | ACCd | IAA (μg mL−1 per OD 630 nm unit) |
| 28P | MG904940 | Klebsiella sp. | 99 | - | 72.64 + 9.06 |
| 37L | MG904942 | Beijerinckia sp. | 99 | + | 53.89 + 5.52 |
| 35P | MG904941 | Klebsiella sp. | 99 | - | 339.26 + 12.59 |
| E37 | MG904943 | Achromobacter xylosoxidans | 99 | - | 5.21 + 0.61 |
PGP: Plant growth promoting trait; ACCd: aminocyclopropane-1-carboxylate deaminase; IAA: indole-3-acetic acid. Values represent means ± standard deviation; (+/ −) indicates presence/absence of the trait. Each measurement was performed in triplicate
Screening of in vitro PGP activities: We observed that the only bacterium capable of using ACC as a nitrogen source was Beijerinckia sp. strain 37 L. Statistically significant differences (data reported as positive) were found in the OD 600 nm of strain 37 L measured in a minimal salt medium (MSM) supplemented with ACC as compared to ammonium sulfate (positive control) and magnesium sulfate (negative control). On the other hand, all four strains were able to produce indoles in tryptic soy broth (TSB) supplemented with tryptophan (100 mM). The maximum concentration of indoles was synthesized by strain 35P, with 339.26 μg mL-1 per OD 630 nm unit (Table 1).
Plant growth promotion assay: Results showed that bacterial inoculation significantly improves (P <0.05) Kikuyu growth compared to the control (Fig. 1.). Average shoot length enhancement was 52, 30, 25, and 20 % compared to the control when 37L, 35P, E37, and 28P were applied, respectively. Similarly, increases higher than 100 % were observed in shoot dry weight in inoculated treatments. For example, the use of 37L and 28P stimulated shoot dry weight by 170 and 131 % in comparison to the control. In contrast, root development was significantly influenced in length only. The application of strains 37L and E37 increased root length by 134 % and 100 % with values of 53.95 cm plant-1 and 46.16 cm plant-1 compared to 23 cm plant-1 of the control treatment. Furthermore, we observed a positive effect in root dry weight, without statistically significant differences between evaluated treatments. Furthermore, microorganisms 35P and 37L showed 0.714 and 0.69 g plant-1, whereas the control only showed 0.48 g plant-1.
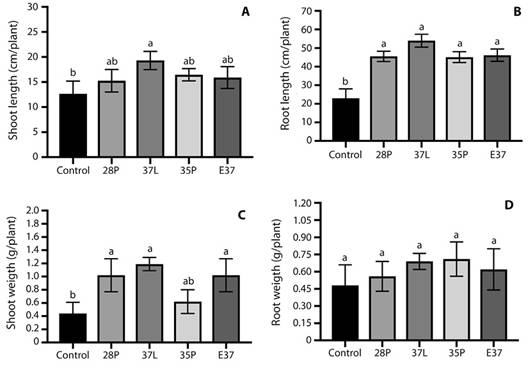
Fig. 1 Effect of inoculation with Klebsiella sp. 28P, Beijerinckia sp. 37L, Klebsiella sp. 35P, and Achromobacter sp. E37 on Pennisetum clandestinum development expressed as: (A) Shoot length, (B) Root length, (C) Shoot weight, and (D) Root weight, under greenhouse conditions. Error bars represent ± standard deviation. Letters denote significant differences based on the HSD Tukey test.
Discussion
Based on the positive impact of using PGPR we conducted this study to evaluate the effect of four bacterial strains isolated from a silvopastoral system in the Colombian high elevation tropics on Kikuyu grass growth. Initially, we studied whether our microorganisms exhibited two mechanisms related to plant growth promotion in morphometric variables through biochemical tests under in vitro conditions (Table 1). The first plant growth promoting feature evaluated was the ability of the bacteria to consume 1-aminocyclopropane-1-carboxylate (ACC) as a source of nitrogen by the enzyme ACC deaminase. We observed that Beijerinckia sp. 37L was the only strain that showed significant growth in presence of ACC. The technique used allows the indirect observation of the synthesis of the ACCd enzyme. According to Glick (2014), the main role of the enzyme ACC deaminase is to mitigate the negative effects of biotic and abiotic stresses in plants by preventing deleterious levels of ethylene in plant tissues. Furthermore, other researchers have supported the premise that hydrolysis of ACC improves root elongation (Nascimento, Rossi, Soares, McConkey, & Glick, 2014).
Production of phytohormone-like compounds such as indole-3-acetic acid (IAA), is a bacterial response to the interaction with the substances produced by the plant in the soil rhizosphere. IAA is involved in the root system and cell development (Sukumar et al., 2013). All our strains showed a remarkable ability to release IAA from tryptophan as a precursor, ranging from 5.21-339.26 μg mL-1 per OD 630 nm unit. Under natural conditions, we find L-tryptophan in organic compounds synthesized by plant roots which can be used by PGPR for IAA bioproduction (Majeed, Kaleem-Abbasi, Hameed, Imran, Rahim, 2015). It is important to note that Beijerinckia sp. 37L was able to produce both ACCd and indole compounds, and its inoculation generated the greatest beneficial effect on Kikuyu roots. In this sense, and considering the abovementioned results, we hypothesize that the root system of the Kikuyu grass (biomass and length) is influenced by both mechanisms of the Beijerinckia sp. 37L strain. Overall, our results strongly suggested that the four strains studied exhibit PGP attributes and that their inoculation in crops might beneficially influence plant growth. Hence, these microorganisms were subject to plant assays under greenhouse conditions with Kikuyu grass.
The greenhouse experiment allowed us to demonstrate the positive influence of bacterial inoculation on Kikuyu grass growth. Interestingly, we observed a significant effect on root length, shoot length, and shoot dry weight, especially in the case of inoculating with Beijerinckia sp. 37L. The average percentage increases in root length, root dry weight, shoot length, and shoot dry weight in all the evaluated strains were 105, 34, 37, and 134 %, respectively. Similar observations have been described in the few investigations reported on the use of PGPR for forage pastures. Criollo, Obando, Sánchez and Bonilla (2012) showed that both Stenotrophomona sp. 4K and Pseudomonas sp. 5B improve root dry weight and shoot dry weight in P. clandestinum by 50 %, respectively. In addition, other studies in Brazil have demonstrated the effective use of the bacterium Azospirillum brasilense for biological nitrogen fixation in forage grasses, and thus, achieved reduced rates in the use of chemical fertilizers (Hungria, Nogueira, & Silva-Araujo, 2016; Sudhakar, Chattopadhyay, Gangwar, & Ghosh, 2000).
Our results were obtained in soils from sites where Kikuyu grass is one the most predominant pastures. This soil did not receive applications of mineral fertilizers or bacterial inoculations for the previous three years. The objective of using these conditions was to observe the plant growth promotion effect from the bacterial inoculation in Kikuyu grass to simulate real conditions to those applied commercially. Moreover, some researchers suggest that it is not enough to perform only laboratory tests under controlled conditions to observe the beneficial effect of PGPR on plants (Collavino, Sansberro, Mroginski, & Aguilar, 2010). An explanation of the results found might be based on previous reports that infer that the positive effect on plant growth is attributed via one or more growth promoting characteristics of the strains inoculated (Romero-Perdomo et al., 2017; Rojas-Tapias, Bonilla, & Dussán, 2014). Interestingly, the strain with the greatest growth-promoting effect on P. clandestinum grass, Beijerinckia sp. 37L, was the only bacterium that exhibited the two PGP traits evaluated. Extensive studies are needed on the strain Beijerinckia sp. 37L using molecular biology and bioinformatic tools to elucidate the intrinsically associated metabolic mechanism in growth promotion of Kikuyu grass.
In conclusion, the four strains evaluated in this study generated a beneficial effect on the growth of Kikuyu grass, but Beijerinckia sp. strain 37L showed the most significant influence on both the biomass and the length of P. clandestinum. Moreover, the biochemical characterization allowed us to observe that our microorganisms exhibit plant growth promotion properties, which might explain indirectly the results observed in the greenhouse experiments. Field tests are necessary to study the response of Kikuyu grass when inoculated with Beijerinckia sp. strain 37L, measuring bromatological and nutritional parameters.












 uBio
uBio 

