Introduction
A biofilm is defined as a consortium of microorganisms (including phyto and zoo biota) associated with an organic matrix and adhered to a fixed submerged surface (Pandey, Bharti, & Kumar, 2014; Gatune, Vanreusel, & De Torch, 2017). The matrix of extracellular polymeric substances (EPS) is excreted by the microorganisms of the consortium, mainly benthic microalgae and bacteria, and serves to improve the adherence of the organisms, forming the community and facilitate the interactions among them (Barranguet et al., 2005). A biofilm can be formed on diverse types of substrate and many studies have been focused on determining the efficiency in the biofilm production, testing substrates such as dead leaves, wood, roots, soil, stones, bamboo, plastic, glass, fabrics, among others (Danilov, & Ekelund, 2001; Christenson & Sims, 2012). Depending on the type and nature of the substrate, the composition and number of microorganisms in the biofilm and its stability may vary significantly. The artificial substrates introduced into water environments to promote biological colonization have been widely used to characterize the algal communities inhabiting those environments, as well as the colonization patterns, succession, and productivity dynamics of the algal communities and pollution (Kardel, Carrano, Blersch, & Kaur, 2015; Kristein, Wichels, Krohne, & Gerdts, 2018). The microorganisms constituting the consortium are important for transferring organic materials among diverse trophic levels, improving the efficiency of the trophic chain and maintaining the water quality (Thompson, Abreu, & Wesielesky, 2002).
Microalgae are a promising source of aquatic pollution remediation, biomass for biofuels and a source of protein, besides they have an important role in aquaculture; they are widely used as feed for the larvae of crustaceans and fishes and for larval, juvenile and adult mollusks (Martínez- Córdova, López, & Enríquez, 2014). This is because of their adequate biochemical composition, as they are rich in macronutrients such as protein (25 - 50 %), carbohydrates (up to 40 %) and lipids (approximately 20 %); additionally, they contain fiber, starch, cellulose, vitamins (mainly B complex and C), pigments (carotenoids), and secondary metabolites with an important role as active compounds. The dried biomass, organic matter and biochemical composition of the diverse microalgae species may vary greatly depending on environmental conditions, including light intensity, salinity, temperature, and nutrient availability, among others (Brown, Jeffrey, Volkman, & Dunstan, 1997; Olivera, 2002; Li et al., 2007; Cabello-Paisini, Macias, Abdala, Korbee, & Figueroa, 2011; Fimbres-Olivarría, 2011).
Diverse microalgal specie have been used for aquaculture; among the diatoms, one of the most important genera is Navicula, which contains planktonic and benthic species (López- Elías et al., 2013). Many studies have demonstrated that benthic diatoms are the main components of marine biofilms when enough light and nutrients are present (Patil & Anil, 2005). The main benthic microalgae belong to the genera Achnanthes, Amphora, Cymbella, Navicula, Licmophora and Oscillatoria, with Navicula being one of the most abundant (Khatoon, Yusoff, Banerjee, Shariff & Mohamed, 2007; Dobretsov, 2010).
Benthic microalgae usually adhere to fixed submerged surfaces forming biofilms. Therefore, cultivating them on substrates of different nature can influence its capacity for adhesion and nutritional composition. The present study was focused on assessing the biofilm-forming capacity of two benthic microalgae using three substrates.
Materials and methods
Selected strains and experimental design: The selected strains were Navicula incerta and Navicula sp. The strain of N. incerta was provided by the collection of the Center for Scientific Research and Higher Education of Ensenada, México (CICESE) with code number NVI1, while Navicula sp. was obtained from the collection of the Department of Scientific and Technological Research of the University of Sonora (DICTUS) at Hermosillo, Sonora, México.
Two experimental trials were performed, one for each species. A one-way completely randomized experimental design with four replicates per treatment was performed (n = 4). The treatments consisted of each one of the substrates: black plastic mesh (High Density Polyethylene - HDPE), white jute fabric, and wood (tongue depressor), which were selected considering the price, availability, and facility of management. The surface evaluated for each substrate were: 16 380 cm2 for plastic mesh, 874.8 cm2 for wood and 17 550 cm2 for fabric, and they were arranged as curtains pending from a wood stick into aquariums with 45 L of marine filtered water, fertilized with F/2 sterile medium, with a concentration of 106 (M of sodium metasilicate Na2SiO3 (Guillard & Ryther, 1962). Microalgae were stocked at a density of 20 000 cells/mL for Navicula sp. and 50 000 cells/mL for N. incerta. They were grown under controlled conditions of laboratory: temperature (20-22 °C), enough aeration and constant light. Cold-light fluorescent lamps of 60 W were provided to maintain a continuous irradiance around 260 μmol m-2/seg-1 in the culture, which last five days. Every 24 h the number of cells per milliliter was counted, taking randomly 1 cm2 of the corresponding substrate (n = 118), which was vigorously re-suspended in 1 ml of sterile marine water, and observed in a Neubauer chamber using an optical microscope (Carl Zeiss Axiostar plus) with the objective 10X (López- Elías, Huerta, Murguía & Mercado, 2012). For the calculus the next formula was used:
#cells/ ml = (total number of cells/number of squares counted) (104).
Analysis of Biofilms: At the end of the trial, the dry matter, organic matter and ash of the formed biofilms were evaluated. The entire biomass attached into each substrate was removed and re-suspended in 5 L of sterile marine water. Subsamples of 300 ml from each treatment (n = 20) were filtered through Whatman GF/C 47 mm paper filters and weighed with a digital balance (OhausR); they were then dried at 65 °C for 8 h in an incubator oven (CSE Chicago Surgical Electrical Co), weighed again, incinerated in a muffle oven (TerlabMR) at 480 °C for 16 h and weighed once more (López- Elías et al., 2012).
For the determination of biochemical composition of the biofilms, the protein analysis (n = 21) was performed according to Lowry, Rosebrough, Farr, and Randall, (1951), and lipids (n = 21) the method of Pande, Khan, and Venkitasubramanian, (1963) was applied; both modified by López- Elías et al. (2012). The carbohydrate content was calculated as the rest of the dry matter minus the protein, lipids and ash.
For the statistical analysis of data, a one-way ANOVA was performed with a confidence level of P < 0.05. When significant differences were observed among means of any of the variables, a posteriori Tukey test was applied to order and rank the means. The software JMP for SAS (SAS, 2010) was used for the analysis.
Results
Dry and organic matter: For Navicula sp., the greatest quantity of dry matter associated with the biofilm was recovered from the fabric (6.64 ( 0.76 g/m2), followed by the plastic net, while the wood had the lowest amount. For N. incerta, the greatest quantity of dry matter was obtained also from the fabric (6.11 ( 0.62 g/m2), followed by the plastic net and wood (Table 1). For organic matter, the pattern was similar: for N. incerta, a significantly greater quantity was found on the fabric (3.22 ( 0.16 g/m2), followed by the plastic. For Navicula sp., the greatest amount was also recovered from the fabric (1.56 ( 0.20 g/m2), followed by the plastic, while the wood had the lowest amount for both species. The type of wood selected was too smooth, and the biofilm that formed over it had poor stability and disaggregated in a short time. The fabric substrate did not maintain its consistency during the trial, and part of it was incorporated into the biofilm and counted as biomass. Additionally, it was difficult to recover that biomass because of the porosity of the material. The plastic mesh has a non-smooth texture and is very porous, which permits an acceptable adherence of the microorganisms and an excellent stability during all the trial.
TABLE 1 Dry and organic matter (g/m2), and cellular density (cells/m2) of the biofilms formed by Navicula sp. and N. incerta (in plastic, fabric and wood substrates)
| Specie | Substrate | Dry matter | Organic matter | Cell density |
| Plastic | 2.83a ±0.23 | 1.32b ± 0.17 | 1.24a x 109 ± 6.2x108 | |
| Navicula sp. | Fabric | 6.64b ± 0.76 | 1.56b ± 0.20 | 4.54 a x 108 ± 1.4x108 |
| Wood | 0.3a ± 0.09 | 0.02a ± 0.01 | 1.43 a x 108 ± 5.8x107 | |
| Plastic | 4.55b ± 0.31 | 2.53b ± 0.19 | 1.1 b x 109 ± 1.4x108 | |
| N. incerta | Fabric | 6.11b ± 0.62 | 3.22b ± 0.16 | 8.8 ab x 108 ± 9.5x107 |
| Wood | 0.2a ± 0.05 | 0.073a ± 0.02 | 5.6 a x 108 ± 6.6x107 | |
| ANOVA P < 0.05 Specie Substrate | 0.003 0.003 | 0.045 0 | 0.234 0.003 |
Different letters in the same column indicate significant differences at P < 0.05.
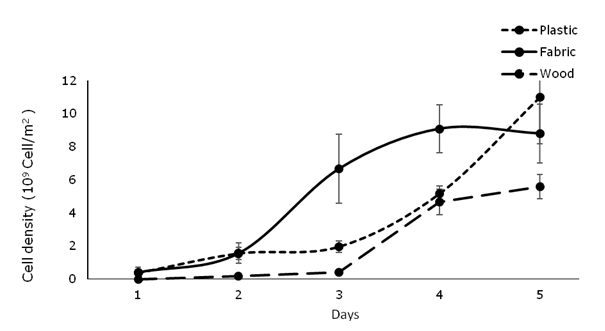
Number of cells: Regarding the number of microalgal cells adhered to the surfaces, the greatest values were recorded for Navicula sp., and the plastic net was the most effective substrate (1.24 x 109 cells/m2) (F = 1.585, P = 0.233). Significant differences in the number of cells adhered to the biofilm were observed among species and substrates, with the higher values for N. incerta (1.1 x 109 cells/m2 (F = 7.003, P = 0.003) when the substrate was plastic net, and the lowest for Navicula sp. (1.43 x 108 cells/m2) when the substrate was wood (Fig. 1).
Substrates effectiveness: In the present study, the biofilm formed over the wood was not stable along the cultivation time, due to its smooth texture, which makes that the biofilm not attached at all. The fabric, despite its porosity, which permits that an acceptable amount of matter adhered to the biofilm, difficult recovering the algal biomass grown in the pores; additionally, the material of the fabric disintegrated along the trial. The plastic mesh has a non-smooth texture and is very porous, which permits an acceptable adherence of the microorganisms and an excellent stability during all the trial.
Biochemical composition: Regarding the biochemical composition of the formed biofilm, significant differences were found between the two microalgae as well as among the substrates evaluated. The protein content was significantly higher (F = 6.043, P = 0.018) in biofilms formed by Navicula sp. on the fabric and by N. incerta on the plastic net compared to those formed by N. incerta on the fabric and Navicula sp. on the plastic net. The lipid concentration was significantly higher (F = 87.646, P = 0) for Navicula sp. regardless of the substrate. The highest carbohydrate content was observed for N. incerta. (F = 13.612, P = 0.001) on the wood and fabric (Table 2).
TABLE 2 Final biochemical composition (%) of the biofilm formed by Navicula sp. and N. incerta in plastic, fabric and wood substrates
| Specie | Substrate | Protein | Lipids | Carbohydrates |
| Plastic | 11.90a ± 1.95 | 8.72a ± 0.8 | 37.06a ± 3.37 | |
| Navicula sp. | Fabric | 24.19b ± 2.21 | 12.38a ± 0.9 | 36.67a ± 3.10 |
| Wood | 21.55b ± 1.95 | 11.23a ± 0.8 | 35.65a ± 2.74 | |
| Plastic | 21.17b ± 1.95 | 5.38a ± 0.8 | 33.45a ± 2.74 | |
| N. incerta | Fabric | 2.73a ± 0.5 | 3.05a ± 1.38 | 54.19b ± 4.74 |
| ANOVA P < 0.05 | Wood | 11.68a ± 1.9 0.988 | 2.37a ± 0.8 0.306 | 54.19b ± 4.74 0.003 |
| Specie Substrate | 0.018 | 0 | 0.001 |
Different letters in the same column indicate significant differences at P< 0.05.
Discussion
In the present study, the rough surfaces showed to be more efficient for biofilm formation with benthic diatoms, as previously documented by other authors (Fernandes Da Silva et al., 2008), disagreeing to the reported by Sweat and Johnson (2013) who found that benthic diatoms have a greater ability to colonize smooth surfaces. The surface texture is an important factor that influences microalgae attachment to different substrates. Wrinkled and porous surfaces are associated with a greater adherence of organisms and organic matter due to a larger area and major protection against hydraulic forces (Babu, 2011). Cellulose- based materials as jute, achieved greater attachment than synthetic polymers as HDPE; however, the downside to porous materials such as jute fabric in our study was the difficulty in harvesting the algal biomass growing in the pores (Christenson & Sims, 2012). The adherence and stability of the biofilm greatly depends also on factors such as the type of culture, the culture medium and the substrate (Johnson & Wen, 2010; Shen, Zhang, Xu, Lin, 2015; Dang & Lovell, 2016; Miao et al. 2019). Some environmental parameters, including irradiance, temperature, salinity and nutrient content, can also influence the colonization patterns (Tyler & Allen, 2011).
Diverse materials have been assessed as biofilm surfaces for mobile microalgae such as Chlorella sp. or benthic species; these substrates include polystyrene foam, carton, nylon, fabrics, glass, bamboo, and many others (Johnson & Wen, 2010). Azim et al. (2002) demonstrated that crystal tubes and bamboo, due to their higher densities, were able to support a more diverse peryphitic community than cane bagasse and wood. Similarly, Khatoon et al. (2007) reported that bamboo, PVC and plastic sheets had adequate peryphyton colonization in shrimp aquaculture ponds in Malaysia. Hashimoto, Vasquez, Kitamura, and Satuito (2016) evaluated diatom communities associated with biofilms in vertically submerged glass surfaces in the Sea of Japan, and they reported that Navicula and Nitzschia were the dominant genera in the study.
Some characteristics of the substrates have a significant effect on the colonization patterns in benthic microalgae; for instance, the texture and porosity of tissue favor adherence and biofilm formation (Viau et al., 2013; Kardel et al., 2015). In aquaculture, biofilms have been proven to maintain or improve water quality and the production response of diverse farmed species, mainly fish (Keshavanath et al., 2001; Mata, Luza, & Riquelme, 2017) and shrimp (Kent, Browdy, & Letter, 2011). For this activity, a wide variety of substrates have been used, including biodegradable materials (bamboo, wood, and diverse fabrics), no degradable materials (fiberglass, glass bottles, nylon, PVC, and plastic sheets), and specially designed materials known as AquamatsTM (Ferreira, Lara, Wilson, & Abreu, 2016).
Johnson and Wen (2010) conducted an investigation to produce biofuels from the microalga Chlorella sp., and they reported that the biomass and lipid content were strongly influenced by the type of substrate used, with polystyrene foam being the best material for that purpose (25.65 g/m2 of dry matter and 2.31 g/m2 of lipid content). Christenson and Sims (2012) found higher concentrations of microalgal biomass on the surfaces of natural polymers (cotton and jute) compared to that of synthetic polymers (nylon, polypropylene and acrylic). The disadvantage of porous materials, such as polyurethane, jute, vegetal foam and nylon foam, is the difficulty in recovering the biomass that embeds into the pores (Johnson & Wen, 2010).
It is common to find differences in the biochemical composition of microalgae among species and even between the same species depending on the culture conditions. Flores- Vergara, (1998) found that for benthic microalgae cultured under different conditions of light intensity and temperature, the concentration of protein ranged from 11 to 69 %, the carbohydrates from 2 to 40 %, and the lipids from 1.8 to 45 %. Similarly, Fimbres- Olivarría et al. (2015) reported concentration values in Navicula sp. ranging from 12 to 22 % for protein, 3 to 4 % for carbohydrates, and 14 to 35 % for lipids. Courtois, Porta, Viera, Fernández, and Izquierdo (2012) found values in N. incerta of 6 to 8 % for lipids, 13 % for protein and 20 to 27 % for carbohydrates.
The major component of both species in this study was the carbohydrates. The high carbohydrate content found in all the treatments (26 - 42 %) could be attributed to the presence of diverse extracellular polymeric substances (EPS), mainly polysaccharides excreted by the microalgae, which are rich in glucose and galactose (Leal et al., 2013; Klein et al., 2014; Van Colen, Underwood, Serôdio, & Peterson, 2014). It has been reported that benthic microalgae have a high polysaccharide content (Leal, Miranda, Curbelo, & Hernández, 2010) and the production of these components is affected by the time of cultivation, the nutrient concentration and the substrate (Shen et al., 2015). The EPS play an important role in the formation process of the biofilms by facilitating adhesion and providing nutrients for bacteria and other microorganisms, as well as protection against hydraulic forces (Wingender et al., 1999; Decho, 2000).
In the present study, the biochemical profiles of some of the biofilms formed by both species on a particular substrate, specifically the protein content of Navicula sp. on the fabric and N. incerta on the plastic net (approximately 24 and 20 %, respectively), seem suitable as complementary food sources for farmed fish and shrimp.
From the results obtained in this study, we can conclude that: the two microalgae could form biofilms on the three evaluated substrates; however, the amount of adhered dry and organic matter and the number of microalgal cells associated with the biofilms varied significantly. The plastic net was considered the best substrate due to its biochemical composition, the stability of the biofilm and the easiness in recovering the material. The proximate biochemical composition of the biofilms also varied widely among species and substrates. In some of the cases, the biochemical composition of the biofilm was adequate to be considered a complementary feed source and, in the future, they could be incorporated in larvae cultures as shrimps. We suggest that the beneficial effects of this biofilms need to be analyzed before commercial applications.












 uBio
uBio