How tall can terricolous lichens grow? What determines that growth? Research to answer these two simple questions is not abundant, perhaps because lichens have traditionally been divided into “crustose”, “foliose” and “fruticose” growth forms according to their shape and height, a practice that artificially hides a growth continuum (Grube & Hawksworth, 2007; Tehler & Irestedt, 2007).
But even though we need to go beyond growth forms to better identify growth factors, it must be borne in mind that they are useful ecological concepts when researchers want to generalize and predict: for example, crustose lichens can colonize more extreme habitats (Rogers, 1990) while foliose and fruticose species are better competitors in less demanding environments (Armstrong, 1993; Armstrong & Welch, 2007). In tropical latitudes, the lower temperatures and higher photosynthetic rates of mid altitudes favor foliose and fruticose species (Ceron & Quintero, 2009), but only crustose species can grow in the more demanding conditions of high altitudes (Rai, Khare, Baniya, Upreti, & Gupta, 2015). Also in the tropics, crustose lichens are more abundant in primary forests; while fruticose and foliose species are most abundant in disturbed forest, thanks to their endobionts, structure and physiology (Benítez, Aragón, González, & Prieto, 2018).
According to Rogers (2006), a possible key factor in how tall lichens can grow is the amount of rain. However, the relationship between lichens and water goes beyond rain levels and, in some lichens, incorporates fog, dew and atmospheric water that interact with temperature and light to define carbon fixation and thallus growth (Gauslaa, 2014; Colesie, Williams, & Büdel, 2017).
Paramos are highland tropical environments where low temperatures prevent tree growth, superficially resembling higher latitudes (Kappelle & Horn, 2005; 2016). The Costa Rican paramo has over 200 described species of lichens but there are few studies about them (Sipman, 2005). Here I compare lichen height, relative humidity, and temperature, in the two slopes of a Costa Rican paramo.
Materials and methods
The study was done from February through December of 2005 in a site with two slopes, one oriented to the Pacific, the other to the Caribbean, in Cerro Buena Vista (better known as Cerro de la Muerte), Costa Rica. The slopes are at 9°33’33.62” N & 83°45’20.78” W (Pacific) and at 9°34'40.83"N & 83°45’6.78” W (Caribbean).
A 200 m transect, marked at 1 m intervals, was set with rope on each slope and a digital random number generator (random.org) chose the points where polyvinyl chloride plastic frame markers (50 x 50 cm) were placed, as recommended by Root & McCune (2012) for the study of small scale factors in lichens. There were 30 frames in the Caribbean slope and 31 frames in the Pacific slope; and frames were not moved during the study period, so they were fixed sampling points where measurements were repeated on every trip.
Photographs of frames, and the lichens inside them, are freely available for corroboration: https://doi.org/10.5281/zenodo.1296967. All raw data can also be downloaded for critical review and reanalysis: https://doi.org/10.5281/zenodo.1296956.
On each visit, I inserted a dual probe (temperature and relative humidity) inside the largest lichen thallus from each plastic frame, and, a couple seconds later, under its thallus, touching the substrate. These single-time records were fed to an electronic datalogger (HOBO U-Shuttle, ONSET, Model: U-DT-1). I also measured lichen height over ground with a ruler and recorded the substrate type (soil or rock) on each visit. When time permitted, I measured one or two additional lichens per frame, for a larger sample. Measurements were made consistently between all sampling months, between 09:00 h and 15:00 h in all visits. The number of dual lichen/soil measurements of temperature and relative humidity (i.e. 4 data for measurement), for February, April, August, October and December (2015) were, respectively, 34, 33, 51, 51 and 51 datasets for the Caribbean; and 31, 33, 43, 44 and 44 datasets for the Pacific slope (for tabulated details, see https://doi.org/10.5281/zenodo.1296956).
Hypotheses
1. Lichens on hard ground grow shorter because conditions are more difficult (as proposed by Clair, Johansen, & Rushforth, 1993).
2. Lichens on warmer ground grow taller because of faster physiological reactions (as proposed by Benedict, 1990).
3. Lichens on wetter ground grow taller (as proposed by Rogers, 2006).
4. Caribbean slope lichens grow higher than Pacific slope lichens because they have more water (see Herrera, 1985 for Pacific versus Caribbean climate).
5. Lichens grow taller on the rainy season because of higher levels of available water (Herrera, 1985; Rogers, 2006).
Results
The substrate was soft (i.e. dirt and organic matter) in 360 measurements and hard (i.e. rock) only in 33 measurements; there were no statistical differences in relative humidity, temperature, or lichen height, between soft and hard soil (Kruskal-Wallis test, P > 0.05 for all combinations of variables).
Crustose lichens in the studied area grew less than a millimeter and their height cannot be measured with a ruler, but the mean height of foliose lichens was 15 mm (Standard Error 1.0 mm), and for fruticose it was 82 mm (Standard Error 7.0 mm).
There were 139 measurements in which the lichen was fruticose, 123 where it was crustose and 107 where it was foliose, i.e. none of the growth forms dominated numerically the random quadrats (χ² = 4.16, P = 0.1249).
There were 13 identifiable species in the Caribbean slope and 11 identifiable species in the Pacific slope (Table 1).
Table 1: Lichen species versus slope where they were present
| Species | Morphotype | Caribbean | Pacific |
| Cladia aggregata (Sw.) Spreng. (?) | Fruticose | ||
| Cladonia andesita* Hedwigia Beiblätter (1899) | Fruticose | ||
| Cladonia coccifera* (L.) Willd (1787) | Fruticose | ||
| Cladonia confusa* Sant (1942) | Fruticose | ||
| Cladonia didyma* (Fée) Vain (1887) | Fruticose | ||
| Cladonia sp.* | Fruticose | ||
| Cora sp. | Foliose | ||
| Dibaeis absoluta (Tuck.) Kalb & Gierl (1993) | Crustose | ||
| Diploschistes cinereocaesius (Sw.) Vain (1921) | Crustose | ||
| Everniastrum sp. | Fruticose | ||
| Hypotrachyna sp. | Foliose | ||
| Peltigera sp. | Foliose | ||
| Siphula pteruloides Nyl (1859) | Fruticose | ||
| Stereocaulon cf. ramulosum? Th. Fr. (1857) | Fruticose | ||
| Stereocaulon strictum var. explanatum Th. Fr. (1857) | Fruticose | ||
| Stereocaulon tomentosum Fr. (1825) | Fruticose | ||
| Trapeliopsis sp. | Fruticose | ||
| Usnea sp. | Fruticose | ||
| Unidentified | Not recorded |
* Cladonia has a dimorphic thallus.
In the next sections, figures are presented only when there were statistically significant patterns.
Seasonal Pattern: From December to February there were higher temperatures in both soil and lichens, and a reduction in the mean height of the lichen colonies (Kruskal-Wallis ANOVA, P > 0.05; Figure 1).
From April through October, the soil and the lichens were moister and cooler; and lichen colonies were taller (Figure 1).
Effect of slope: There were no significant slope differences in temperature, but there was an important difference in relative humidity. In the Caribbean slope, which was wetter, both soil and lichens were moister, and lichens were almost twice as tall as in the Pacific slope of the site (Kruskal-Wallis, P < 0.001 in all cases; Figure 2).
Growth form: Ground temperature and relative humidity were similar to those of the crustose lichens growing on it; but were cooler and moister under foliose and fruticose lichens (Figure 3).
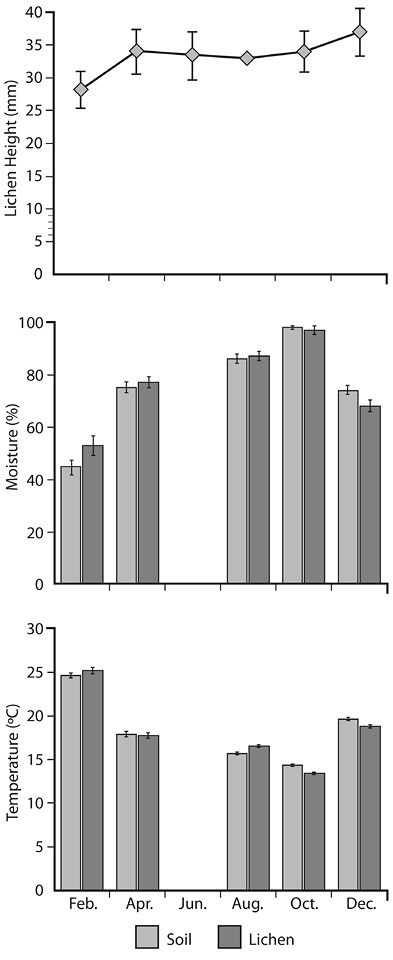
Figure 1 Yearly trends of mean temperature, relative humidity and lichen height in the Buena Vista paramo. Error bars represent the standard error of the mean (June values are interpolations because there was no sampling in that month, thus they have no error bars).
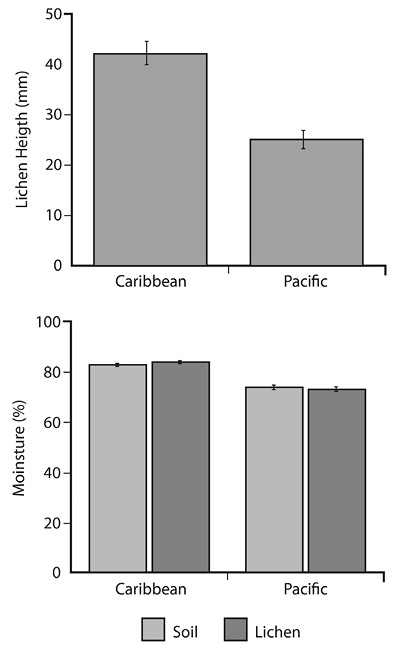
Figure 2 Relative humidity of soil and lichens, and lichen height, in the Cerro de la Muerte paramo (Caribbean versus Pacific versants; error bars represent the standard error of the mean).
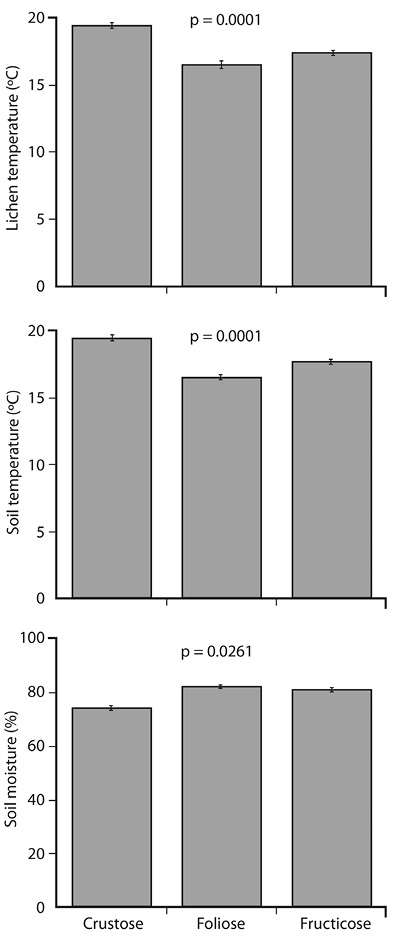
Figure 3 Temperature (soil and lichens) and soil relative humidity for each lichen growth form, in the Cerro de la Muerte paramo (Caribbean versus Pacific versants; error bars represent the standard error of the mean; p values indicate that bars of different height represent significantly different mean values for those variables).
Discussion
The lichenoflora of the Costa Rican paramos, has about 200 described species and are dominated by foliose and fruticose forms (Sipman, 2005). The species have wide geographic ranges that include many of the neotropical highlands, possibly as a result of geographic paramo continuity during the Pleistocene (Sipman, 2005; Kappelle & Horn, 2005; 2016). Apparently this is the first study to analyze temperature and relative humidity in Costa Rican paramo lichens.
The results are in agreement with the hypotheses that lichens grow taller on (1) warmer ground, (2) wetter ground, (3) the moister Caribbean slope, and (4) the season with heavier rainfall, as expected according to the findings of, among others, Benedict (1990), and Rogers (2006). Benedict’s experiments with Xanthoparmelia spp. showed that warmer temperatures, and increased moisture available for photosynthesis, correlated with a positive net carbon assimilation rate that was rapidly translated into lobe elongation (Benedict, 1990). Rogers’ study of 61 Australian lichen species along a 1 500 km transect found that taller species dominated wetter regions and shorter species dominated the dry interior (Rogers, 2006).
The data did not match one hypothesis, though: in contrast with softer ground, rock-hard substrates fluctuate abruptly in temperature and do not absorb water well (Clair et al., 1993; Giordani et al., 2014), so I expected less vertical growth in lichens growing on rock. Apparently from these results, atmospheric factors are more important than substrate in the determination of temperature, relative humidity and growth of lichens. Some temperate and tropical lichens can use water from dew, fog or humid air for growth that exceeds what may be expected from substrate alone (Lange, Green, & Heber, 2001; Lange, Buedel, Meyer, Zellner, & Gerhard, 2004; Lange, Green, Melzer, Meyer, & Zellner, 2006) but only future research could identify with certainty the cause of these results for the Costa Rican paramo lichens.
The finding that crustose species, which do not raise much from the ground, basically have the same temperature and relative humidity as the substrate, is not surprising. There are no equivalent tropical data that I know of, but Cao, Zhang, Zheng, Liu, and Zhou (2015) reported similar results in Antarctic lichens: species with a tufted thallus were better protected from external conditions than those with low lying thalli. And the classic studies by Kershaw and colleagues in Canada found that tall lichens have thermic and moisture gradients, from the top layer, which closely matches environmental fluctuations, to the lower levels, where conditions are milder and moisture is often high, and the taller the lichen, the larger the gradient (Kershaw, & Field, 1975; Kershaw, & Smith, 1978).
Overall, these findings suggests that physiologically available water is the main determinant of lichen vertical growth in the Buena Vista paramo. Based on these and other findings from lichens in Buena Vista and elsewhere (Mehlen, 1969; Bässler et al., 2015; Bokhorst, Asplund, Kardol, & Wardle, 2015; Maestre et al., 2015), I recommend future work on the hypothesis that taller and more tufted lichens -and their microcommunities- will be more resistant to climate change than those with flat, low growth thalli.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. A signed document has been filed in the journal archives












 uBio
uBio 

