Over past years, several native and non-native mosquito species are leading to novel epidemiological scenarios with high and dramatic transmission of infectious diseases such as yellow fever, dengue, chikungunya or Zika, among others (Medlock et al., 2012). The environmental changes caused by anthropic activities have a significant impact on the abundance and community composition of diverse species of mosquitoes (Ferraguti et al., 2016). For example, some mosquito species of the genera Aedes and Culex have been able to shift from natural larval habitats (e.g., fitotelmata and rock pools) to anthropic environments containing artificial containers. In urban settlements, artificial recipients (e.g., rain catch basins, discarded tires, tins, flower vases, and water storage containers) have become the most important breeding site for new invasive species such as Aedes albopictus (Skuse, 1894) (Bonizzoni, Gasperi, Chen, & James, 2013) and for the widespread yellow fever mosquito Aedes aegypti (Linnaeus, 1762). Same kind of domestic containers also provide important larval habitat for synanthropic Culex mosquitoes (Subra, 1981).
Within these urbanised habitats, cemeteries offer high density spots with great proliferation of culicids (Vezzani & Schweigmann, 2002). The reason is based on four basic requirements: energy supply (carbohydrates provided by funeral flowers), food (blood meal is provided by visitors and caretakers that are frequently around as well as by domestic and wild animals including dogs, cats, other small mammals, and birds), shelter (diverse vegetation types, ornamental vegetation and human-created structures) and water-filled containers (artificial containers provided by visitors/local authorities, gravesites, mausoleums and natural sources such as tree holes and bromeliads) (Vezzani, Velázquez, Soto, & Schweigman, 2001; Vezzani, 2007).
Two additional factors contribute to complicate the picture. Cemeteries are frequently located in urban or periurban areas, often closely located to important human foci (e.g. residential areas, churches, hospitals, universities, and schools, among others), which increase the risk of transmission of mosquito-borne diseases to a high density of human populations. Secondly, cemeteries are neglected in standard and routine adult mosquito control campaigns by insecticide spraying, which potentially support vector population refugia (Abe, McCall, Lenhart, Villegas, & Kroeger, 2005). These reasons make cemeteries as highly suitable habitats for several species of culicids adapted to urban human environments.
Although the interest on mosquito studies is rising, little updated information on this insect group is available in the Dominican Republic, with the exception of some studies published recently (Alarcón-Elbal et al., 2017; Rodríguez Sosa, Vásquez Bautista, Fimia-Duarte, Guerrero, & Alarcón-Elbal, 2018; Rueda, Rodríguez Sosa, Vásquez Bautista, Guerrero, & Alarcón-Elbal, 2018). First, because the characterization of mosquito breeding sites is limited. A better knowledge of these sites occupied by vector species would provide epidemiological information of paramount relevance. Cemetery surveys of immature mosquitoes are of great interest because these areas possess a variety of artificial-containers, which may be colonised by diverse species who have adapted to them. This is evidenced by almost 30 mosquito species found in cemeteries in the American continent including the invasive species A. aegypti and A. albopictus, and the urban Culex pipiens Linnaeus, 1758 and C. quinquefasciatus Say, 1823 as the most frequent ones (reviews Natal, Gonçalves, & Taveira, 1997; Vezzani, 2007). Second, as features in breeding sites influence the development success of immature mosquito species, more studies evaluating the effects of multiple biotic and abiotic variables are needed. The selection of containers or survivorships of larvae/pupae in culicids, depends on different factors: water volume, container size, location, container colour, vegetation cover, water chemical characteristics, size of water surface, type of material, solar exposition, temperature, organic matter, detritus, microorganisms, and intra and interspecific competition, among others (Vezzani, 2007; Getachew et al., 2015; Rey & Lounibos, 2015). Evaluating some of these micro-environmental variables in association with the different species and the occurrence of mosquito infestations levels are crucial for a better understanding of the mosquito vectors and essential for an efficient application of control methods.
In order to contribute to the knowledge of the mosquito community species and ecological factors involved on these important container-inhabiting mosquitoes, an urban cemetery in Jarabacoa (Dominican Republic) was studied. We examined the association between features of the containers (material of the container, presence of flowers, water availability, and height) and the levels of infestation of the immature stages of mosquitoes.
Materials and methods
Study area: The study was performed in Jarabacoa (La Vega Province, Dominican Republic), which has a population of ca. 32 600 inhabitants within an area of ca. 660 km2. Jarabacoa has a typical tropical rainforest climate with 1 340 mm of annual rainfall, 22.9 ºC of annual daily mean temperature and Köppen climate classification: Af. The Municipal Cemetery of Jarabacoa (size 3 535 m2 = 0.35 ha) is located just in the town centre (central point: 19°07’27.21” N & 70°38’33.39” W, 520 masl). The design and plant use is homogeneous along the cemetery, with sections separated by sidewalks and in general scarce vegetation structure. Reflecting the cultural practices, funeral and sympathy vases containing flowers was frequently provided by visitors in the cemetery during all the year.
Sampling, material processing and identification: Four working days were needed to examine and sample the entire cemetery. Two time periods were selected as representative of different climatic conditions, March 2017 (dry season, typically cooler, daily mean temperature (T) of 21.3 ºC and 112.3 mm of precipitations) and August 2017 (rainy season, typically warmer, daily mean T: 24.1 ºC and 155.5 mm of precipitations).
All water filled containers (positive or negative) were counted and classified according to the type of material. Information about immature stages, water volume, presence of flowers inside the containers and height from the ground was recorded. All individuals of larvae and pupae in positive containers were collected with Pasteur pipettes on plastic trays. Then, immature stages of mosquitoes were transferred alive into mosquito breeders (Bioquip Products, USA) with their own water to be reared in the laboratory. The immature pupae were raised to adults so the species could be accurately identified. In general, the identification of immature stages was preferably carried out with IV instar larvae. Both adults and immature stages were identified using the key of González Broche (2006). Voucher specimens are deposited in the Laboratory of Entomology (UAFAM, Jarabacoa, Dominican Republic).
Due to the close resemblance of the type of containers, these were classified according to the material of which they are made of as follows: a) plastic, b) derived from sedimentary rocks (cement, ceramic, and mud), c) glass, d) metal (bronze, copper, and aluminium) and, e) others (bamboo, cardboard, granite, foam, and timber). The water volume of the container was measured by decanting into a graduated cylinder. The water volume filled in the containers was categorised into the following water levels: 0-100, 101-500, 501-1 000 and > 1 001 ml. The presence of flowers (or plants, hereafter as flowers) was recorded as positive (flowers inside the containers) or negative (no flowers). The height (position of the containers respect to the ground level) was categorised into the following heights scales: 0-50, 51-100, 101-150, > 151 cm.
Out of the 1 207 potential artificial containers found, 239 (19.8 %) were excluded for further analysis due to logistic problems and/or inability to access, giving a total of 968 containers included in the study.
Statistical analysis: Non-parametric tests were performed after no-normality distribution was assumed. U-Mann-Whitney test was conducted to determine the influence of the presence of plants on the density of culicids. Kruskall-Wallis tests were performed to compare medians between immature stages inhabiting containers, to compare the density of culicids with the type of material and to compare the density with the height. The association between the water volume and the density of culicids was studied with the Spearman correlation coefficient. Mann-Whitney U comparison with Bonferroni correction was followed for multiple testing adjustment. The mean squares of each season (monthly variation) was analysed by F-statistic. The occurrence of immature stages (larvae and pupae) for both trapping periods in the cemetery was represented by Least Square Means (LS Means) based on a linear model (ANOVA).
All statistical tests focused on the three most common species identified (A. aegypti, A. albopictus, and C. quinquefasciatus), the total larvae of these species, total pupae of these species, and the total Culicidae including the larvae and pupae of these studied species. Data is expressed for both trapping periods pooled, otherwise is specified. Container indices for the material types were compared using the chi-squared test (difference between proportions).
The Container Index (CI) = (positive containers infested/number of inspected containers x 100) was calculated for the total immature stages and for each type of material (Webb, 2008). The total density of the containers in the cemetery was calculated as the total number of containers (including those inaccessible)/area of the cemetery.
The significance level was set to alpha = 0.05. All statistical tests were conducted using IBM SPSS V22.O software (IBM, 2016). Graphs were made using Microsoft Excel.
Results
In total 968 containers were studied for both trapping periods (483 in March and 485 in August) with a density of 3.48 per ha. Out of 968 containers, 677 (69.9 %) contained water, of which 203 (21.0 % of the total) were positive for immature mosquitoes (117 and 86 in March and August, respectively).
Abundance: In total 7 758 immature stages (97.9 % of the total catches) found in water-filled containers were accurately identified to four species of culicids. Up to 7 141 specimens (90.5 %) were recorded as larvae and the remaining as pupae. C. quinquefasciatus and A. aegypti accounted for 50.5 %, and 47.1 %, respectively, while A. albopictus and C. nigripalpus Theobald, 1 901 represented less than 2.5 % of the total catches (Table 1). Of the 203 containers with immature mosquitoes, A. aegypti was found in 158 containers (77.8 %), C. quinquefasciatus in 76 containers (37.4 %), A. albopictus in 30 containers (14.7 %) and C. nigripalpus in 4 (1.9 %). The mean of A. albopictus immatures (Mean, M ± Standard Deviation, SD = 5.0 ± 3.1) per inhabited container was significantly inferior (Kruskall-Wallis = 6.4; P = 0.007) compared to A. aegypti (23.1 ± 8.5) and C. quinquefasciatus (51.6 ± 12.1).
Monthly variation: Significantly more specimens were collected in March than in August (F = 3.723; P = 0.006). When comparing the abundance between both periods, A. aegypti was the predominant species during March whereas C. quinquefasciatus dominated in the warmer sampling period. The abundance of A. albopictus increased from March to August. C. nigripalpus was recorded only in August (Table 1; Figure 1).
Table 1: Immature stages of Culicidae recorded for both trapping periods in the cemetery of Jarabacoa, Dominican Republic
| Species | March | August | Both periods | |
| C. quinquefasciatus | 1 824 | 2 097 | 3 921 | (50.5 %) |
| A. aegypti | 2 599 | 1 058 | 3 657 | (47.1 %) |
| A. albopictus | 34 | 117 | 151 | (1.9 %) |
| C. nigripalpus | 0 | 29 | 29 | (0.4 %) |
| Total culicids | 4 457 | 3 301 | 7 758 | |
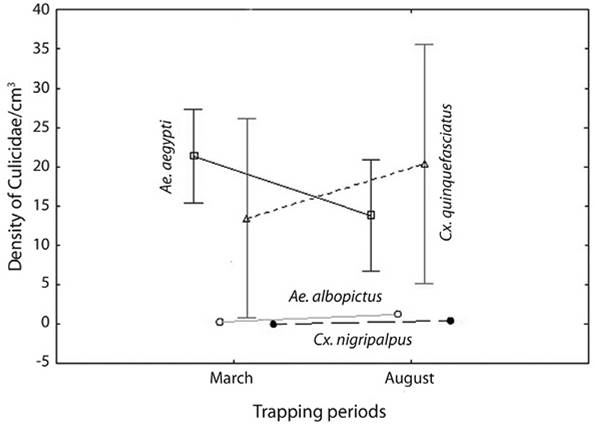
Figure 1 Ocurrence of immature stages (larvae and pupae) for both trapping periods in the cemetery of Jarabacoa, Dominican Republic (represented by LS means and vertical bars denote 0.95 confidence intervals).
Water volume: In total, 853 (476 and 377 for March and August, respectively) out of 968 were water-filled containers. The water volume ranged from 10 to 10 000 cm3 (M ± SD = 735.5 ± 632 cm3). No correlation was found between water volume and density of immature stages in A. aegypti(Spearman test r = 0.122; P = 0.144), total larvae (r = 0.166; P = 0.066), total pupae (r = 0.149; P = 0.198). In contrast, a weak positive correlation was found in C. quinquefasciatus (r = 0.302; P = 0.007) and total culicids (r = 0.330; P = 0.006). Overall, a greater proportion of immature stages was recorded for 101-1 000 ml, in contrast to ≤100 ml containers, which supported the lowest infestations, e.g., C. quinquefasciatus (Average Range, AR = 63.5 in > 101 ml and AR = 115.1 in > 1 001 ml) and in total culicids (AR = 85.9 in > 101 ml and AR = 111.1 in > 1 001 ml). A weak negative correlation was found in A. albopictus immatures (r = -0.314; P = 0.007) showing the occurrence of the lowest infestations in water-filled containers with > 1 001 ml compared to the other ranges. The mean height of the containers for each type of material can be consulted in Table 2.
Table 2: Characteristics of immature culicids recorded for both trapping periods (pooled) in the cemetery of Jarabacoa, Dominican Republic
| Material 1 | H (cm) | WV (cm³) | WFC | Flower | CI | nº specimens |
| Plastic | 69.6 | 784.6 | 372 | 72 | 23.4 | 4 956 |
| Rock | 65.9 | 636.8 | 134 | 36 | 25.9 | 2 412 |
| Glass | 105.4 | 95.8 | 70 | 2 | 3.8 | 178 |
| Metal | 6.25 | 346.8 | 21 | 2 | 12.9 | 119 |
| Other | 0.0 | 2 037.5 | 6 | 2 | 2.0 | 93 |
H = Mean height; WV = Mean water volume; WFC = Water-filled containers; Flower: Containers with water and flowers; CI = Container Index (%).
1Rock (cement, ceramic and, mud), metal (bronze, copper and, aluminium), and other (bamboo, cardboard, granite, foam and timber).
Presence of flowers: In total, 114 (56.7 %), 56 and 58 containers in March and August respectively, had flowers. Significantly higher number of total culicids were recorded in flower pots containing plants (Mann-Whitney U test = 3 953.5; P = 0.008). This difference was significant either for total larvae (U test = 4 059.5; P = 0.016) or total pupae (U = 4 275.5; P = 0.042). Among the four species, the density of immature stages of C. quinquefasciatus was greater in presence of flowers (U = 3531.0; P = 0.000). In contrast, containers without flowers were favourable to A. aegypti immature stages (U = 4257; P = 0.049). The number of containers for each type of materials can be consulted in Table 2.
Type of material and distribution: Out of 203 positive containers, 62.1 % (126) were plastic material, 32.5 % (66) were derived from sedimentary rocks, 2.4 % (5) from glass, 1.9 % (4) from metal and 0.9 % (2) from other materials. Out of 7 758 immature mosquitoes collected from the positive containers, 4 957 (64 %) were retrieved from plastic containers, 2 412 (31 %) from sedimentary rocks, 178 (2.3 %) from glass, 119 (1.5 %) from metal and 93 (1.2 %) from other diverse materials (Figure 2). The number of specimens was three times greater in plastic containers that in rock containers in March, but similar numbers were observed in August (Figure 2).
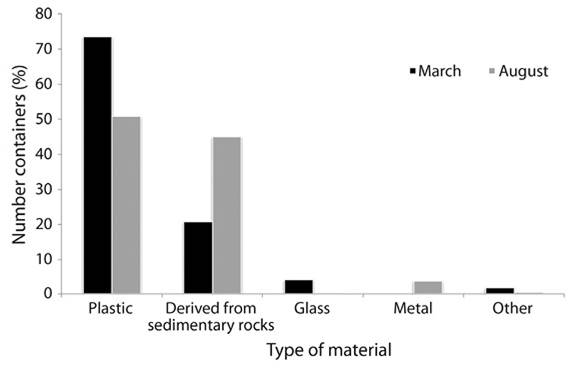
Figure 2 Distribution of immature stages (larvae and pupae) according to the type of material for both trapping periods in the cemetery of Jarabacoa, Dominican Republic.
Container Index (CI): March resulted in a CI = 23.07 % and August in CI = 18.7 %. Within type of material, plastic containers had a CI = 23.4 %, materials derived from rock (CI = 25.9 %), metal (CI = 12.9 %), and other materials including glass (CI = 5.8 %) (Table 2, Figure 3). The CI between March and August for plastic and derived from rocks were not statistically different (Kruskall-Wallis χ 2 = 12.4 and 16.1, respectively; d.f. = 1; P ≥ 0.05) but varied significantly between both trapping periods for glass, metal and other materials (all P ≥ 0.05; Figure 3).
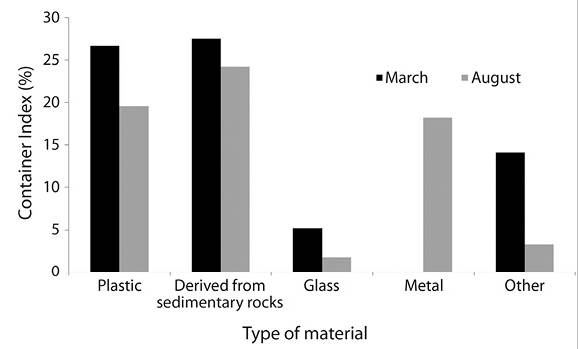
Figure 3 Container Index according to the type of material for both sampling periods in the cemetery of Jarabacoa, Dominican Republic.
Height from the ground: Height of containers ranged from 0 to 300 cm (M ± SD = 67.9 ± 68.3cm). No correlation was found between the height and the density of total larvae (Spearman test r = -0.135; P = 0.055), total pupae (r = -0.013; P = 0.858) and total culicids (r = -0.125; P = 0.075). However, statistical differences in total culicids were found when Kruskal-Wallis test was performed (χ 2 = 14.0, P = 0.016) and greater densities of vectors were found at ground level (0 cm than from 50 to > 151cm). The mean height of the containers for each type of material can be consulted in Table 2.
Discussion
This study highlights the importance of cemeteries as foci with high density of vector species. In previous works, the roles of cemeteries have been reported as potential breeding sites not only for dengue and chikungunya vector species like A. aegypti and A. albopictus but also for other species. Infected Aedes triseriatus (Say, 1832) with La Crosse encephalitis arbovirus were collected in cemeteries and consequently, these could serve as an ideal bridge to spread vector populations and virus amplification between rural native sites and urban environments (Trout Fryxell et al., 2015). The cemetery in this study supported breeding environment in the urban centre of Jarabacoa. In 500 m radius, there is a City Hall and diverse buildings with intense movement of people such as two bus stations, various entertainment venues, cultural centres, a hospital, a police department and a gas station, thus increasing the risk of arbovirus transmission to the population (Abe et al., 2005). Considering that the dispersal of Aedes and Culex adult mosquitoes could reach several hundred meters, it is likely that this cemetery serve as one of the most important sources of mosquitoes for the municipality of Jarabacoa.
The four species found in our survey have been incriminated in the transmission of diseases to humans worldwide. The role of both aedine species as vectors, particularly A. aegypti, was evidenced after the epidemics of dengue and chikungunya in the Caribbean region and subsequently corroborated by the detection of field-collected A. aegypti infected with both viruses in Martinique (Service, 2012; Farraudière et al., 2017). Aedes albopictus has been reported as a vector of dengue in American countries such as Brazil (de Figueiredo et al., 2010) and Mexico (Ibáñez-Bernal et al., 1997), and showed to be a competent vector of chikungunya (Vazeille et al., 2007). Regarding chikungunya arbovirus, recent studies carried out in the Dominican Republic, Martinique and Guadeloupe suggest that deaths caused by this virus are not as rare as had been suggested previously (Freitas, Alarcón-Elbal, Paulino-Ramirez, & Donalisio, 2018; Freitas, Alarcón-Elbal, & Donalisio, 2018). The presence of the Southern house mosquito C. quinquefasciatus should not be neglected, as it likely plays an important role in maintaining viruses within bird populations and is responsible for transmitting the filarial nematode Wuchereria bancrofti, which is still endemic in the Hispaniola (Addiss & Chuke, 2002). Culex nigripalpus is involved in the transmission of Venezuelan equine encephalitis virus (Mendéz et al., 2001), St. Louis encephalitis virus (Day, Curtis, & Edman, 1990) and West Nile virus (Turell et al., 2005).
The presence of A. aegypti, A. albopictus, and C. quinquefasciatus into a lesser extent, have been well-documented in cemeteries worldwide (reviewed in Vezzani, 2007). However, only two reports for C. nigripalpus in Venezuela (Barrera, Machado-Alison, & Bulla, 1979) and Florida (Leisnham, LaDeau, & Juliano, 2014) exist. Regarding monthly variation, the dry season was a little more productive in immature stages of culicids than the wet season. We found some differences in the abundance of vectors between both sampling periods. Aedes aegypti abundances were clearly superior to its conspecific A. albopictus in both study periods. This contrasts, the fact that A. albopictus is a more recent invasive species with interspecific competitive superiority characteristics. The differences in the abundance between A. aegypti and C. quinquefasciatus between both sampling periods might respond to rainfall patterns (Leisnham et al., 2014), although data seems insufficient to establish conclusions. This is not unusual as tropical areas have continuous periods of raining and mosquito fauna proliferates all year around. Although mosquitoes are more abundant during the wet season (Barrera, Machado-Alison, & Bulla, 1979; Shultz, 1989; 1993), the availability of artificial breeding containers in cemeteries is ensured both by the water-filled vases provided by humans and rains along the whole year, including the dry season. In particular, changes in mosquito’s density might be reinforced to special commemorative dates where new flowerpots are placed (Barrera et al., 1982; Gonçalves, 1989; Natal et al., 1997). As noted by Vezzani and Albicócco (2009), there was no clear correlation between precipitation indicated and CI values.
The number of immatures per inhabited breeding site varied between species. Aedes aegypti and C. quinquefasciatus, the dominant species breeding in artificial containers in the study, coexisted although the latter was found in lower numbers. The number of A. aegypti immatures per infested container were slightly higher compared to previous reports (Vezzani & Schweigmann, 2002; Abe et al., 2005). A lower mean number of A. albopictus per infested conspecific container was reported here. Whether these differences in the numbers are related to interspecific interactions, food competence or distinct oviposition patterns and/or subsequent success in the survival of immature are not yet fully understood but might be considered as possible explanations (Vezzani & Albicócco, 2009).
In relation to the micro-environmental features associated with breeding mosquitoes, the water volume content had an influence in the infestation levels of A. aegypti and C. quinquefasciatus. In this respect, it seems that higher numbers are record in larger vases than lower ones in accordance to other published works in cemeteries (Vezzani, Velásquez, & Schweigmann, 2004; Abe et al., 2005). As opposed to our study, the latter did not found immature stages of A. aegypti in containers with less than 100 ml of water. The opposite pattern was observed for A. albopictus, possibly responding to their natural tendency of laying eggs in smaller volumes of water under natural conditions. Flower vases usually store lower water volume in comparison with other studies with access to multiple-sized containers.
Vases with flowers provide a variable habitat, which can be used by different mosquito species as organic matter gains importance. For example, Aedes spp. are typically found when water is clean, and later as water becomes more organic due to flowers, Culex spp. becomes predominant (Barrera et al., 1979; 1981; Natal et al., 1997; Vezzani et al., 2001; Vezzani et al., 2004; Abe et al., 2005; Vezzani, 2007). Our study documented that C. quinquefasciatus densities were higher in containers with flowers, which presumably have higher organic matter in the water. In contrast, immature A.aegypti density was greater in absence of flowers, which is in line with the results reported by Abe et al. (2005).
Our results failed to show an association between type of material and infestation for the species studied. This is not surprising due to the high opportunistic behaviour and ecological plasticity showed by A. aegypti and A. albopictus which are able to breed in almost any type of water-filled container (even with eutrophic water) as has been shown recently in Cuba (Diéguez et al., 2015; 2016). It is reasonable to say that the sources of Aedes immature stages depend on the local habits of water and not on the material of the container as mentioned by Kittayapong & Strickman (1993). In this case, A. aegypti showed an overall preponderance for some materials, particularly in favour of plastic type, although varies depending on studies (Abe et al., 2005; Vezzani & Schweigmann, 2002; García, Micieli, Achinelly, & Marti, 2002; Barja-Simon, Le Goff, Callata, Walter, & Bremond, 2009; Devera, Devera, & Velázquez, 2013). In contrast, in Venezuela, cement and ceramic containers overcome the rest of the materials (Castillo, Brown, Castillo, Caprazo, & Sánchez, 2016; Traviezo-Valles et al., 2016). However, it is important to be careful in the interpretation as one material could constitute the most available breeding site and therefore this will not indicate a specific attraction for it (Abe et al., 2005). Less literature is available to compare C. quinquefasciatus and A. albopictus. The fact that some materials are less suitable for breeding could be explained by high temperature in the water of the vase, the overheating of the inner surface where eggs are laid as well as by release of toxic particles as apparently occur with some types of metallic materials (i.e. copper) (Romi et al., 2000; Vezzani & Schweigmann, 2002). This could explain the low CI recorded in glass containers and other varied materials.
The CI values obtained from Jarabacoa cemetery is in line with other studies carried out in Argentina and Venezuela, in which levels of infestation in cemeteries varied widely between 0 to 50 % (Vezzani et al., 2001; Vezzani & Schweigmann, 2002; Abe et al., 2005; Devera et al., 2013). The container availability (density of containers per area) was moderately superior in our study with respect to those reported in Vezzani et al. (2001). Note that these authors used the CI as the positive containers/water-filled containers which in this case resulted in our CI index even higher (40.3 %). CI seems to dependant on other factors such as the environment (landscape), seasonality (periods of time), exposure of sunlight (sunlight/shaded), cultural habits (commemorative dates) and different type of cemeteries (Natal et al., 1997; Vezzani et al., 2001; Vezzani & Schweigmann, 2002; Vezzani & Albicócco, 2009; Devera et al., 2013). Therefore, the full understanding of the attraction of female mosquitoes to a specific material is the result of diverse factors that remain partly unknown due to the complexity of the variables involved.
Although no correlation was found between height and density levels in immature mosquitoes, greater densities of immature stages were found at ground level than at higher heights. Frequently, the burial system in cemeteries provides different levels that serve as wall niches for breeding. The vertical distribution of larvae and pupae of A. albopictus in a cemetery of Spain was not related to height from the ground: Similarly, Martins et al. (2010) did not found significant differences when compared different heights of breeding sites and noted the high capacity of Aedes spp. to colonize variables heights.
For the control of container breeding sites, integration of multiple methods is desirable. Considering that disposable plastic containers are the most common material by far in the present cemetery and in other studies mentioned, a simple measure to reduce the density of artificial breeding sites would rely on their elimination. Replacing water by sand and/or draining water weekly to avoid breeding sites is also plausible although challenging as container by container treatment is time-consuming and requires permanent attention. Novel approaches such as adding water crystals to flowering vases impeding the piling water, treat vases with a special material that turns water into a gel preventing mosquito development, keeping flowers fresh or even banning the use of natural flowers as occur in some cemeteries in Cuba, should also be considered.
Since permanent cement vases (i.e. graves or stationary jars) are the second most abundant container, other alternative measures are required, for example, the application of copper or larvicide-based control techniques. Adequate application of electric wire in flower pots with A. albopictusresulted very promising in reducing the immature stages in cemeteries of Europe (Romi et al., 2000; Eritja & Herreros, 2017). The use of the larvicide temephos in an environment with high availability of potential breeding sites was effective in reducing A. aegypti populations in a cemetery of Argentina (Vezzani et al., 2004). Spinosad, is also an effective larvicide against A. aegypti and A. albopictus in semi-rural cemeteries of Mexico. The efficacy of Bti (Bacillus thuringiensis var. israelensis) seems to perform poorly when comparing with the latter ones (Marina et al., 2011; 2012). Pyriproxyfen larvicide application has demonstrated to be very effective to control urban mosquitoes in Brazil. The dust-particles in simple dissemination stations are transferred by female mosquitoes (mosquito-driven dissemination) to artificial breeding sites (Abad-Franch et al., 2015). This approach is promising for A. aegypti, A. albopictus or Culex spp. at small spatial scales, an scenario compatible with cemeteries. Other alternatives as biological control with nematodes, green algae or cyclopoid copepods resulted little effective to control mosquito immatures in cemeteries (Natal et al., 1997; Pons, Sans, Gomez, & Calliari, 2008).
Using these appropriate control agents delivered by local authorities on the entrances of cemeteries sounds reasonable although for its success, public health education is essential to create knowledge and awareness of the residents on mosquito-borne diseases. We believe that in low-income countries of the Caribbean region, simple, cheap and easy implementation techniques may have better chances to be successful than novel and more sophisticated techniques such as those involving transgenic or Wolbachia-infected mosquitoes (Araújo, Carvalho, Loshino, Costa-da-Silva, & Capurro, 2015). However, these techniques will prosper in a long-term perspective and will overcome the cost of reapplication and availability of qualified staff.
Unfortunately, in large-scale cemeteries, hand application of control agents would be impracticable (Vezzani, 2007). When the previous efforts fail or under the risk of potential epidemics, residual chemical control is the most efficient strategy available. Insecticides (adulticides) are commonly used as standard or routine practices from modified-cars provided by the Ministry of Health in the Caribbean islands. However, the particular lay-out of most cemeteries would obstruct conducting these duties by motorised-cars. Again, is highlighted that the solution usually is an integration of several techniques simultaneously or paralleled. This scenario becomes even more complicated when considering the existence of clandestine cemeteries or without appropriate staff maintenance, leading to densely vegetated areas and disposable/rubbish potential containers which favoured a continuous proliferation of mosquitoes (Barja-Simon, Le Goff, Callata, Walter, & Bremond, 2009). At this point, it is worth noting that inadequate disposal of garbage (solid waste) not only produces a strong environmental deterioration but also contributes to the proliferation of A. aegypti and A. albopictus as noted Borge de Prada et al. (2018) in the Dominican Republic.
In conclusion, it is important to emphasize to Dominican authorities that control of mosquito vector, environmental management and health education programmes would be unsuccessful unless public support for these measures are secured (Abe et al., 2005). The arrival of invasive species and their spread to urban environment seem unstoppable and thus, integration of new methods should be taken into consideration. In an attempt to reduce potential breeding sites for immature stages in cemeteries, this study suggests the importance of a community-based physical activity intervention, in which both local authorities (gardeners and cemetery keepers) and visitors get involved in the removal or treatment of disposable water-filled containers. Finally, this study also contributes to targeting mosquito breeding sites for vector control efforts highlighting environmental variables as strategic points to be considered by public health authorities to implement control strategies.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. A signed document has been filed in the journal archives












 uBio
uBio 

