Identifying priority areas for the pres- Mittermeier, van Dijk, Rhodin, & Nash, ervation of biodiversity is a focal objective 2015; Noss et al., 2015). One appealing stratof conservation planning (Myers, Mittermei- egy involves the management of wide-ranging er, Mittermeier, Da Fonseca, & Kent, 2000; ‘umbrella’ species, under the assumption that habitat preservation for species with large spatial requirements would provide simultaneous protection for co-occurring species (Frankel & Soulé, 1981; Roberge & Angelstam, 2004; Hurme, Monkkonen, Sippola, Ylinen, & Pentinsaari, 2008). The risk inherent to this approach is that umbrella species may be improperly selected, thereby limiting ecosystem-wide benefits.
At present, the effectiveness of the umbrella approach and benefit for co-occurring species (hereafter beneficiary species) is equivocal (Berger, 1997; Simberloff, 1998; Sergio et al., 2008; Caro, 2010). Empirical evidence of the utility of large carnivores as umbrella species is scarce because surprisingly few studies have appropriately evaluated their umbrella effectiveness (Noss, Quigley, Hornocker, Merrill, & Paquet, 1996; Caro, 2010). One notable exception concluded that jaguars (Panthera onca) were an effective umbrella species for cooccurring mammals in Latin America (Thornton et al., 2016).
Nuclear Central America (NCA) provides a unique opportunity to evaluate the effectiveness of a large carnivore as an umbrella species in a heterogeneous tropical landscape. Due to their large home ranges and presence in diverse habitats, areas designated for the conservation of jaguars would presumably meet the space requirements for beneficiary species besides mammals. Jaguars inhabit areas across a broad elevational gradient in NCA (Briones-Salas, Lavariega, & Lira-Torres, 2012; Castañeda, Pereira, & Solís, 2011; Sanderson et al., 2002) where a recent study, from Chiapas, Southern Mexico, estimated their home ranges at 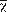 = 306.5 ± 169.81 km2 (de la Torre, Núñez, & Medellín, 2017). Jaguars usually favor lowlands but they are often detected in montane habitats, including recent records at 1 758 m in Chiapas (de la Torre, Rivero, Camacho, Álvarez-Márquez, & Medellín, in press) and 2 200 m in western Honduras (Castañeda, 2016).
= 306.5 ± 169.81 km2 (de la Torre, Núñez, & Medellín, 2017). Jaguars usually favor lowlands but they are often detected in montane habitats, including recent records at 1 758 m in Chiapas (de la Torre, Rivero, Camacho, Álvarez-Márquez, & Medellín, in press) and 2 200 m in western Honduras (Castañeda, 2016).
We selected NCA as our study area due to the region’s high endemism of amphibians and reptiles (Campbell, 1999; Townsend, 2014; Johnson, Wilson, Mata-Silva, García-Padilla, & DeSantis, 2017), classification as part of the Mesoamerican biodiversity hotspot (MBH) (Myers et al., 2000), and priority status for the range-wide jaguar network (Rabinowitz & Zeller, 2010). We were able to verify distributions mapped by the IUCN and compare international and regional status assessments of these threatened taxa in NCA due to our familiarity with the region’s amphibians and reptiles (Puschendorf, Castañeda, & McCranie, 2006; McCranie & Castañeda, 2007; GarcíaPadilla & Mata-Silva, 2014; Johnson et al., 2017). Also, we have systematically assessed jaguar presence in NCA since 2009 (Castañeda et al., 2011; Figel, 2011; Calderón-Quiñónez, 2013; Chávez, Zarza, de la Torre, Medellín, & Ceballos, 2016; Petracca et al., 2017; de la Torre et al., in press).
The jaguar network aims to preserve jaguar populations (categorized as jaguar conservation units - JCUs) and maintain connectivity in fragmented, human-use landscapes (Rabinowitz & Zeller, 2010; Olsoy et al., 2016). JCUs are defined as either 1) areas with a stable, diverse prey base and adequate habitat capable of maintaining at least 50 breeding jaguars; or 2) areas with fewer than 50 breeding jaguars but with sufficient habitat and prey to support the species if their populations increased under favorable conditions (Sanderson et al., 2002). The corridors connecting JCUs are intended to maintain connectivity and facilitate jaguar dispersal between suitable habitat patches (i.e. JCUs), increasing the likelihood of gene flow and maintenance of genetic diversity (Rabinowitz & Zeller, 2010). The Rabinowitz and Zeller (2010) analysis identified corridors connecting all JCUs range-wide with one notable exception: a gap was identified between the Sierra de las Minas JCU in southeast Guatemala and the Pico Bonito/Texiguat JCU in north-central Honduras. This transboundary disconnect highlights the urgency of conservation measures needed in NCA.
The objective of our study was to quantify co-occurrence of jaguars and sympatric herpetofauna (Amphibia, Reptilia) endemic to NCA. Endemic species (species unique to a defined geographic location, biome, or habitat type) often face greater threats due to their relatively small distributions, which are one of the strongest predictors of extinction risk (Purvis, Gittleman, Cowlishaw, & Mace, 2000; Sodhi et al., 2008). Substantiation of multi-taxa dependence on habitat under the ‘umbrella’ of the jaguar network would facilitate the identification of overlooked areas and strengthen justification for NCA as a global priority for conservation. Given the well-documented association between jaguar presence and surface water (Leopold, 1959; Zeller, Nijhawan, Salom-Pérez, Potosme, & Hines, 2011), we predicted greater overlap for amphibians than reptiles, due to amphibians’ greater dependence on water.
Materials and methods
Study site: As defined by Schuchert (1935), NCA comprises the mainland area between the Isthmus of Tehuantepec in Southern Mexico and the Nicaraguan Depression in Northern Nicaragua, excluding Belize and the Yucatan Peninsula (Fig. 1). Within this region, our study area spanned ~ 370 000 km² across four countries: Mexico, Guatemala, Honduras, and Nicaragua. Elevations ranged from 0-4 220 m.
NCA is a topographically and ecologically diverse region with biogeographic barriers (e.g. volcanoes, mountain ridges, valleys) recognized to influence species distributions and promote high endemism (Carr, 1950; Campbell, 1999; Townsend, 2014; Suárez-Atilano, Burbrink, & Vásquez-Domínguez, 2014). The Isthmus of Tehuantepec and Nicaraguan depression, which represent the Northern and Southern limits of our study area, respectively, are widely recognized as geographical barriers restricting gene flow (Hardy, González-Cózatl, Arellano, & Rogers, 2013; Pérez-Consuegra & Vásquez-Domínguez, 2015).
Ground-truthing the Jaguar Conservation Network: Our umbrella analysis of the NCA jaguar network included field-validating (ground-truthing) portions of putative jaguar corridors in Guatemala, Honduras and Chiapas, Mexico. In these corridors, we conducted camera-trap surveys and systematic interviews with people living or working in areas believed to be occupied by jaguars (Castañeda et al., 2011; Figel, 2011; Calderón-Quiñónez, 2013; Chávez et al., 2016; Petracca et al., 2017; de la Torre et al., in press). A primary objective of the ground-truthing was to estimate the probability of jaguar presence in 36 km² sampling units (sensuZeller et al., 2011), or to verify jaguar presence within specific areas (de la Torre et al., in press). For Nicaragua, we only included the Rio Platano-Bosawas JCU in the analysis because all corridors in this country are located south of our study area.
Evaluating jaguars as umbrella species: To evaluate the umbrella effectiveness of the jaguar in NCA, we measured the extent of overlap in three networks, each ~ 65 900 km² in spatial extent: the Rabinowitz and Zeller (2010) modeled jaguar network, the ground-truthed jaguar network, and a randomly selected portion of the Mesoamerican Biological Corridor (MBC). The ground-truthed jaguar network refers to our field-based assessment of jaguar presence in NCA (Castañeda et al., 2011; Figel, 2011; Calderón-Quiñónez, 2013; Chávez et al., 2016; Petracca et al., 2017; de la Torre et al., in press). To generate the random network, we randomly selected portions of the MBC until its total area equaled the spatial extent of the JCUs and corridors. We included the Rabinowitz and Zeller (2010) network to demonstrate the importance of ground-truthing modeled corridors and we included the MBC under the assumption that an ecologicallybased analysis would be more informative from a management perspective.
We restricted our analysis to endemic herpetofauna (rather than including Mammalia or other classes) because taxonomic similarity may positively influence conclusions of umbrella effectiveness (Fleishman, Blair, & Murphy, 2001; Hurme et al., 2008; Branton & Richardson, 2011). Also, NCA is a global hotspot for population declines of amphibians, which are the most threatened class of vertebrates worldwide (Stuart et al., 2008; Hoffmann et al., 2010). For reptiles, NCA contains a greater density of threatened species than any other region in the Western Hemisphere (Tingley, Meiri, & Chapple, 2016).
To estimate the umbrella effectiveness of jaguars, we downloaded species distribution vector polygons (shapefiles) from the IUCN Red List of Threatened Species website (IUCN, 2017) and imported them into GoogleEarth Pro © as raster images. We excluded all historical distributions, polygons where the species’ presence is uncertain, and polygons comprised of < 5 presence points. We then overlapped the Rabinowitz and Zeller (2010) modeled jaguar network, the ground-truthed jaguar network, and the random MBC network with the shapefiles of herpetofauna distributions, and we calculated the area of the distribution of each species that overlapped each network (Fig. 2).
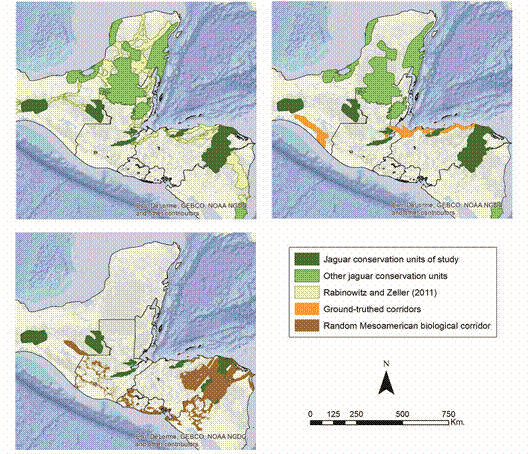
Fig. 2 The networks used for our overlap analysis on jaguars and endemic herpetofauna in Nuclear Central America.
The polygons are generally “extent of occurrence” (EOO) maps because the IUCN considers these distributions to represent a species’ EOO, which they define as “the area contained within the shortest continuous imaginary boundary which can be drawn to encompass all the known, inferred or projected sites of present occurrence of a taxon, excluding cases of vagrancy” (IUCN, 2012). EOO is measured by a minimum convex polygon (“the smallest polygon in which no internal angle exceeds 180 degrees and which contains all the sites of occurrence” (IUCN, 2012). Thus, EOO maps represent distribution boundaries, not occupancy.
We defined a regional endemic as any species with > 50 % of its EOO inside our predetermined NCA study area. Any habitat for species that fell outside the boundaries of our NCA study area was not included in our estimates of umbrella overlap. For species with > 50 % of their EOO inside the NCA, we excluded all portions of the EOO in the Yucatan Peninsula, Southern Nicaragua, or west of the Isthmus of Tehuantepec. Thus, our umbrella analysis was restricted to the region between the Isthmus of Tehuantepec in Southern Mexico and the Nicaraguan Depression, excluding Belize and the Yucatan Peninsula.
For each sampled network, we estimated 1) total numbers of herpetofauna species’ EOOs overlapped; 2) overlap for species classified by their IUCN risk status (Critically Endangered, Endangered, Vulnerable, Near Threatened, Data Deficient, Least Concern); 3) JCUs and corridors containing the greatest proportions of overlap; and 4) the proportion of the species’ EOO overlapped by the network. For species with EOOs that extended beyond our study area (i.e. west of the Isthmus of Tehuantepec), we only included the percentage of its EOO in our NCA study area. We applied one-way ANOVA to test statistical differences between the ground-truthed and random networks overlapping the EOOs of the most threatened (i.e. Critically Endangered and Endangered) amphibians and reptiles.
Results
Umbrella results: Our analysis included 304 regional endemics - 173 amphibians and 131 reptiles. The IUCN classified 105 (60.7 %) of these amphibians and 34 (26 %) of these reptiles as Critically Endangered or Endangered. Species overlap, numbers of species with 100 % overlap, and the average proportion of species’ EOOs were greatest in the ground-truthed network (Table 1). The ground-truthed network had significantly higher coverage for Critically Endangered and Endangered amphibians than the randomly generated MBC network (one-way ANOVA, F 1,208 = 20.82, P < 0.001). Between the two networks, results did not differ significantly for Critically Endangered and Endangered reptiles (one-way ANOVA, F 1,66 = 2.25, P = 0.14). Reptiles were less represented than amphibians in terms of number of species overlapped despite having, on average, EOOs 4 times larger.
Table 1 Summaries of reptile and amphibian distributions overlapped by each network
| Amphibians (n = 173) | Ground-truthed | Rabinowitz/Zeller | Random |
|---|---|---|---|
| Species overlap (% of total) | 104 (60.1) | 96 (55.5) | 79 (45.7) |
| # of species with 100 % overlap (% of total) | 19 (11) | 14 (8.1) | 4 (2.3) |
| Avg. proportion of species’ EOO inside network | 29.6 | 26.1 | 12.04 |
| # of CR species overlapping network (% of total) | 30 (17.3) | 30 (17.3) | 22 (12.7) |
| # of EN species overlapping network (% of total) | 30 (17.3) | 28 (16.2) | 20 (11.6) |
| Reptiles (n = 131) | Ground-truthed | Rabinowitz/Zeller | Random |
| Species overlap (% of total) | 83 (63.3) | 74 (56.5) | 70 (53.4) |
| # of species with 100 % overlap | 14 (10.7) | 11 (8.4) | 8 (6.1) |
| Avg. proportion of species’ EOO inside network | 24.4 | 21.1 | 17.7 |
| # of CR species overlapping network (% of total) | 6 (4.6) | 6 (4.6) | 5 (3.8) |
| # of EN species overlapping network (% of total) | 9 (6.9) | 9 (6.9) | 10 (7.6) |
Among amphibians, the greatest benefit was observed for Craugastoridae (n = 48 species) and Hylidae (n = 42). Jaguars served as a less effective umbrella for amphibian families Bufonidae (n = 14) and Plethodontidae (n = 60) (Fig. 3). 19 amphibians, including 11 critically endangered species (Bolitoglossa diaphora, Craugastor cruzi, Craugastor fecundus, Craugastor trachydermus, Isthmohyla insolita, Ixalotriton parvus, Oedipina tomasi, Plectrohyla chrysopleura, Plectrohyla exquisite, Plectrohyla lacertosa and Ptychohyla sanctaecrucis), five endangered species (Craugastor montanus, Exerodonta chimalapa, Oedipina gephyra, Plectrohyla lacertosa and Ptychohyla spinipollex), and two vulnerable species (Dendrotriton megarhinus, Fig. 4, and Dendrotriton xolocalcae) occur exclusively within the groundtruthed jaguar network.
Fig. 3 Boxplots of the proportion of the extent of occurrences of endemic amphibians (white boxes) and reptiles (shaded boxes) overlapped by the ground-truthed jaguar network in Nuclear Central America. Boxplots display the median (vertical black lines), upper and lower quartiles (open boxes), and variability outside those quartiles (whiskers).
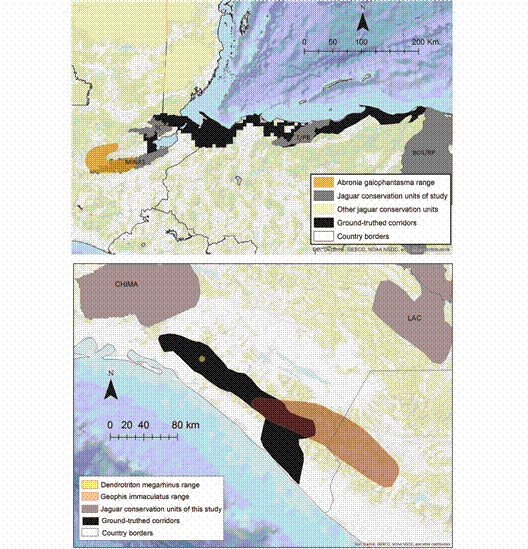
Fig. 4 The Long-Nosed Bromeliad Salamander (Dendrotriton megarhinus) occurs exclusively within the ground-truthed jaguar corridor in Chiapas. Also shown are examples of overlap between JCUs/corridors and the distributions (EOOs) of the Brilliant Arboreal Alligator Lizard (Abronia gaiophantasma) and Downs’ Earth Snake (Geophis immaculatus).
SC = Sierra de Santa Cruz, T/PB = Texiguat/Pico Bonito, MINAS = Sierra de las Minas, BOS/RP = Bosawas/Rio Platano.For reptiles, Dipsadidae (n = 39) and Dactyloidae (n = 14) were the beneficiary families with the greatest average overlap. The least overlap was observed for Anguidae (n = 21) and Colubridae (n = 14). 15 reptiles, including two critically endangered species (Bothriechis guifarroi and Rhadinella tolpanorum), three endangered species (Lepidophyma lipetzi, Norops amplisquamosus and Norops cusuco), and three vulnerable species (Geophis nephodrymus, Omoadiphas aurula, and Rhadinella pegosalyta) occur exclusively within the ground-truthed NCA jaguar network.
The JCUs with the greatest overlap of endemic amphibian and reptile distributions in the NCA jaguar network were the Chimalapas (n = 59) in Southern Mexico and the Sierra de las Minas in Guatemala (n = 46). Per unit area, however, the greatest overlap of endemic distributions was found in the Sierra Santa Cruz (Table 2). Among corridors, the Sierra Madre de Chiapas contained the greatest number of endemic amphibians and reptiles (n = 33). Accounting for area, the 551 km² corridor connecting the Sierra de las Minas and Santa Cruz in eastern Guatemala had the highest endemism (Table 3).
Table 2 Area, protected status, deforestation rate and endemic herpetofauna overlap in Nuclear Central American JCUs
| JCU | Country | Area (km²) | Area of JCU protected (%) | Annual deforest. rateǂ | Endemic amphibian overlap | Endemic reptile overlap | Total herp. overlap in JCUs | EOOs of endemic herps/100 km² |
|---|---|---|---|---|---|---|---|---|
| SC | GT | 1 063 | 0 (0)* | > 10 | 21 | 13 | 34 | 3.20 |
| T/PB | HN | 1 715 | 1 715 (100) | 1.5-2.5 | 23 | 18 | 41 | 2.39 |
| MINAS | GT | 2 085 | 1 687 (80.9) | 1.5-2.5 | 24 | 22 | 46 | 2.21 |
| CHIMA | MEX | 10 777 | 1 907 (17.7) | 2.5-5 | 27 | 32 | 59 | 0.55 |
| LAC | MEX | 7 324 | 5 127 (70) | 5-10 | 11 | 11 | 22 | 0.30 |
| BOS/RP | HN/NIC | 25 210 | 23 496 (93.2) | 5-10 | 9 | 11 | 20 | 0.08 |
| TOTAL | - | 48 174 | 33 932 | - | 115 | 107 | 222 | - |
SC = Sierra de Santa Cruz, T/PB = Texiguat/Pico Bonito, MINAS = Sierra de las Minas, CHIMA = Chimalapas, LAC = Lacandona, BOS/RP = Bosawas/Rio Platano. ǂ From Olsoy et al. 2016, supplementary data .
*In 2018, funding was secured for protected area establishment in the Sierra de Santa Cruz (World Land Trust, 2018).
Table 3 Area, protected status, deforestation rate and endemic herpetofauna overlap in Nuclear Central American corridors
| Corridor* | Area (km²) | Area of corridor protected (%) | Annual deforest. rateǂ | Endemic amphibian overlap | Endemic reptile overlap | Total herp. overlap | EOOs of endemic herps/100 km² |
|---|---|---|---|---|---|---|---|
| IZ_GT | 551 | 156 (28.3) | 5 - 10 | 9 | 4 | 13 | 2.36 |
| E_GT | 3 007 | 1 398 (46.5) | > 10 | 15 | 5 | 20 | 1.43 |
| SM_CHP | 7 598 | 2 531 (33.3) | 2.5 - 5 | 20 | 13 | 33 | 1.30 |
| W_HN | 3 392 | 1 108 (32.7) | 1.5 - 2.5 | 22 | 9 | 31 | 0.91 |
| E_HN | 3 150 | 508 (16.1) | 1.5 - 2.5 | 7 | 1 | 8 | 0.25 |
| Total | 17 698 | 5 701 | - | 73 | 32 | 105 | - |
*IZ_GT = Guatemala corridor on western edge of Lake Izabel, Guatemala, W_HN = Western Honduras corridor, E_HN = Eastern Honduras corridor, E_GT = Eastern Guatemala corridor, SM_CHP = Sierra Madre de Chiapas corridor.
ǂ From Olsoy et al. 2016, supplementary data.
Discussion
Our analysis represents the first multitaxon evaluation of the jaguar’s umbrella value. We demonstrate the utility of a single-species conservation strategy to serve as a reasonably effective umbrella for co-occurring reptiles, and as a highly effective umbrella for threatened amphibians. NCA is the epicenter for species richness of threatened reptiles (Tingley et al., 2016; Johnson et al., 2017) but amphibian distributions more commonly overlapped habitat in the ground-truthed jaguar network.
The jaguar’s imperfect efficacy as an umbrella for herpetofauna in NCA is not surprising. No single species can be expected to serve as an effective umbrella for all other species in the community, much less across a broad regional landscape (Noss et al., 1996; Caro, 2010). Ideally, suites of focal species would be selected (i.e. jaguar, mountain lion Puma concolor, tapir Tapirus bairdii, whitelipped peccary Tayassu pecari) each representing different habitat types and grouped according to the dominant threats to their viability. Each species in such an umbrella suite can be used to determine the “spatial and compositional attributes that must be present in a landscape and their appropriate management regime” (Lambeck, 1997).
Nonetheless, given the immense areas necessary to maintain viable populations of jaguars and most large carnivores, they are considered among the most effective single-species umbrellas (Carroll, Noss, & Paquet, 2001; Caro, 2010). In the central coast of California, for example, a reserve network with core areas and corridors designed for mountain lions captured 77 % of serpentine soils harboring rare and endemic plants, 88 % of old-growth coast redwood (Sequoia sempervirens) forest, most oak woodlands, and 79 % percent of watersheds with extant populations of steelhead trout (Oncorhynchus mykiss) (Thorne, Cameron, & Quinn, 2006). For this reason, as well as for their assumed or demonstrated importance as keystone species, ecologists interested in protecting entire biotic communities have long placed emphasis on carnivore conservation (e.g. Shelford, 1933; Leopold, 1949; Terborgh et al., 1999).
Additionally, this study illustrates the importance of incorporating data from national scientists to improve regional conservation and management strategies for jaguars. The ground-truthed corridors used in our analysis represent the most current information about jaguar use of corridors within the NCA jaguar network. For instance, de la Torre et al. (in press) demonstrate how the Sierra Madre de Chiapas corridor and the mountain ranges in neighbouring areas between the Mexico and Guatemala border likely have greater potential to link the Chimalapas region with the Lacandona region than previously proposed corridors (i.e. Rabinowitz & Zeller, 2010). Similarly, the western Honduras corridors spanning the north coast are more likely to link JCUs between Guatemala and Honduras (Castañeda et al., 2011).
The Guatemala-Honduras transboundary segment of the NCA network is one of the most critical linkages of the range-wide jaguar network because it comprises part of a highly threatened segment of the ~ 525 km distance between the two largest JCUs in Mesoamerica: the trans-national Maya Forest JCU spanning the Mexico-Guatemala-Belize border and the Rio Platano-Bosawas JCU along the HondurasNicaragua border (Sanderson et al., 2002). The urgency of conservation measures in this region is increasing because JCUs in Guatemala and Honduras experienced the greatest rates of habitat loss among Mesoamerican countries from 2000-2012 (Olsoy et al., 2016). In Guatemala, the Sierra de Santa Cruz experienced the greatest extent of habitat loss among JCUs range-wide, losing 11.37 % of its forest cover from 2000-2012 (Olsoy et al., 2016). Consequently, only one-third of the Honduras-Guatemala transboundary connection is believed to support jaguar movement (Calderón-Quiñónez, 2013; Wultsch et al., 2016).
From 2001 to 2010, deforestation in the moist forest biome - which comprises ~ 78 % of the ground-truthed jaguar network in NCA (Table 4) - mainly occurred along the Caribbean slopes of Nicaragua (8 574 km2), Guatemala (4 816 km2), and Honduras (263 km2) (Redo, Grau, Aide, & Clark, 2012). Despite the extensive deforestation, JCUs still represent the largest intact habitat for amphibians and reptiles, both of which are threatened by habitat loss more than any other stressor (Sodhi et al., 2008; Johnson et al., 2017).
Table 4 Ecoregion coverage of the ground-truthed jaguar network in Nuclear Central America
| Ecoregion | km² | % of NCA jaguar network |
|---|---|---|
| Central American Atlantic Moist Forests | 31 757 | 52 |
| Peten-Veracruz Moist Forests | 15 628 | 25.7 |
| Central American Pine-Oak Forest | 3 206 | 5.3 |
| Central American Montane Forest | 1 820 | 3 |
| Miskito Pine Forest | 1 727 | 2.8 |
| Chiapas Pine Forest | 1 608 | 2.6 |
| Chimalapas Montane Forest | 1 583 | 2.6 |
| Mesoamerican Gulf-Caribbean Mangrove | 1 518 | 2.5 |
| Pantanos de Centla | 668 | 1.1 |
| Sierra Madre de Oaxaca Pine-Oak Forest | 516 | 0.85 |
| Southern Pacific Dry Forest | 337 | 0.55 |
| Motagua Valley Thornscrub | 269 | 0.44 |
| Chiapas Depression Dry Forest | 183 | 0.3 |
| Central American Dry Forest | 93 | 0.15 |
Our findings highlight the need to prioritize research and conservation in several key areas for both jaguars and herpetofauna. The Sierra de las Minas and Sierra de Santa Cruz in Guatemala and Chimalapas region in southeastern Mexico, in particular, are crucially important JCUs and both contained the highest species richness of endemic amphibians and reptiles in NCA. At the time of our study, the Sierra de Santa Cruz was the only unprotected JCU in NCA. However, international efforts led by the Rainforest Trust and World Land Trust have secured funding to protect part of the region (Rainforest Trust, 2014; World Land Trust, 2018).
Beyond NCA, other areas with high endemism - such as the western Sierra Madre de Mexico and Tropical Andes - should be prioritized for more in-depth analyses of the jaguar’s umbrella value. We recognize that inadequate data on rare or infrequently detected species can limit inferences about occurrence and bias assessments of conservation status in NCA and elsewhere (Ficetola et al., 2014). Nonetheless, the IUCN maps often represent the best available data and management decisions should include all species, not only datasets on the best known or most easily detected species (Zipkin, Royle, Dawson, & Bates, 2010). The jaguar’s umbrella value could increase as more corridors are ground-truthed and distributions of rare and cryptic amphibians and reptiles are updated.
Ethical statement: authors declare that they all agree with this publication and made significant contributions; that there is no conflict of interest of any kind; and that we followed all pertinent ethical and legal procedures and requirements. A signed document has been filed in the journal archives.












 uBio
uBio