Galls are structures produced by plants in response to the activity of several types of organisms, such as nematodes, mites, bacteria, fungi and mainly insects (Mani, 1964; Shorthouse, Wool, & Raman, 2005). Among the latter, the orders Diptera and Hymenoptera contain the highest number of gall-inducing species (Espírito-Santo & Fernandes, 2007; Mani, 1964).
Plant galls arise mostly by hypertrophy (overgrowth) and hyperplasia (overproliferation) of vegetal cells, and usually by the formation of tissues that are absent in ungalled host plants (Mani, 1964; Raman, 2011). Galls display great complexity and an incredible variety of forms, allowing insects to take nutrients and shelter simultaneously (Shorthouse et al., 2005). All plant organs are susceptible to gall induction by insects, the leaves being the most frequently attacked. This pattern has arisen in different localities and vegetation types at a global scale (Felt, 1940; Mani, 1964; Shorthouse & Rohfritsch, 1992; Blanche & Ludwig, 2001; Nieves-Aldrey, Ibáñez, & Medianero, 2008; Kuzmanich, Altamirano, & Salvo, 2015; Mendonça & Stiling, 2017). A few studies have reported stems as the most affected organs (Veldtman & McGeoch, 2003; Carneiro, Borges, Araújo, & Fernandes, 2009; Coelho, Carneiro, Branco, Borges, & Fernandes, 2013; Fernandes et al., 2002; Toma & Mendonça, 2013). The resultant galls have been classified according to their shapes, the organs they affect and other features, in a high number of morphological types (Isaias, Carneiro, Oliveira, & Santos, 2013; Arriola, Melo, & Isaias, 2015).
Some local and regional patterns have been observed in the distribution of insect galls on host plant families and galled plant organs. Regarding the taxonomy of the host plant, some local features of vegetation such as species composition, plant density and richness, together with historical factors, such as the number of species of each plant family occurring in the region, may have contributed to the richness and radiation patterns of the galling insects observed (Gonçalves-Alvim & Fernandes, 2001; Araújo, Scareli-Santos, Guilherme, & Cuevas-Reyes, 2013; Araújo, 2017; Bergamini, Bergamini, Santos, & Araújo, 2017; Mendonça & Stiling, 2017). Thus, in different regions of the world, different families of plants have been mentioned as the most attacked by gall inducing insect species. For example in North America and Europe, the dominant family of galled plants is Fagaceae (Mani, 1964), while in South America, the most attacked plant families are Asteraceae and Fabaceae (Fernandes & Santos, 2014; Mani, 1964).
In the Southern part of the Neotropical region, knowledge of insect gall communities is rather poor. In Argentina, most of the taxonomic studies were performed in the early twentieth century (Kieffer & Jörgensen, 1910; Tavares, 1915; Brèthes, 1916; Jörgensen, 1917; Houard, 1933). Nonetheless, the number of studies dealing with taxonomic and ecological aspects of galling insects in Argentina is growing (Fernandes et al., 2002; Carabajal De Belluomini, Castresana, Salim, & Notario, 2009; Martinez, Altamirano, & Salvo, 2011; Quintero, Garibaldi, Grez, Polidori, & Nieves-Aldrey, 2014; Kuzmanich et al., 2015; Malcom, Oggero, Arana, Tordable, & Boito, 2015; Altamirano, Valladares, Kuzmanich, & Salvo, 2016). In spite of these advances, the information is still fragmented and incomplete. In this context, the goals of the present study were to advance the inventory of galls induced by insects on plants in Córdoba (central Argentina) and to analyze some taxonomic and ecological aspects of these plant-insect interactions. We particularly focused on the frequency of plant-insect taxonomic associations and on the plant organs most commonly attacked by gall-inducing insects, using information from field surveys and a literature review.
Materials and methods
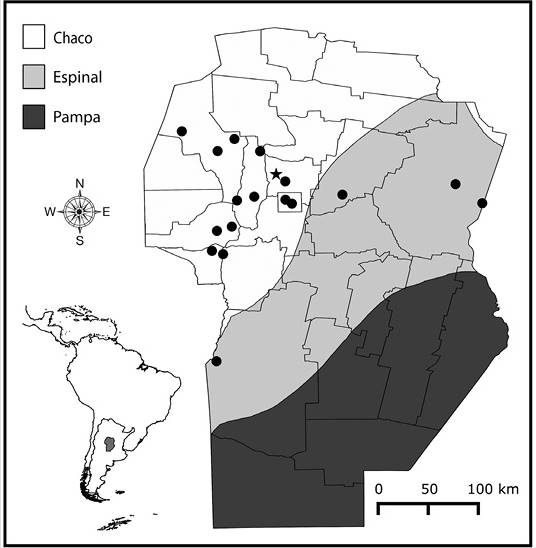
Study Area: The study area includes the province of Córdoba (Argentina), (31˚ 25’ S & 64˚ 10’ W), which covers ca. 161 000 km2 and comprises three major biogeographical units. The Pampa, located in the Southeast extreme, is dominated by grasslands, whereas the Espinal and Chaco biogeographical provinces cover most of the province and are dominated by seasonally dry forests (Cabrera, 1976). The Pampa grasslands, the Espinal forests and the Eastern lowland area of Chaco are now reduced to small and isolated patches increasingly surrounded by a matrix of soybean, while the Chaco in the Western lowland area of Córdoba is, in general, covered by closed and open forests and shrublands in different successional stages (Hoyos et al., 2013; Cabido et al., 2018). Finally, the mountain region of Chaco (commonly named as Chaco Serrano), is composed of a complex matrix of native open and closed woodlands, shrublands and grasslands, including also monospecific stands of exotic woody species (Cingolani, Renison, Zak, & Cabido, 2004; Giorgis et al., 2017). This area constitutes the main reservoir of biodiversity in Córdoba (Zak & Cabido, 2002).
A highly seasonal subtropical climate with important variation along the latitudinal and altitudinal gradient characterize the studied area (see Tecco et al. 2016 and Cabido et al. 2018 for more details). Precipitation is coupled with temperature to define the growing and flowering season, which mainly occur between October and April (Giorgis, Cingolani, Teich, & Renison, 2010; Giorgis, Cingolani, Gurvich, & Astegiano 2015).
Field surveys and literature review: Two types of field surveys were performed: i) systematic surveys restricted to a region of Chaco Serrano, located approximately 35 km North of Córdoba City (31º 07’ S & 64º 23’ W), which contains 26 sites that were visited five times each in two consecutive growing seasons (October to April, 2014-2016). In each site and sampling date, all plants in plots of 20 x 20 m were carefully observed to detect gall occurrence; and ii) complementary surveys, which consisted in just one or two visits to 17 sites in the Espinal and Chaco provinces (Figure 1). In each site and date, the vegetation was searched for insect galls for approximately one hour (Price et al., 1998), totalizing a number of 109 sampling hours. In both systematic and complementary surveys, galls were collected and taken to the laboratory in order to rear adult insects. Part of the material (insects and galls) was kept in alcohol 70 % for dissection and posterior conservation. Voucher specimens of gall morphotypes obtained by field surveys were deposited at the Entomological Collection of the National University of Córdoba.
The identification of the galling insects was done to the lowest possible taxonomic level through identification keys (White & Hodkinson, 1985; Stehr, 1987; Burckhardt & Basset, 2000; Nieves-Aldrey & Blas, 2015) and in comparison with reference material, using compound (Olympus CX31) and stereoscopic (Zeiss, Stemi dV4) microscopes. Gall morphotypes were classified in one of the following categories: clavate, conical, cylindrical, fusiform, globoid, lenticular, rosette, bivalveshaped, hornshaped, leaf fold, marginal roll and pocket shaped, according to Isaias et al. (2013). Other categories were proposed when necessary. A comprehensive literature search of electronic databases (Scopus, Scielo and Google Scholar) was conducted, searching all published papers containing simultaneously the phrases “insect gall” and “Córdoba, Argentina”, in the period from 1980 to 2017. Additionally, the largest entomological libraries of the country (located at La Plata and Bernardino Rivadavia Museums and Darwinion Botanic Institute) were visited to get access to unavailable digital resources, particularly those published at the beginning of the century. Species names were updated following several taxonomic databases available on the internet (http://www.floraargentina.edu.ar, https://www.hemiptera-databases.org/psyllist/, http://eol.org/).
Results
In the Córdoba province, a total of 99 gall morphotypes on 58 plants species belonging to 21 families and 44 genera were recorded through both field surveys and a literature review (Table 1). From all gall records, 55 % were registered for the first time in Cordoba province and 49 % constitute new records for Argentina (Table 1). Sixteen of the morphotypes registered through field surveys were previously mentioned in the literature for the province, whereas 28 % of the interactions were recorded as literature citations, not corroborated by field observations. A total of 18 published studies provided information useful to the inventory.
Table 1: Insect galls from central Argentina
| Host plant | Host plant family | Inducer insect | Organ | Gall shape 1 | Sources 2 |
|---|---|---|---|---|---|
| Dicliptera squarrosa Nees | Acanthaceae | Diptera, Cecidomyiidae | Stem | Globoid | * |
| Iresine diffusa Humb. & Bonpl. ex Willd. | Amaranthaceae | Diptera, Cecidomyiidae | Stem | Globoid | * |
| Lithraea molleoides (Vell.) Engl. | Anacardiaceae | Hemiptera, Calophya clavuligera Burckhardt & Basset | Leaf | Lenticular | 4X |
| Schinus fasciculata (Griseb.) I. M. Johnst. | Anacardiaceae | Hemiptera, Tainarys sordida Burckhardt | Leaf | Marginal roll | 4; 14$ |
| Schinus fasciculata (Griseb.) I. M. Johnst. | Anacardiaceae | Calophya catillicola Burckhardt & Basset | Leaf | 4; 14$ | |
| Schinus fasciculata (Griseb.) I. M. Johnst. | Anacardiaceae | Calophya duvauae (Scott) Burckhardt | Leaf | Conical | 14 X |
| Schinus fasciculata (Griseb.) I. M. Johnst. | Anacardiaceae | Lepidoptera, Cecidoses eremita Curt. | Stem | Globoid | 3; 10; 11; 12; 14; 17$ |
| Schinus fasciculata (Griseb.) I. M. Johnst. | Anacardiaceae | Lepidoptera, Dicranoses congregatella Brèthes | Stem | Cylindrical | 14; 17$ |
| Schinus fasciculata (Griseb.) I. M. Johnst. | Anacardiaceae | Unknown | Bud | Cylindrical | * |
| Schinus fasciculata (Griseb.) I. M. Johnst. | Anacardiaceae | Unknown | Stem | Amorphous | * |
| Aspidosperma quebracho-blanco Schltdl. | Apocynaceae | Diptera, Anasphodiplosis aspidospermae (Blanch.) | Bud | Cylindrical | 2; 9; 10; 17 X |
| Ambrosia elatior L. | Asteraceae | “Prob. Aphididae” | Leaf | Marginal roll | 10; 17$ |
| Angelphytum aspilioides (Griseb.) H. Rob. | Asteraceae | Diptera, Cecidomyiidae | Stem | Fusiform | * |
| Baccharis aliena (Spreng.) Joch.Müll. | Asteraceae | Lepidoptera | Stem | Fusiform | 10; 17 X |
| Baccharis articulata (Lam.) Pers. | Asteraceae | Unknown | Stem | Fusiform | * |
| Baccharis coridifolia DC. | Asteraceae | Diptera, Baccharomyia cordobensis (Kieff. & Jörgen.) | Stem | Fusiform | 9; 10; 11; 12 X |
| Baccharis coridifolia DC. | Asteraceae | Diptera, Cecidomyiidae | Stem | Fusiform | * |
| Baccharis flabellata Hook. & Arn. | Asteraceae | Unknown | Stem | Globoid to fusiform # | * |
| Baccharis pingraea DC. | Asteraceae | Unknown | Leaf | Lenticular | * |
| Baccharis rufescens Spreng. | Asteraceae | Diptera, Tephritidae | Stem | Fusiform | * |
| Baccharis rufescens Spreng. | Asteraceae | Lepidoptera | Stem | Fusiform | * |
| Baccharis rufescens Spreng. | Asteraceae | Unknown | Stem | Globoid | * |
| Baccharis rufescens Spreng. | Asteraceae | Unknown | Stem | Rosette | * |
| Baccharis salicifolia (Ruiz & Pav.) Pers. | Asteraceae | Baccharomyia ornaticornis (Kieff. & Jörgen.) | Stem | Fusiform | 9; 10; 11; 12; 17$ |
| Baccharis salicifolia (Ruiz & Pav.) Pers. | Asteraceae | Diptera, Geraldesia sp. | Leaf | Fusiform | 8 X |
| Baccharis salicifolia (Ruiz & Pav.) Pers. | Asteraceae | Diptera, Rhoasphondylia crassipalpis (Kieff. & Jörgen.) | Stem | Globoid | 9; 10; 11; 12; 17 X |
| Baccharis salicifolia (Ruiz & Pav.) Pers. | Asteraceae | Hemiptera, Trioza cf. steinbachi (Com. Pers. Burckhardt D.) | Leaf | Marginal roll | 10; 13; 17 X |
| Baccharis salicifolia (Ruiz & Pav.) Pers. | Asteraceae | Unknown | Stem | Globoid | * |
| Baccharis salicifolia (Ruiz & Pav.) Pers. | Asteraceae | Unknown | Stem | Rosette | * |
| Chromolaena arnottiana (Griseb.) R.M. King & H. Rob. | Asteraceae | Diptera, Cecidochares sp. | Stem | Cylindrical | * |
| Conyza sumatrensis (Retz.) E. Walker | Asteraceae | Diptera, Cecidomyiidae | Stem | Fusiform | * |
| Gnaphalium cabrerae S. E. Freire | Asteraceae | Unknown | Stem | Globoid | * |
| Porophyllum ruderale (Jacq.) Cass. | Asteraceae | Diptera, Cecidomyiidae | Stem | Globoid | * |
| Pseudognaphalium cheiranthifolium (Lam.) Hilliard & B.L. Burtt | Asteraceae | Diptera, Tephritidae | Bud | Fusiform | 10; 17$ |
| Senecio pampeanus Cabrera | Asteraceae | Unknown | Stem | Globoid | * |
| Vernonia mollissima D. Don ex Hook. et Arn. | Asteraceae | Diptera, Cecidomyiidae | Stem | Fusiform | * |
| Zexmenia buphtalmiflora (Lorentz) Ariza | Asteraceae | Unknown | Stem | Globoid | * |
| Zexmenia buphtalmiflora (Lorentz) Ariza | Asteraceae | Unknown | Bud | Globoid | * |
| Berberis ruscifolia Lam. | Berberidaceae | Hemiptera, Psylloidea | Leaf | Globoid | 10; 17 X |
| Wahlenbergia linarioides (Lam.) A. DC. | Campanulaceae | Diptera, Cecidomyiidae | Stem | Globoid to fusiform # | * |
| Celtis ehrenbergiana (Klotzch) Liebm. | Celtidaceae | Diptera, Cecidomyiidae | Stem | Globoid | *; 7 X |
| Celtis ehrenbergiana (Klotzch) Liebm. | Celtidaceae | Diptera, Cecidomyiidae | Leaf | Globoid | * |
| Celtis ehrenbergiana (Klotzch) Liebm. | Celtidaceae | Diptera, Cecidomyiidae | Stem | Fusiform | *; 7 X |
| Croton argentinus Müll. Arg. | Euphorbiaceae | Diptera, Cecidomyiidae | Leaf | Fusiform | 10; 17$ |
| Croton lachnostachyus Baill | Euphorbiaceae | Unknown | Leaf | * | |
| Tragia dodecandra Griseb. | Euphorbiaceae | Diptera, Cecidomyiidae | Bud | N/A | 10; 17$ |
| Acacia aromo Gillies ex Hook. & Arn. | Fabaceae | Hymenoptera, Eschatocerus acaciae Mayr. | Stem | Globoid | 6; 16$ |
| Acacia aromo Gillies ex Hook. & Arn. | Fabaceae | Unknown | Thorn | Fusiform | * |
| Acacia caven (Molina) Molina | Fabaceae | Eschatocerus acaciae Mayr. | Stem | Globoid | 6; 16$ |
| Acacia caven (Molina) Molina | Fabaceae | Diptera, Cecidomyiidae | Leaf | Globoid | * |
| Acacia caven (Molina) Molina | Fabaceae | Unknown | Thorn | Fusiform | * |
| Acacia caven (Molina) Molina | Fabaceae | Unknown | Stem | Fusiform | * |
| Acacia caven (Molina) Molina | Fabaceae | Unknown | Stem | Globoid | *, 13 X |
| Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart | Fabaceae | Diptera, Allodiplosis crassa Kieff. & Jörgen. | Bud | Globoid | 9; 10; 12; 17 X |
| Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart | Fabaceae | Diptera, Cecidomyiidae | Stem | Globoid | 10; 17$ |
| Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart | Fabaceae | Diptera, Cecidomyiidae | Bud | Globoid | 10; 17$ |
| Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart | Fabaceae | Lepidoptera | Stem | Fusiform | 10; 17$ |
| Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart | Fabaceae | Lepidoptera | Stem | Fusiform | 10; 17$ |
| Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart | Fabaceae | Unknown | Stem | Globoid | * |
| Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart | Fabaceae | Unknown | Stem | Fusiform | 10; 17$ |
| Prosopis alba Griseb. | Fabaceae | Eschatocerus acaciae Mayr. | Stem | Globoid | 5; 16 X |
| Prosopis alba Griseb. | Fabaceae | Diptera, Cecidomyiidae | Leaf | Globoid | *; 5X |
| Prosopis alba Griseb. | Fabaceae | Diptera, Cecidomyiidae | Stem | Globoid | * |
| Prosopis alba Griseb. | Fabaceae | Diptera, Cecidomyiidae | Stem | Fusiform | *; 5 X |
| Prosopis alba Griseb. | Fabaceae | Unknown | Petiole | Fusiform | * |
| Prosopis alba Griseb. | Fabaceae | Unknown | Bud | Globoid | * |
| Prosopis chilensis (Mol.) Stuntz. | Fabaceae | Eschatocerus acaciae Mayr. | Stem | Globoid | 6; 16$ |
| Prosopis nigra (Griseb) Hieron. | Fabaceae | Eschatocerus acaciae Mayr. | Stem | Globoid | 6; 16$ |
| Lepechinia floribunda (Benth.) Epling | Lamiaceae | Unknown | Stem | Fusiform | * |
| Minthostachys verticillata (Griseb.) Epling | Lamiaceae | Diptera, Cecidomyiidae | Stem and Petiole | Globoid | 18 X |
| Heimia salicifolia (Kunth) Link | Lythraceae | Unknown | Stem | Fusiform | * |
| Nassella neesiana (Trin. & Rupr.) Barkworth | Poaceae | Unknown | Stem | Cylindrical | * |
| Monnina dictyocarpa Griseb. | Polygonaceae | Diptera, Cecidomyiidae | Flower | N/A | 10; 17$ |
| Monnina dictyocarpa Griseb. | Polygonaceae | Diptera, Cecidomyiidae | Leaf | N/A | 10; 17$ |
| Ruprechtia apetala Wedd. | Polygonaceae | Diptera, Cecidomyiidae | Stem | Globoid to fusiform # | * |
| Condalia buxifolia Reissek | Rhamnaceae | Unknown | Stem | Fusiform | * |
| Condalia microphylla Cav. | Rhamnaceae | Diptera, Cecidomyiidae | Leaf | Fusiform | 10; 17$ |
| Condalia microphylla Cav. | Rhamnaceae | Diptera, Cecidomyiidae | Bud | Fusiform | 10; 17$ |
| Condalia microphylla Cav. | Rhamnaceae | Lepidoptera | Bud | Fusiform | 10; 12; 17$ |
| Condalia montana A. Cast. | Rhamnaceae | Unknown | Stem | Fusiform | * |
| Condalia montana A. Cast. | Rhamnaceae | Unknown | Bud | Fusiform | * |
| Condalia montana A. Cast. | Rhamnaceae | Unknown | Bud | Globoid | * |
| Malus domestica Borkh. | Rosaceae | Hemiptera, Eriosoma lanigerum Hausm. | Stem | N/A | 10; 17$ |
| Prunus persica Stokes | Rosaceae | Hemiptera, Myzus persicae persicae (Sulzer, 1776) | Leaf | N/A | 10; 17$ |
| Populus deltoides subsp. Monilifera (Aiton) Eckenw. | Salicaceae | Hemiptera, Pemphigus populitransversus Riley | Petiole | Globoid | 10; 17$ |
| Jodina rhombifolia Hook. & Arn. | Santalaceae | “Insecta” | Stem | Amorphous | 10; 17 X |
| Lycium cestroides Schltdl. | Solanaceae | Hymenoptera, Allorhogas cordobensis Martínez | Stem | Cylindrical | 15 X |
| Lycium ciliatum Schltdl. | Solanaceae | Unknown | Stem | Fusiform | * |
| Lycium elongatum X cestroides Hieronymus | Solanaceae | Diptera, Cecidomyiidae | Bud | Globoid | 10; 17$ |
| Physalis viscosa L. | Solanaceae | Diptera,Neolasioptera argentata (Brèthes) | Stem | Fusiform | *; 9; 11; 12 X |
| Solanum argentinum Bitter & Lillo | Solanaceae | Diptera, Cecidomyiidae | Stem | Fusiform to tubular # | 1; 7 X |
| Aloysia gratissima (Gillies & Hook. ex Hook.) Tronc. | Verbenaceae | Unknown | Leaf | Lenticular | * |
| Lantana megapotamica (Spreng.) Tronc. | Verbenaceae | Diptera, Cecidomyiidae | Stem | Globoid | * |
| Lantana megapotamica (Spreng.) Tronc. | Verbenaceae | Unknown | Leaf | Globoid | * |
| Lantana grisebachii Seckt var. grisebachii | Verbenaceae | Unknown | Stem | Fusiform | * |
| Lippia turbinata Griseb. | Verbenaceae | Diptera, Cecidomyiidae | Bud | Fusiform | 10; 17$ |
| Lippia turbinata Griseb. | Verbenaceae | Diptera, Cecidomyiidae | Leaf and Stem | Conical | 10; 17$ |
| Verbena citrodora (Paláu) Cav. | Verbenaceae | Hemiptera, Psylloidea | Leaf | * | |
| Larrea divaricata Cav. | Zygophyllaceae | Unknown | Stem | Fusiform | * |
1. Gall shapes were taken from Isaias et al. (2013); “#” indicates a shape proposed by the authors and “N/A” indicates that information about gall shape was not available.
2. Sources on interactions records: “*” indicates new record of each interaction for Córdoba, “$” indicates interactions mentioned in the bibliography, “X” indicates interactions registered in field samplings and also in literature. References are given in numbers (1) Altamirano et al. (2016), (2) Blanchard (1938), (3) Bréthes (1916), (4) Burckhardt & Basset (2000), (5) Carabajal de Belluomini et al. (2009), (6) Díaz (1980), (7) Fernandes et al. (2002), (8) Gagné (1994), (9) Gagné & Jaschhof (2017), (10) Houard (1933), (11) Jörgensen (1917), (12) Kieffer & Jörgensen (1910), (13) Kuzmanich et al. (2015), (14) Malcolm et al. (2015), (15) Martínez et al. (2011), (16) Nieves Aldrey & Blas (2015), (17) Tavares (1915), (18) Valladares, Zapata, Zygaldo, & Banchio (2002).
The botanical families most frequently involved in interactions with galling insects were Asteraceae (27 morphotypes on 17 plant species), Fabaceae (22 morphotypes on six plant species) and Anacardiaceae (eight morphotypes on two plants species), whereas the rest of the families had fewer than seven morphotypes each (Table 2). Baccharis was the genus displaying the highest number of gall morphotypes (16), with seven species acting as hosts. Other plant genera frequently attacked by galling insects were Acacia, Condalia, Geoffroea, Prosopis and Schinus, which had seven morphotypes each, in three or fewer host plant species. Three plant species may be considered as superhosts (sensu, Veldtman & McGeoch, 2003): Geoffroea decorticans (Gillies ex Hook. & Arn.) Burkart (Fabaceae) and Schinus fasciculata (Griseb.) I. M. Johnst. (Anacardiaceae), which displayed seven gall morphotypes each; and Baccharis salicifolia (Ruiz & Pav.) Pers., with six galling-insect species. For the first time, the plant genera Nassella (Poaceae) and Angelphytum (Asteraceae) were reported as hosting gall-inducing insects.
Table 2: Number of plant host species and number of gall morphotypes per plant families in Córdoba (central Argentina)
| Botanical family | Number of gall morphotypes (%) | Plant host species (%) | Number of plant species 1 |
|---|---|---|---|
| Asteraceae | 27 (27.27) | 17 (29.31) | 269 |
| Fabaceae | 22 (22.22) | 6 (10.34) | 107 |
| Anacardiaceae | 8 (8.08) | 2 (3.45) | - |
| Rhamnaceae | 7 (7.07) | 3 (5.17) | - |
| Verbenaceae | 7 (7.07) | 5 (8.62) | - |
| Solanaceae | 5 (5.05) | 5 (8.62) | 58 |
| Celtidaceae | 3 (3.03) | 1 (1.72) | - |
| Euphorbiaceae | 3 (3.03) | 3 (5.17) | 65 |
| Polygonaceae | 3 (3.03) | 2 (3.45) | - |
| Lamiaceae | 2 (2.02) | 2 (3.45) | - |
| Rosaceae | 2 (2.02) | 2 (3.45) | - |
| Acanthaceae | 1 (1.01) | 1 (1.72) | - |
| Amaranthaceae | 1 (1.01) | 1 (1.72) | - |
| Apocynaceae | 1 (1.01) | 1 (1.72) | - |
| Berberidaceae | 1 (1.01) | 1 (1.72) | - |
| Campanulaceae | 1 (1.01) | 1 (1.72) | - |
| Lythraceae | 1 (1.01) | 1 (1.72) | - |
| Poaceae | 1 (1.01) | 1 (1.72) | 308 |
| Salicaceae | 1 (1.01) | 1 (1.72) | - |
| Santalaceae | 1 (1.01) | 1 (1.72) | - |
| Zygophyllaceae | 1 (1.01) | 1 (1.72) | - |
| Malvaceae | - | - | 39 |
| Caryophyllaceae | - | - | 33 |
| Brassicaceae | - | - | 36 |
| Cactaceae | - | - | 36 |
| Cyperaceae | - | - | 72 |
| TOTAL | 99 (100) | 58 (100) |
1Number of species for the ten most abundant plant families in the region, taken from Zuloaga et al. (1999).
Galls occurring in Cordoba were classified into 11 morphotypes (Table 1), the most common shapes being fusiform (35 % of total gall morphotypes registered) and globoid (34 %) (Table 1). Other forms were represented by 6 % or less of the total gall morphotypes.
Stems were the most affected plant organs, accounting for 58.6 % of the gall morphotypes, whereas a noticeably lower representation was observed for leaf (19.1 %) and bud (14.1 %) galls. Other organs like thorns, petioles, flowers and spines were less affected. Only a very small fraction of galling-insect species was found developing in two different organs (Table 3).
Table 3: Plant organs in which galls are induced by insects, in Córdoba (central Argentina)
| Organs | Number of morphotypes | Relative frequency (%) |
|---|---|---|
| Stem | 58 | 58.6 |
| Leaf | 20 | 20.2 |
| Bud | 14 | 14.1 |
| Thorn | 2 | 2 |
| Petiole | 2 | 2 |
| Flower | 1 | 1 |
| Leaf and stem | 1 | 1 |
| Stem and petiole | 1 | 1 |
Regarding the insects, 41.4 % of the gall-inducing species were Diptera, 11.1 % Hemiptera, 7 % Lepidoptera and 6 % Hymenoptera. Cecidomyiidae was the family with the highest number of galling species (38.3 %) whereas Cynipidae, Tephritidae, Aphididae, Calophyidae and other families were less-well represented. The absence or very low number of insects reared from more than 75 % of the gall morphotypes prevented the taxonomic identification of the insect inductor. Seven percent of the other 25 % of the interactions (in which the insect inductor was identified), belonged to Cecidomyiidae. From these figures, it is evident that there is a need of an increased sampling effort to obtain adults of unidentified species, in order to attain a better knowledge of gall inducing insects in the region.
Discussion
On the basis of the results obtained from field surveys and a literature review, our study provides a list of 99 interactions between species of plants and gall-inducing insects in the Córdoba province. It is interesting to note that more than half of these records constitute new citations for Cordoba, and 49 % are reported for the first time in Argentina, which highlights the scarce knowledge there is about these interactions in the region. The number of galls reported here is rather high in comparison with the ones reported in the few studies available on gall-inducing insects in Argentina, which covered different geographic areas and employed different sampling efforts (Fernandes et al., 2002; Quintero etal., 2014; Kuzmanich et al., 2015).
Our results identify Diptera, particularly the family Cecidomyiidae, as the most species-rich group, which is in agreement with different studies from across the globe (Mani, 1964; Fernandes et al., 2002; Espírito-Santo & Fernandes, 2007; Quintero et al., 2014; Gagné & Jaschhof, 2017; Urso-Guimarães, Castello, Kataoka, & Kochk, 2017). Specific identification of gall midges is very difficult given the scarce knowledge there is about the group in South America (Maia, 2012). In Córdoba, only seven out of 38 gall morphotypes induced by Cecidomyiidae are species properly described, the rest being unknown, even at the generic level.
The plant families hosting the highest number of galls were Asteraceae (27.2 % of interactions) and Fabaceae (22.2 %). Several studies conducted in the Neotropical region reported Asteraceae (Carneiro et al., 2009; Coelho et al., 2013; Arriola et al., 2015; Kuzmanich et al., 2015) and Fabaceae (Fernandes et al., 2002; Coelho et al., 2009; Carvalho-Fernandes, Silva De Almeida-Cortez, & Ferreira, 2012; Urso-Guimarães et al., 2017) as the families most frequently attacked by galling insects. In Córdoba, the families best represented in the vegetation are Poaceae, Asteraceae and Fabaceae, in that order (Zuloaga, Morrone, & Rodriguez, 1999; Giorgis et al., 2011); the predominance of the last two plant families in our records is partially concordant with the “plant family size hypothesis”, which predicts a positive correlation between the high number of plant species and the number of associated gall morphotypes (Fernandes, 1992). It is notable, however, that just one association was recorded between a gall-inducing insect and a species of Poaceae. Similar disproportionate low numbers of gall morphotypes in Poaceae, in spite of a high availability of species in the flora, were observed in Brazil (Maia, 2001; Arriola & Ferreira, 2016). Among Asteraceae, the genus Baccharis had the highest number of species (7) associated with galling insects and displayed the highest number of gall morphotypes (16). The vulnerability of this genus to galling insects has previously been reported in the Neotropical region, and it has been observed particularly for Cecidomyiidae inducers (Fernandes et al., 2014; Gagné, 1994).
Surprisingly, stems were the organs most frequently affected by galling insects in Cordoba. This result disagrees with the general trend of leaves being the preferred organ for galling insects (Mani, 1964; Shorthouse & Rohfritsch, 1992; Quintero et al., 2014; Arriola et al., 2015; Kuzmanich et al., 2015; Maia & Carvalho-Fernandes, 2016). Just a few studies have reported a greater number of galls on plant stems (Fernandes et al., 2002; Veldtman & McGeoch, 2003; Carneiro et al., 2009; Coelho et al., 2013;), and in some of these cases, they were restricted to a single insect taxon, such as Coleoptera (Maia & Oliveira, 2004). Even when some studies explored the mechanisms of gall induction (see Stuart, Chen, Shukle, & Harris, 2012; Giron, Huguet, Stone, & Body, 2016), to our knowledge, no studies to date have explored the mechanisms by which galls tend to be induced in leaves, stems or other plant organs. It is known that young and undifferentiated tissues are necessary for plant gall induction (Rohfritsch, 1992; Weis, 1988). In tropical latitudes, being climate and resources favorable to a continuous growth of the plant, the active meristematic tissues tend to be more available in leaves than in stems throughout the year, and this may be the reason explaining the usually reported predominance of foliar over stem galls (Shorthouse & Rohfritsch, 1992). The opposite tendency could be expected at higher latitudes, where most of the plants display a seasonal foliage loss and regrowth, thus caulinar meristematic tissues became a more stable resource available for gall-inducing insects. However, the scarce studies in which stem galls predominate were conducted at both, tropical (Carneiro et al., 2009; Coelho et al., 2013) and subtropical (Fernandes et al., 2002; Veldtman & McGeoch, 2003; Toma & Mendonça, 2013) localities. It could be also possible that foliar galls are exposed to early leaf-abscission, which may be incremented by hydric stress (Veldtman & McGeoch, 2003) under arid and semiarid climates, as in central Argentina. Moreover, more stable temperatures (Carneiro et al., 2009) and hydric conditions in stems than in leaves could be favoring the induction of galls in stems. Evidence supporting this idea was observed for one species of Erioccoccidae, whose adults normally induce leaf galls, but before leaf fall, they induce a second gall morphotype in stems to undergo dormancy throughout the dry season (Gonçalves, Gilson, & Isaias 2009).
Our study highlights the scarce knowledge that exists about plants and gall-inducing insects in Argentina, especially regarding interactions between plants and gall-inducing Cecidomyiidae, with more than 30 undescribed species noted in our study. The galling insect community deserves further taxonomic and biological studies, especially considering the speed of deforestation of native forests in central Argentina (Hoyos et al., 2013; Cabido et al. 2018). Finally, in our opinion, understanding the mechanisms by which galls tend to predominate in leaves or stems in a given region is certainly a future challenge.












 uBio
uBio