Holothuroidea is a diverse class of wormlike and usually soft-bodied echinoderms. They are commonly found in tropical shallow waters from the intertidal zone to the deep ocean (Hadel, Monteiro, Ditadi, Tiago & Tommasi, 1999; Pawson, 2007) which can comprise up to 90 % of the total biomass of these environments (Pawson, 1966; Hendler, Miller, Pawson & Kier, 1995). In Brazilian coast, these organisms are found rather commonly grouped under rocky outcrops (Mendes, Marenzi & Di Domenico, 2006), usually in contact with the sandy floor, from which they feed during periods of immersion (Tommasi, 1969).
Most holothurians are large organisms, free-living, iteroparous and dioecious. They typically reproduce once a year by spawning their gametes into the sea where fertilization occurs (Hyman, 1955; Smiley, McEuen, Chaffee & Krishnan, 1991). The reproductive cycle in sea cucumbers is often very complex. Gametogenesis and spawning usually follow a distinct seasonal pattern, varying in relation to species and geographic locations (Drumm & Loneragan, 2005). Some abiotic factors such as seawater temperature and photoperiod (Smiley et al., 1991; Ramofafia, Byrne & Battaglene, 2000), lunar rhythms (Battaglene, Seymour, Ramofafia & Lane, 2002; Mercier, Sun, Baillon & Hamel, 2011) and biotic factors such as food availability (Drumm & Loneragan, 2005) have been suggested as exogenous cues affecting the reproduction of the species.
Holothuria (Halodeima) grisea has been harvested in some areas of southeastern-south Brazilian coast (Hadel et al., 1999; A. Marenzi, personal communication, February 19, 2010) several years ago. A sustainable exploitation of this resource necessarily requires a description of the reproduction cycle of this potentially exploitable species to implement a harvest season and a closure during the spawning (Guzmán, Guevara & Hernandez, 2003; Abdel-Razek, Abdel-Rahman, El-Shimy & Omar, 2005).
Though Holothuria (Halodeima) grisea represents a very common sea cucumber along the Brazilian coast, its spatial and temporal distribution, beyond ecological aspects of this species are still poorly understood. In this investigation, the reproductive aspects of a population of Holothuria (Halodeima) grisea was evaluated by macroscopic and histological characteristics of gonads, as well as the participation of the body components in the gametogenesis process.
Materials and methods
Study site
Specimens were collected from a rocky shore located nearby the mouth zone of Guaratuba Bay in the State of Paraná, Southern Brazil (25° 53’ 22.73” S and 48° 33’ 37.81” W) .The climate is classified as mesothermic subtropical with a mean annual rainfall of 2.500 mm (maximum of 5.300 mm), an average annual temperature of 22 °C with a hot rainy summer season and mean air humidity around 85 % (Marone et al., 2004).
The main atmospheric disturbances are cold fronts from the SW-NE direction that originate in Southern America. A typical rainy season initiates in late spring and lasts during most of the summer while the dry season lasts from late autumn to late winter, but is usually interrupted by a short and weak rainy period in early winter (Lana, Marone, Lopes & Machado, 2001). The tides are characterized by diurnal inequality and attain to maximum and minimum amplitudes of approximately 2 and 0.5 m respectively (Knoppers, Brandini & Thamm, 1987).
In the sampling campaigns the water temperature varied from 26 ºC (February) to 19 ºC (April and June) in the studied period. The seawater salinity remained relatively constant, approximately 30.
Field sampling and lab routines
Holothuria (Halodeima) grisea specimens were sampled in February, April, June and October 2008. About twenty to twenty-five specimens larger than 10 cm were randomly collected by hand in each month. Surface seawater temperature and salinity were recorded in situ. All individuals were transferred to the laboratory, relaxed and dissected. The total body drained weight following the opening of the body and the removal of coelomic fluid, the body wall, respiratory tree, haemal system, empty digestive tract and gonads were recorded with an electronic precision scale (0.0001 g). The removed gonads were macroscopically and microscopic examined in order to determinate sex and maturity stage and then fixed in 9 % formaldehyde (Despalatovic, Grubelic, Simunovic, Antolic & Zuljevic, 2004).
The gonadal macroscopic features (gonad color, opacity and consistency of the tubules) were presented on a scale adapted from AbdelRazek et al. (2005) and Ghobadyan, Morovvati, Ghazvineh and Tavassolpour (2012). For the histological examinations, small sections of the gonad tubules were dehydrated, embedded in paraffin wax, sectioned (7 μm-thick) and stained with haemotoxylin and eosin (H / E). The stages of gametogenesis were based on those used in previous studies of sea cucumber gonads (Purwati & Luong-Van, 2003; Drumm & Lonergan, 2005; Rasolofonirina, Vaitilingon, Eeckhaut & Jangoux, 2005; Shiell & Uthicke, 2006). The mature oocytes were measured by the median diameter axis [(length + width) x 0.5] with an ocular micrometer.
Data Analysis
Frequency distribution of the maturity scale in both sexes (growing, mature or post-spawning) was calculated at each month. Sex ratio was estimated considering the total sample over the study period and between months. Statistically significant difference from the expected 1:1 sex ratio was tested using c2 (Chi-square test) (Zar, 1996).
The relationship between total wet weight and organs (body wall, respiratory tree, haemal system, digestive tract, gonadal) is described by the linear function (y = a + bx). To evaluate if the relationship between total wet weight and individual’s organ weight among the months, we use analysis of covariance (ANCOVA) after logarithmic transformation of all the variables (Ebert, Hernandez & Russell, 2011). Regressions coefficients were tested for significant deviations from zero. Slopes of different regression equations were compared with each other and intercepts with the Y-axis with an ANOVA. Besides, we test a post-hoc SNK test and the significance was tested at the 95 % confidence level.
Results
Macroscopic features in gametogenesis stages (Fig. 1 a - d)
Gonads are a single structure, consisting of numerous filamentous tubules basically gathered into one tuft attached to the left side of the dorsal mesentery. The macroscopic appearance in relation to color, opacity and texture of the gonads between the months showed noticeable differences to the naked eye. However, during growth and depleted, no macroscopic morphological differences were observed between sexes.
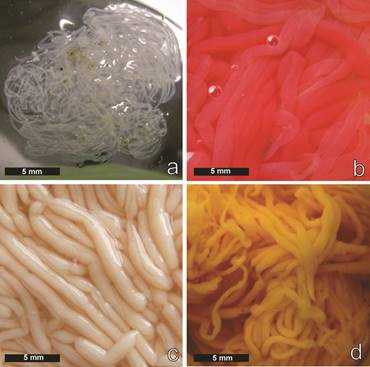
Fig. 1 Holothuria (Halodeima) grisea. Macroscopic features of the gonads at different developmental stages. a, Growth. b, Mature female. c, Mature male. d, Depleted.
Growth: The gonad was characterized as shorter, thinner, branched and with yellowwhite color with an increase in opacity and consistency in both sexes (Fig. 1a).
Mature: The maturing gonad was characterized by long, thick, branched maximum volume tubules and, with rare exceptions, the female gonads showed red color and the male gonads presented a marked cream tone. The structures had an opaque appearance and were quite swollen (Fig. 1 b - c).
Depleted: After spawning, both sexes showed branched, thin, whitish, translucent tubules and with a less consistent texture (Fig. 1 d).
Microscopic features of the gonad maturity stages (Fig. 2 a - f)
Based on the histological analyses, the maturation of the tubules was classified into three stages for females and males:
Growing: the gonad lumen was still filled with nutritive phagocytes. In females, pre and early vitellogenic oocytes had an irregularshaped contour and appeared attached to the tube wall. Some solitary vitellogenic oocytes moved away from the wall and occupied the center of tube lumen among nutritive phagocytes. The size of these germ cells varies according to the developmental stage, and the vitellogenic oocyte has, on average, a diameter of 75.56 µm (SD = 17.69, n = 144) (Fig. 2 a). In males, primary sexual cells (spermatogonia and spermatocyte) occurred as a band close to the tube wall. Few or no spermatozoa occur at the center of the tube surrounded by nutritive phagocytes (Fig. 2 b).
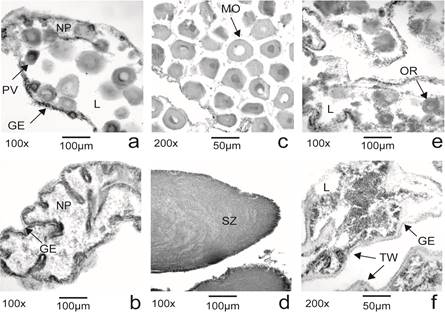
Fig. 2 Holothuria (Halodeima) grisea. Microphotographs of gonadal stages of the males and females at Guaratuba Bay. Females: a Growing, c Maturing and e Post-spawning; Males: b Growing, d Maturing and f Post-spawning (GE germinal epithelium; NP nutritive phagocytes; SZ spermatozoa; L lumen; TW tubule wall; PV pre vitellogenic oocytes; MO mature oocytes; OR residual oocyte).
Maturation: most of the ovarian lumen are filled with mature gametes of polygonal shape exhibiting a great number of cortical granules at the surface. Few primary oocytes are found and nutritive phagocytes cells are rare or absent; the size of the oocytes on average was 76.27 µm (SD = 13.23, n = 75) and the maximum diameter found was 160 µm (Fig. 2 c). In males, most of the tube lumens were filled with mature spermatozoa. A straight band of primary cells was still present, but nutritive phagocytes were absent (Fig. 2 d).
Post-spawning: A few relict oocyte (Fig. 2 e) or spermatozoa (Fig. 2 f) may be found in a reduced tube lumen within sparse nutritive phagocytes. Phagocytosis is evidenced by the presence of strong basophilic agglutination structures. In some male’s gonads, the tubes showed a mixture of nutritive phagocytes, primary cells and spermatozoa (Fig. 2 f). The lumen of the tubules (females) may further include residual oocytes with an average diameter of 75.74 µm (SD = 15.64, n = 61).
Sex ratio and gametogenesis
A total of 94 individuals of Holothuria (Halodeima) grisea were examined: 33 females, 55 males and 6 individuals without gonads. External sexual dimorphism was not observed. The results of the test statistic c² no accepted the null hypothesis (H0) for the sex ratio equal to 1 (c² = 5.5, p = 0.019 df=1). However, analyzing each month the female to male ratio ranged, from February (c² 0.04 df = 1 p = 0.841), April (c² 6.76, df = 1 p = 0.009), June (c² 1.47, df = 1 p = 0.225) and October (c² = 1.19, df = 1 p = 0.275), showing sex ratio different from 1 only at April.
The analysis of gametogenesis of Holothuria (Halodeima) grisea could be characterized by continuous emission of gametes throughout the samplings, despite an increase of individuals during maturation in the February. For both sexes mature gonads were observed mainly in the February and October (summer and spring months), whereas in April (autumn) half of population showed their gonads in growing stage. In June (winter) the majority of individuals were characterized as post-spawning stage (Fig. 3). Individuals lacking the gonad appeared in April and June.
The ANCOVA analysis performed on body compartments data with total wet weight as the covariate, showed body wall and gonads as the most representative organs. Although there is high plasticity between months, this variation was different for each body compartments, but there was no difference in variation between sexes (ANCOVA: F10.170 = 1.076, p = 0.382).
The body wall of Holothuria (Halodeima) grisea represents almost the entire animal weight (~ 80 - 90 %) and showed differences between the samplings (ANCOVA: F3.87 = 14.258, p < 0.001), increasing in April (growth stage) and fall in the June (post-spawning stage) (Fig. 4 a, Table 1).
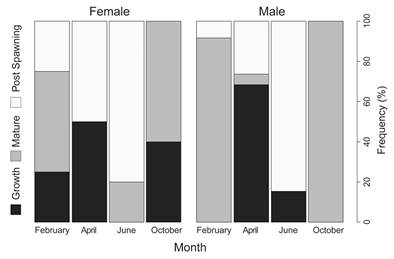
Fig. 3 Frequency distribution (%) of different maturity stages for males and females of Holothuria (Halodeima) grisea in Guaratuba Bay.
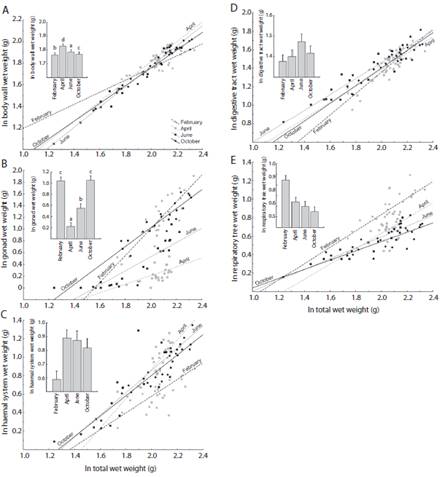
Fig. 4 Holothuria (Halodeima) grisea. Linear models (p < 0.001) and Means (± IC, g), for the variation of the body organs, using the toral wet weight as a covariate (N = 94). Body wall A, gonads B, hemal system C, digestive tract D and respiratory tree E. The letters (a, b, c and d) show the differences of the SNK test from ANCOVA.
Gonads showed significant differences between the samplings (ANCOVA: F3.87 = 7.383, p < 0.001), increased tenfold of size during periods of maturation (October and February), decreasing in April (growth stage) and dropping too in June (post-spawning stage) (Fig. 4 b, Table 1).
Haemal system (ANCOVA: F3.87 = 2.024, p > 0.05) varied by 1 to 30 % of the total weight, although no differences among the slopes, showed a difference between the intercepts (t - value: - 2.047, p > 0.005), the organ is significantly smaller in the February (Fig. 4 c, Table 1).
Digestive tract (ANCOVA: F3.87 = 2.052, p > 0.05) varied by 2 to 4 % of the total weight and showed no difference between the slopes or intercepts (Fig. 4 d, Table 1).
Respiratory tree (ANCOVA: F3.87 = 1.495, p > 0.05) was the organ with lesser participation in relation to the total weight of the individual (1 - 2 %), also did not change (Fig. 4 e, Table 1).
Table 1 Holothuria (Halodeima) grisea. Variance of the body organs between months from Guaratuba Bay-Brazil in 2008, using summary statistics with raw data and ANCOVA with logarithmic data
| - | ln intercept | - | ln slope | - | - | - | - |
|---|---|---|---|---|---|---|---|
| Body Wall | Mean (SD) | p | Mean (SD) | p | r² | n | p |
| February | 0.629 (0.124) | 0.000 | 0.562 (0.059) | 0.000 | 0.786 | 25 | 0.000 |
| April | - 0.030 (0.069) | 0.753 | 0.923 (0.047) | 0.000 | 0.943 | 25 | 0.000 |
| June | - 0.115 (0.061) | 0.074 | 0.943 (0.032) | 0.000 | 0.975 | 23 | 0.000 |
| October | 0.107 (0.100) | 0.286 | 0.824 (0.047) | 0.000 | 0.942 | 21 | 0.000 |
| Gonads February | - 3.557 (0.539) | 0.000 | 2.282 (0.260) | 0.000 | 0.762 | 25 | 0.000 |
| April | - 1.249 (0.664) | 0.072 | 0.730 (0.324) | 0.034 | 0.180 | 25 | 0.034 |
| June | - 1.877 (0.252) | 0.000 | 1.203 (0.135) | 0.000 | 0.791 | 23 | 0.000 |
| October | - 2.195 (0.335) | 0.000 | 1.610 (0.160) | 0.000 | 0.841 | 21 | 0.000 |
| Haemal system February | - 1.232 (0.430) | 0.008 | 0.904 (0.207) | 0.000 | 0.441 | 25 | 0.000 |
| April | - 2.314 (0.491) | 0.000 | 1.591 (0.240) | 0.000 | 0.655 | 25 | 0.000 |
| June | - 1.555 (0.262) | 0.000 | 1.205 (0.140) | 0.000 | 0.779 | 23 | 0.000 |
| October | - 1.399 (0.245) | 0.000 | 1.101 (0.117) | 0.000 | 0.822 | 21 | 0.000 |
| Digestive tract February | - 0.956 (0.245) | 0.000 | 1.558 (0.118) | 0.000 | 0.800 | 25 | 0.000 |
| April | - 0.386 (0.277) | 0.177 | 0.887 (0.135) | 0.000 | 0.650 | 25 | 0.000 |
| June | - 0.263 (0.115) | 0.032 | 0.862 (0.061) | 0.000 | 0.903 | 23 | 0.000 |
| October | - 0.507 (0.182) | 0.011 | 0.955 (0.087) | 0.000 | 0.863 | 21 | 0.000 |
| Respiratory tree February | - 0.943 (0.363) | 0.016 | 0.888 (0.175) | 0.000 | 0.516 | 25 | 0.000 |
| April | - 1.028 (0.386) | 0.013 | 0.829 (0.188) | 0.000 | 0.456 | 25 | 0.000 |
| June | - 0.582 (0.093) | 0.000 | 0.586 (0.049) | 0.000 | 0.868 | 23 | 0.000 |
| October | - 0.463 (0.232) | 0.060 | 0.502 (0.111) | 0.000 | 0.518 | 21 | 0.000 |
Discussion
Currently, it is known that the regulation of the reproductive cycle is not just concern with the timing of spawning, for this is only the climax of considerable preparatory activity, but encompasses all aspects of the organism’s physiology and behavior (Giese, 1959).
Since the spatial and temporal distribution, beyond ecological aspects of Holothuria (Halodeima) grisea are still poorly understood in Paraná coast, we decided to reduce the sampling effort. Although we do not observe all stages gametogenics due to lack of sample, we can characterize a reproductive pattern of H. (H.) grisea with continuous and synchronic emission of gametes across the months. The increase in number of sexually mature individuals in February, may indicate a population strategy in maximize the reproductive synchrony at this time of the year.
Recent studies on the reproduction of Holothuria (Halodeima) grisea in Brazil (available only in meeting proceedings yet) reported that the sea cucumbers show only one annual spawning during the reproductive cycle. Martins and Ventura (2013) in Southeastern (Rio de Janeiro State, Lat. 23º S) observed that both sex are mature between October and January (spring and summer). Leite et al. (2014) in Northeastern (Ceará State, Lat. 3º S), observed mature individuals (with high gonadossomatic index) between December and April, and many individuals of indeterminate sex between June and July.
In spite of annual pattern of reproductive cycle be considered arbitrary (Holland, 1991), many studies describe only one annual spawning during the reproductive cycle, as recorded for temperate species like Psolus fabricii (Düben & Koren, 1846) (Hamel, Himmelman & Dufresne, 1993) from eastern Canada and tropical species like Isostichopus badionotus (Selenka, 1867) (Lima, Ventura & Campos-Creasey, 2001) from southeast Brazil, Holothuria (Mertensiothuria) leucospilota (Brandt, 1835) (Druum & Loneragan, 2005) from Cooks Island and Holothuria (Metriatyla) scabra Jaeger, 1833 (Rasolofonirina et al., 2005) from south-west of Madagascar.
For Caribbean waters, Holothuria (Halodeima) mexicana Ludwig, 1875 and Isostichopus badionotus were reported in mature gonad stages throughout the year, suggesting that individuals may reproduce continuously or asynchronously several times during the year (Guzmán et al., 2003).
An important environmental factor influencing the cycle of this species is the water temperature, especially in sites with marked seasonality as the coast off Paraná (Lat. 25º S). Maximal reproduction was observed for Holothuria (Halodeima) grisea during the warmest part of the year, when the temperature was high. Similarly, the spawning of Isostichopus badionotus from Brazil occurs during the summer, when the temperature reaches its maximum of 30 ºC (Lima et al., 2001).
The sex-ratio of the studied population was near 1:1 in almost all months, with the exception of April, where the number of males was higher than females. This unbalanced sex ratio is also observed in some species (Simunovic & Grubelic 1998; Shiell & Uthicke 2005), but deviations from unity in sex ratios among large aspidochirotes are rare, and typically associated with species that undergo asexual reproduction (Harriott 1982; Uthicke et al. 1998, 1999). However, given that asexual reproduction was not observed for the Holothuria (Halodeima) grisea, and may be related to the low number of samples collected.
Hamel et al. (1993) studied the relation of organs (respiratory tree, intestine and gonads) with seasonal changes and increased metabolic demands for gametogenesis in Psolus fabricii in Eastern Canada, found differences in body organs between males and females especially for gonadal index, that in males was more than twice that of females. For our results however body components variations were similar in males and females, which may indicate similarities in the metabolic demands for the species since in that case there is a greater requirement for sex.
The highest values observed for the development of gonads occurred in periods when there was lower participation of the body wall. Such opposition may indicate that there is an exchange of energy between these organs.
Prim, Lawrence and Turner (1976) and Giese (1976) suggested that the body wall of holothurians has a great storage potential, mainly for proteins, and according with Lawrence and Lane (1982) resorption of the body wall should enable echinoderms to maintain basic metabolism during periods of nutrient deprivation. This assumption is generally made for thick-skinned holothurian species (Lawrence, 1972; Prim et al., 1976), which eviscerate and then regenerate the gut within 1 to 3 months (Jespersen & Lutzen, 1971).
Unlike the temperate species, the body components of Holothuria (Halodeima) grisea such as respiratory tree, haemal system and digestive tract had little contribution to the weight of the animal. However, the gonads represent a large part of the weight of individual. The size of gonads was ten times higher in February than October collections. April and June showed small variation of the gonads size, which does not necessarily indicate a pattern of the species in the region. Other studies also showed significant differences in the gonad size during the year, as is Holothuria (Mertensiothuria) leucospilota (Drum & Loneragan, 2005) and Psolus fabricii (Hamel et al., 1993).
The percentage of individuals with mature gonads, as well as the gonad index, can be used to estimate the reproductive effort or energy invested in reproduction (Conand, 1993). Furthermore, the body weight could also be important for interpreting interspecific variations. We observed a strong antagonism between the index of the body wall and the gonads. Though some authors as Chao, Chen and Alexander (1995) recognize that the gonad index is a reliable indicator to study the sexual reproduction of Holothuroidea. We do not recommend the index alone to determine the maturation for females since, as observed in this study, the constant presence of mature cells in the female individuals masked the interpretation of the index (without differences between samplings). For males the highest values of the gonadal index in October and February coincided with the maturation of individuals and the maximum drop of the index indicated that April to June are the most important periods for the issuance of gametes.
Gonads showed a great variation in size, stage of development and coloration, both in relation to species and between males and females from the same species. In this study the gonads of Holothuria (Halodeima) grisea had differences to color, opacity and texture in relation to reproductive condition between the sexes. Purwati and Luong-Van (2003) and Abdel-Razek et al. (2005) confirm the differences in staining pattern in mature individuals of Holothuria (Mertensiothuria) leucospilota and Holothuria (Halodeima) atra Jaeger, 1833, respectively. For other species as Stichopus chloronotus Brandt, 1835 (Hoareau & Conand, 2001) and Cucumaria frondosa (Gunnerus, 1767) (Hamel & Mercier, 1996), there were no such changes; therefore, histological examination is still the safest method to describe the reproductive cycle of holothurians.
For most echinoderms, life strategies are characterized by the energy requirement for one or both physiological trends, especially in the case of females, who use too much energy to produce larger gametes (Lawrence, 1987). However, this reproductive effort is strongly conditioned by life strategies developed by the population of a given environment. This hypothesis can be verified comparing the average diameter of mature oocytes of tropical and temperate species; e.g., Hamel et al. (1993) reported mature oocytes with diameter between 400-600µ for the species Psolus fabricii. While tropical species such as Holothuria (Metriatyla) scabra (Rasolofonirina et al., 2005), Holothuria (Mertensiothuria) leucospilota (Drumm & Loneragan, 2005) and Holothuria (Halodeima) grisea produce oocytes around 70 - 160 µ.
In conclusion, the period of maturation and variation in body organs was similar between the sexes. These similarities in the metabolic demands of the population could be confirm a strategy to maximize the reproductive synchrony. This species is important for the Brazilian coast, since it is becoming rare in some places and has the potential for commercial exploitation as a food source and for it extraction with pharmaceuticals purposes (Alvarado, Ortiz, Benavides & Toral-Granda, 2013). In spite the lack of information and sample efforts, our results represents the first official information about gametogenesis process of this species and contribute to sustainable management of this population.












 uBio
uBio 

