Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.62 suppl.1 San José Feb. 2014
Lack of correlation between vertical distribution and carrier frequency, and preference for open spaces in arboreal katydids that use extreme ultrasound, in Gorgona, Colombia (Orthoptera: Tettigoniidae)
Abstract
Male Tettigoniidae emit sound to attract conspecific females. The sound is produced by stridulation. During stridulation the forewings open and close, but it is during the closing stroke that the scraper contacts the file teeth to generate the predominant sound components, which are amplified by adjacent wing cells specialized in sound radiation. The sounds usually exceed the sonic boundary and might occur above 40 kHz, reaching extreme ultrasonic frequencies of 150kHz in some species. Here we test the hypothesis that Tettigoniidae species should prefer microhabitats that favour efficient signal transmission, i.e. that there is a relationship of sound frequency with the vertical distribution of the species (from ground to canopy) at Gorgona National Natural Park, Colombia. We sampled 16 trees and four different altitudinal levels between 1 and 20m above the understory vegetation. We placed collecting blankets separated by vertical distances of 5m, and knocked insects down using the technique known as fogging. We found no correlation between vertical distribution and carrier frequency, but there was a preference for open spaces (below the canopy and above the understory) in species using extreme ultrasound. This is the first quantitative description of the vertical distribution in neotropical species of the family Tettigoniidae and its relationship to the calling song frequency. Rev. Biol. Trop. 62 (Suppl. 1): 289-296. Epub 2014 February 01.
Key words: canopy insects, stridulation, fogging, bioacoustics, ultrasound, vertical distribution.
Resumen
Los machos de Tettigoniidae producen sonido frotando las alas anteriores para atraer a sus hembras coespecíficas con fines reproductivos (mecanismo conocido como estridulación). Un borde esclerotizado (o raspador) en el ala derecha es frotado sobre una vena modificada con dientecillos en el ala izquierda. Durante la estridulación las alas abren y cierran, pero es durante el cierre que el raspador contacta los dientecillos de la lima y produce vibraciones que son amplificadas por celdas adyacentes especializadas para radiar sonido. Sonidos que superen los 20 000Hz, se consideran ultrasónicos, en los Tettigoniidae, la mayoría de los cantos superan el umbral sónico y pueden ocurrir a más de 40 000Hz, con casos extremos de hasta 148 000Hz. La esencia de este trabajo es el estudio de esta gama espectacular de frecuencias de la familia Tettigoniidae y su relación con la distribución vertical de los individuos en las especies que habitan en Gorgona. Se muestrearon 16 árboles a cuatro niveles diferentes de altitud entre 1 y ~20m sobre el sotobosque, instalando sabanas colectoras a intervalos de 4-5m. Los insectos se colectaron mediante el método de nebulización térmica. El análisis indica que no hay correlación entre la distribución vertical de las especies y la frecuencia del canto. Sin embargo se observó una preferencia por espacios abiertos en especies que cantan a frecuencias extremas. Este estudio representa la primera descripción cuantitativa de la distribución vertical de Tettigoniidae Neotropicales y su relación con la frecuencia del canto.
Palabras clave: insectos canopy, estridulación, nebulización, bioacústica, ecografía, distribución vertical.
The diversity of insects that produce sound in the tropics challenges our understanding of animal communication mechanisms and their evolution. The use of extremely high frequencies by South American katydids suggests unknown acoustic mechanisms responsible for the acoustic energy and the tuning of these signals (Montealegre-Z, Morris & Mason, 2006). Typically, calling songs are is produced by males to attract conspecific females (Morris, 1999). This sound is produced in most species of Ensifera by the friction of the forewings or tegmina (stridulation). The right wing has a specialized area of the anal margin that forms a scraper or plectrum, while the left wing has a modified vein with teeth on its ventral surface, called a file (Bailey, 1970). The opening and closing movement of the wings causes the contact and rubbing the plectrum on the file teeth. The successive impacts between the plectrum and individual teeth of the stridulatory vein (file) induce vibration in both wings and consequent sound radiation (Montealegre-Z & Mason, 2005).
Among the families of ensiferan Orthoptera, crickets (Gryllidae) tend to produce pure tones (musical sounds at a single frequency) of relatively low frequency (about 5kHz), having a correspondence between the frequency of sound produced and the rate of impacts between the plectrum and teeth on the stridulatory vein (Bennet-Clark, 1999; Bennet-Clark, 2003; Bennet-Clark & Bailey, 2002; Koch, Elliott, Schaffner & Kleindienst, 1988). Katydids on the other hand use more diverse signals and exploit a large range of frequencies (Heller, 1988, Pierce, 1948; Suga, 1966 ; Montealegre-Z, 2009). Some species produce only pure tones, some use broadband loud sounds (Morris, Mason, Wall & Belwood, 1994) and at frequencies ranging from very low, as in crickets, to extremely high ultrasonic frequencies above 100kHz (Mason & Bailey, 1998; Montealegre-Z et al., 2006; Morris et al., 1994; Römer, Spickermann & Bailey, 1998). In these species, the speed of wing movement required to move the plectrum along the file at a rate corresponding to the sound frequency is too high to be achieved by simple muscular contraction. These species make use of a mechanism that combines muscle-driven wing movement with stored elastic energy from bending cuticular structures (Montealegre-Z et al., 2006).
The detection of these signals is also an important consideration. The typical role of singing is to communicate over long distances (Robinson & Hall, 2002), but ultrasonic signals are transmitted with difficulty as the distance increases between transmitter and receiver. High frequency sounds experience significant excess attenuation, particularly in tropical habitats where transmission is quite affected by moisture and vegetation (Bass, Sutherland & Zuckerwar, 1990). This means that high frequency sounds are not transmitted well and may therefore require highly sensitive detection systems and / or sophisticated production mechanisms to achieve the required acoustic power.
Previous work in Amazonian forests and other rain forests in Colombia, where the Tettigoniidae are abundant (Montealegre-Z, Guerra & Morris, 2003; Montealegre-Z & Morris, 1999; Montealegre-Z & Morris, 2003; Nickle & Castner, 1995), have brought us to study both aspects of the acoustic behavior of these insects - high frequency signaling, and mechanisms of production and detection of ultrasound. We have recently discovered a species that produces pure tone signals (musical signals) at 130-150kHz-the highest frequency currently known among arthropods (Montealegre-Z et al., 2006). It belongs to the genus Ultrasonus extremus (Listrocelidinae). Very little is known, however, of the behavior and physiology of these animals.
Because environmental interference with sound propagation should be greater for high frequencies, we set out to test the hypothesis that the vertical distributions of katydid species within the rainforest canopy should be correlated with song frequency-i.e. species should prefer microhabitats that favour efficient signal transmission. Using a fogging technique (Erwin, 1989), a section of forest in Gorgona National Natural Park (Colombia) was sampled at different height levels to collect individuals of the arboreal katydid species. The songs of these species were known and recorded in advance and this allowed us to establish an analysis of the relationship between dominant frequency and the vertical location.
Materials and methods
Study site: This study was conducted in the Gorgona National Natural Park, Department of Cauca, Colombia, which is located in the southern Colombian Pacific (2°47’- 3°6’ N and 78°6’ - 78°18’ W), at 35km from the coast. The park includes a land area of 13.8km2 and comprises the islands Gorgona and Gorgonilla. Elevations vary from 0m to 338m (Chamorro, 1990), the average temperature is 27ºC, average annual rainfall is 6891.4mm, and the ecosystem is classified as tropical rainforest (Rangel & Rudas, 1990).
Call recordings: In December 2003 and May 2007 we obtained recordings from the vast majority of species collected in this study. Collected specimens belonging to species of which recordings were not available underwent anatomical analysis to estimate the frequency of song based on the size of the generator. The dimensions of the generator or mirror predict with precision the fundamental frequency of song in Tettigoniidae (Montealegre-Z, 2009), according to the linear equation Ln fc=e4-(1.2*Ln Ml where fc is the carrier frequency of the calling song (in Hz) and Ml represents the mirror length (in m). This correlation is not affected by the phylogenetic history of the katydids (Montealegre-Z, 2009).
The ultrasonic songs were identified in the field using ultrasonic detectors. Collected males were recorded in the lab at Gorgona with ultrasound sensitive equipment, Brüel & Kjær (B&K) type 1/8” (4138) or 1/4” (4939), connected to amplifier B&K 2606 or B&K Nexus (Type 2690), respectively. The acoustic signals were digitized using laptop computers equipped with high-speed cards (National Instruments BNC-2110, Austin, TX, USA, 16bit) for data acquisition. This allowed to create a data base of sound recorded at the habitat temperature. From these recordings we calculated song carrier frequency and correlated this with measurements of vertical distribution.
Collection of specimens: On three different field trips to PNN Gorgona (2003, 2006, and 2007) singing individuals were located by ear or with the help of a bat detector (Ultrasound Advice, U30). Animals calling from the ground to up to 10m were tracked by climbing trees using a ladder and or climbing equipment. The vertical position of the singing individuals was measured using a scaled rope. Canopy-dwelling-animals (i.e.>10m elevation) were occasionally collected in light traps, but most specimens were collected through fogging (see below). In November 11-20, 2007, we sampled arthropods from 16 trees in the eastern sector of the island Gorgona by the technique of insecticidal spraying (fogging), adapted from the protocol of Pimienta (2005). In each tree nylon collecting sheets (2x2m) were installed in horizontal layers between 1.20 and ~20m, with a vertical distance of 4-5 m between layers (Fig. 1ABE). Sheets were place in a way such that the outfit did not prevent individuals from the tree tops to fall into one of the lower layers. In the center of each sheet a collection container with 80% ethanol was installed. Fogging was conducted between 00:00-02:00, a period during which the microclimate (especially low wind speed) facilitated the sampling.
The arthropods were knocked out by a biodegradable pyrethroid insecticide (Permost VPM®) applied with thermal spray resonant pulse principle (Golden Eagle Electric Star XL, Model 2610, series 3), which expels a cloud of insecticide-laden mist with a size of atomized particles of 0.5-50microns (Fig. 1DC). The insecticide cloud was applied directly to the vegetation of each sampling height for 2min using tree climbing equipment; in cases where the collecting sheets were only 1 meter high, the fogging was carried out from the ground to the canopy. After fogging, we waited 2h to allow the insecticide to take effect. After this time, the arthropods were “swept” into the container and preserved in 90% ethanol in labelled vials. Labels included tree number, sheet number and sheet vertical height.
Tettigoniidae specimens were identified at species level. Collected insects of other taxa out of the interest of this project were deposited in the collection of the Museum of Entomology, Universidad del Valle (MEUV) to be studied by experts in particular groups.
Statistical analysis: Because the collection of specimens was performed at different ranges (height or levels), there is no certainty of the preferred height (above the understory vegetation), at which the specimen was perched at the moment of the fogging, but rather we have an idea of his altitude range. Because sheets were placed in a way that every one only gets insects from a specified height interval, we have information of the distributional vertical range of each insect. If a species was found in two different ranges, it was assigned to the upper most range. Thus the vertical distribution was divided into five levels or categories: 0.0-1.0m 1.5-5.0, 5.5-10.0, 10.5-15.0, and 15.5->20m. To determine whether there is a type of correlation between vertical distribution and frequency of the song we performed a Kruskal-Wallis test comparing the five altitude intervals.
Results
The manual and fogging techniques allowed the collection of 136 specimens of the family Tettigoniidae, which belong to 27 species (Table 1). Of these 27 species, 14 species prefer altitudes below 10m (52%), three were found between 10 and 14m, and nine preferred altitudes near 15m (33.3%) (Fig. 2A).
Individuals of 16 of these species were already captured during our work in the PNN Gorgona in the last seven years by manual collection. During these studies we determined with confidence the vertical distribution from the understory vegetation up to 10m, and also studied the acoustic behaviour of these 16 species.
With the new collection method using layered fogging we have confirmed these observations and 11 more species were collected. The vertical distribution for these nine species (see gray boxes Fig. 2) was unknown, however the main components of the call in some of them were studied (e.g., Phlugis sp., Baliophyllum sp.). Therefore the collection of specimens with fogging permitted us to determine at least a range of vertical distribution for them.
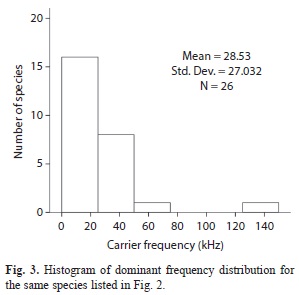
The calling songs and their physical characteristics, especially frequency, were analyzed for 19 of these species from recordings obtained directly in the PNN Gorgona in caged specimens, at room temperature of 26-27ºC (Table 1). For the other species known only from individuals collected by fogging it was not possible to record the song features as the specimens were knocked down, or died immersed in the alcohol of the collection sheets jars. In this case the frequency of the calling song was estimated based on the above-mentioned equation. The species that were subjected to this treatment are highlighted in Table 1 with an asterisk. Fig. 3 shows a histogram of the distribution of carrier frequencies in the population sample. Most carrier frequencies are below 50kHz, with only a few species singing between 50 and 100 kHz. One species used extreme ultrasounds between 137 and 148kHz (see Table 1 for discrete values).
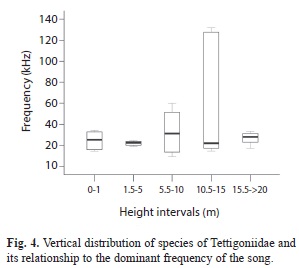
No significant differences in calling song frequency were observed between the different vertical distributions of the species (Kruskal-Wallis: Chi-Square=3.98, df=4, P=0.41, Fig. 4). This means that insects with different sound frequencies, low or high, may share similar microhabitats at similar altitudes. However, we observed that species with extreme frequencies (>100kHz, e.g., Ultrasonus sp.) prefer habitats above 12m but below the canopy where the vegetation is not cluttered.
Discussion
This work constitutes the first study of the physical structures of singing in Neotropical Tettigoniidae and their correlation with the vertical distribution of the species.
We found no correlation between vertical distribution of species and their carrier frequencies. This results are in agreement with Diwakar & Balakrishnan (2007) who did not find significant correlation between calling heights and call features in Ensifera species of a tropical forest in India. However, frequency diversity and shared micro-habitats are apparently related to the exploitation of sound transmission channels that help prevent specific acoustic interference. We showed that 52% of the species prefer heights below 10m, and the possibility of using frequencies between 15-30 kHz appears to increase with increasing height intervals from 0-1m, 5.5-10m to 10-15m. Songs with frequencies near to 20kHz were very common in the range of 1.5-5m. Then the song characteristics, especially the temporal patterns (duration, repetition, etc.) might work better as specific recognition system. It is uncommon to find species using frequencies between 50 and 100kHz in the sampled population (Table 1). Very few species are reported in the literature to sing within this range (Montealegre-Z, 2009).
The preference for vertical distribution at around 15m could be correlated with ultrasonic physical characteristics of the habitat at this level of altitude. At ground level and understory (0-5m) acoustic signals tend to suffer more attenuation than in the upper parts (Marten, Quine & Marler, 1977; Römer, 1993; Römer & Lewald, 1992). At a height of ca. 10m the attenuation of sound seems to be the lowest (Marten et al., 1977). Individuals of Ultrasonus extremus were found in bromeliads and other epiphytic plants around or above 15m. This genus has the most extreme ultrasonic signals ever recorded in arthropods (Montealegre-Z et al., 2006).
We can only speculate that this interval of altitude could provide climatic, physical and spatial conditions, optimal for the transmission of sounds with extreme high frequency. It is possible that at this altitude, with little vegetation and a large acoustic space, environmental channels favour the propagation of ultrasonic sounds over long distances. This effect is called temperature inversion or acoustic lensing, and would represent an airborne analogue of the channels of salinity used by whales to transmit their signals at very long distance (Mercado & Frazer, 1999). Measurements of acoustic conditions at this stratum of the rainforest are required to directly test this hypothesis.
In summary, studying the production, detection and behavioral mechanisms associated with extreme ultrasound signals permits us to identify the principles that govern the transduction and use of ultrasonic frequencies in small animals. Our findings on the vertical distribution of extreme ultrasonic species also highlight the importance of particular physical pressures in the evolution of such acoustic signals in tropical rainforests. Our results might also help to identify the physical principles that have allowed the same basic mechanisms generating the amazing range of signals in Ensifera that communicate acoustically. Many rainforest species are interesting because they allow us to explore the wide range of acoustic signals.
Acknowledgments
This study was supported by National Geographic (Grant No. 7928-05) and National Science and Engineering Research Council of Canada (grant Nº. 238882) of Andrew Mason. We thank Fernando Vargas-S. for helping during fieldwork at PNN Gorgona. The Institute Alexander von Humboldt Fernando Gast, and Fabio Lozano contributed to the thermal spray loan. We are particularly thankful with the Colombian Ministry of Environment for allowing us to work in PNN Gorgona (research permit No. DTSO-G-31) and for providing export permits. Special thanks to Nancy Murrillo-Bohorquez, Margarita Gnecco-Ortiz, Luz Aida Angel-Parra and Aleyda Martinez, and to the functionaries Hector Montaño and Belisario Solis for their invaluable help in the field.
References
Bailey, W. J. (1970(. The mechanics of stridulation in bush crickets (Tettigonioidea, Orthoptera) I. Tegminal generator. Journal of Experimental Biology, 52: 495-505. [ Links ]
Bass, H. E., Sutherland, L. C. & Zuckerwar, A. J. (1990). Atmospheric absorption of sound-update. Journal of the Acoustical Society of America, 88: 2019-2021. [ Links ]
Bennet-Clark, H. C. (1999). Resonators in insect sound production: How insects produce loud pure-tone songs. Journal of Experimental Biology, 202: 3347-3357. [ Links ]
Bennet-Clark, H. C. (2003). Wing resonances in the Australian field cricket Teleogryllus oceanicus. Journal of Experimental Biology, 206: 1479-1496. [ Links ]
Bennet-Clark, H. C. & Bailey, W. J. (2002). Ticking of the clockwork cricket: The role of the escapement mechanism. Journal of Experimental Biology, 205: 613-625. [ Links ]
Diwakar, S. & Balakrishnan, R. (2007). Vertical stratification in an acoustically communicating ensiferan assemblage of a tropical evergreen forest in southern India. Journal of Tropical Ecology, 23: 479-486. [ Links ]
Chamorro, C. (1990). Suelos. In J. Aguirre & J. O. Rangel (Eds.), Biota y ecosistemas de Gorgona (pp. 65-72). Fondo FEN, Bogotá, Colombia. [ Links ]
Erwin, T. L. (1989). Canopy arthropod biodiversity: A chronology of sampling techniques and results. Revista Peruana de Entomología, 32: 71-77. [ Links ]
Heller, K. G. (1988). Bioakustik der europäischen Laubheuschrecken. Margraf, Weikersheim. [ Links ]
Koch, U. T., Elliott, C. J. H., Schaffner, K. H. & Kleindienst, H. U. (1988). The mechanics of stridulation of the cricket Gryllus campestris. Journal of Comparative Physiology, 162: 213-223. [ Links ]
Marten, K., Quine, D. & Marler, P. (1977). Sound transmission and its significance for animal vocalization. Ii. Tropical forest habitats. Behavioral Ecology and Sociobiology, 2: 291-302. [ Links ]
Mason, A. C. & Bailey, W. J. (1998). Ultrasound hearing and male-male communication in Australian katydids (Tettigoniidae : Zaprochilinae) with sexually dimorphic ears. Physiological Entomology, 23: 139-149. [ Links ]
Mercado, E. & Frazer, L. N. (1999). Environmental constraints on sound transmission by humpback whales. Journal of the Acoustical Society of America, 106: 3004-3016. [ Links ]
Montealegre-Z, F. (2009). Scale effects and constraints for sound production in katydids (Orthoptera: Tettigoniidae): Generator morphology constrains signal parameters. Journal of Evolutionary Biology, 22: 355-366. [ Links ]
Montealegre-Z, F., Guerra, P. A. & Morris, G. K. (2003). Panoploscelis specularis (Orthoptera: Tettigoniidae: Pseudophyllinae): Extraordinary female sound generator, male description, male protest and calling signals. Journal of Orthoptera Research, 12: 173-181. [ Links ]
Montealegre-Z, F. & Mason, A. C. (2005). The mechanics of sound production in Panacanthus pallicornis (Orthoptera : Tettigoniidae : Conocephalinae): The stridulatory motor patterns. Journal of Experimental Biology, 208: 1219-1237. [ Links ]
Montealegre-Z, F. & Morris, G. K. (1999). Songs and systematics of some Tettigoniidae from Colombia and Ecuador, part I. Pseudophyllinae (Orthoptera). Journal of Orthoptera Research, 8: 163-236. [ Links ]
Montealegre-Z, F. & Morris, G. K. (2003). Uchuca giglio-tos, Dectinomima caudell and their allies (Orthoptera: Tettigoniidae: Conocephalinae). Transactions of the American Entomological Society, 129: 503-537. [ Links ]
Montealegre-Z, F., Morris, G. K. & Mason, A. C. (2006). Generation of extreme ultrasonics in rainforest katydids. Journal of Experimental Biology, 209: 4923-4937. [ Links ]
Morris, G. K. (1999). Song in arthropods. In K. G. Davey (Ed.), Encyclopedia of reproduction, (Vol. 4, pp. 508-517). Academic Press, San Diego. [ Links ]
Morris, G. K., Mason, A. C., Wall, P. & Belwood, J. J. (1994). High ultrasonic and tremulation signals in neotropical katydids (Orthoptera, Tettigoniidae). Journal of Zoology, 233: 129-163. [ Links ]
Nickle, D. A. & Castner, J. L. (1995). Strategies utilized by katydids (Orthoptera: Tettigoniidae) against diurnal predators in rainforests of northeastern Peru. Journal of Orthoptera Research, 4: 75-88. [ Links ]
Pierce, G. W. (1948). The songs of insects: With related material on the production, propagation, detection, and measurement of sonic and supersonic vibrations. Harvard University Press, Massachusetts, USA. [ Links ]
Pimienta, M. C. (2005). Protocolo para la caracterización de insectos de dosel. Segundo informe, contrato de prestación de servicios No. 173/04. Instituto Alexander von Humboldt, Bogotá, Colombia. [ Links ]
Rangel, O. & Rudas, A. (1990). Aspectos microclimáticos. In J. Aguirre & J. O. Rangel (Eds.), Biota y ecosistemas de Gorgona (pp. 41-51). Fondo FEN, Bogotá, Colombia. [ Links ]
Robinson, D. J. & Hall, M. J. (2002). Sound signalling in Orthoptera. Advances in Insect Physiology, 29: 151-278. [ Links ]
Römer, H. (1993). Environmental and biological constraints for the evolution of long-range signalling and hearing in acoustic insects. Philosophical Transactions of the Royal Society Biological Sciences, 340: 179-185. [ Links ]
Römer, H. & Lewald, J. (1992). High-frequency sound transmission in natural habitats: Implications for the evolution of insect acoustic communication. Behavioral Ecology and Sociobiology, 29: 437-444. [ Links ]
Römer, H., Spickermann, M. & Bailey, W. (1998). Sensory basis for sound intensity discrimination in the bushcricket Requena verticalis (Tettigoniidae, Orthoptera). Journal of Comparative Physiology, 182: 595-607. [ Links ]
Suga, N. (1966). Ultrasonic production and its reception in some neotropical Tettigoniidae. Journal of Insect Physiology, 12: 1039-1050. [ Links ]
Bass, H. E., Sutherland, L. C. & Zuckerwar, A. J. (1990). Atmospheric absorption of sound-update. Journal of the Acoustical Society of America, 88: 2019-2021. [ Links ]
Bennet-Clark, H. C. (1999). Resonators in insect sound production: How insects produce loud pure-tone songs. Journal of Experimental Biology, 202: 3347-3357. [ Links ]
Bennet-Clark, H. C. (2003). Wing resonances in the Australian field cricket Teleogryllus oceanicus. Journal of Experimental Biology, 206: 1479-1496. [ Links ]
Bennet-Clark, H. C. & Bailey, W. J. (2002). Ticking of the clockwork cricket: The role of the escapement mechanism. Journal of Experimental Biology, 205: 613-625. [ Links ]
Diwakar, S. & Balakrishnan, R. (2007). Vertical stratification in an acoustically communicating ensiferan assemblage of a tropical evergreen forest in southern India. Journal of Tropical Ecology, 23: 479-486. [ Links ]
Chamorro, C. (1990). Suelos. In J. Aguirre & J. O. Rangel (Eds.), Biota y ecosistemas de Gorgona (pp. 65-72). Fondo FEN, Bogotá, Colombia. [ Links ]
Erwin, T. L. (1989). Canopy arthropod biodiversity: A chronology of sampling techniques and results. Revista Peruana de Entomología, 32: 71-77. [ Links ]
Heller, K. G. (1988). Bioakustik der europäischen Laubheuschrecken. Margraf, Weikersheim. [ Links ]
Koch, U. T., Elliott, C. J. H., Schaffner, K. H. & Kleindienst, H. U. (1988). The mechanics of stridulation of the cricket Gryllus campestris. Journal of Comparative Physiology, 162: 213-223. [ Links ]
Marten, K., Quine, D. & Marler, P. (1977). Sound transmission and its significance for animal vocalization. Ii. Tropical forest habitats. Behavioral Ecology and Sociobiology, 2: 291-302. [ Links ]
Mason, A. C. & Bailey, W. J. (1998). Ultrasound hearing and male-male communication in Australian katydids (Tettigoniidae : Zaprochilinae) with sexually dimorphic ears. Physiological Entomology, 23: 139-149. [ Links ]
Mercado, E. & Frazer, L. N. (1999). Environmental constraints on sound transmission by humpback whales. Journal of the Acoustical Society of America, 106: 3004-3016. [ Links ]
Montealegre-Z, F. (2009). Scale effects and constraints for sound production in katydids (Orthoptera: Tettigoniidae): Generator morphology constrains signal parameters. Journal of Evolutionary Biology, 22: 355-366. [ Links ]
Montealegre-Z, F., Guerra, P. A. & Morris, G. K. (2003). Panoploscelis specularis (Orthoptera: Tettigoniidae: Pseudophyllinae): Extraordinary female sound generator, male description, male protest and calling signals. Journal of Orthoptera Research, 12: 173-181. [ Links ]
Montealegre-Z, F. & Mason, A. C. (2005). The mechanics of sound production in Panacanthus pallicornis (Orthoptera : Tettigoniidae : Conocephalinae): The stridulatory motor patterns. Journal of Experimental Biology, 208: 1219-1237. [ Links ]
Montealegre-Z, F. & Morris, G. K. (1999). Songs and systematics of some Tettigoniidae from Colombia and Ecuador, part I. Pseudophyllinae (Orthoptera). Journal of Orthoptera Research, 8: 163-236. [ Links ]
Montealegre-Z, F. & Morris, G. K. (2003). Uchuca giglio-tos, Dectinomima caudell and their allies (Orthoptera: Tettigoniidae: Conocephalinae). Transactions of the American Entomological Society, 129: 503-537. [ Links ]
Montealegre-Z, F., Morris, G. K. & Mason, A. C. (2006). Generation of extreme ultrasonics in rainforest katydids. Journal of Experimental Biology, 209: 4923-4937. [ Links ]
Morris, G. K. (1999). Song in arthropods. In K. G. Davey (Ed.), Encyclopedia of reproduction, (Vol. 4, pp. 508-517). Academic Press, San Diego. [ Links ]
Morris, G. K., Mason, A. C., Wall, P. & Belwood, J. J. (1994). High ultrasonic and tremulation signals in neotropical katydids (Orthoptera, Tettigoniidae). Journal of Zoology, 233: 129-163. [ Links ]
Nickle, D. A. & Castner, J. L. (1995). Strategies utilized by katydids (Orthoptera: Tettigoniidae) against diurnal predators in rainforests of northeastern Peru. Journal of Orthoptera Research, 4: 75-88. [ Links ]
Pierce, G. W. (1948). The songs of insects: With related material on the production, propagation, detection, and measurement of sonic and supersonic vibrations. Harvard University Press, Massachusetts, USA. [ Links ]
Pimienta, M. C. (2005). Protocolo para la caracterización de insectos de dosel. Segundo informe, contrato de prestación de servicios No. 173/04. Instituto Alexander von Humboldt, Bogotá, Colombia. [ Links ]
Rangel, O. & Rudas, A. (1990). Aspectos microclimáticos. In J. Aguirre & J. O. Rangel (Eds.), Biota y ecosistemas de Gorgona (pp. 41-51). Fondo FEN, Bogotá, Colombia. [ Links ]
Robinson, D. J. & Hall, M. J. (2002). Sound signalling in Orthoptera. Advances in Insect Physiology, 29: 151-278. [ Links ]
Römer, H. (1993). Environmental and biological constraints for the evolution of long-range signalling and hearing in acoustic insects. Philosophical Transactions of the Royal Society Biological Sciences, 340: 179-185. [ Links ]
Römer, H. & Lewald, J. (1992). High-frequency sound transmission in natural habitats: Implications for the evolution of insect acoustic communication. Behavioral Ecology and Sociobiology, 29: 437-444. [ Links ]
Römer, H., Spickermann, M. & Bailey, W. (1998). Sensory basis for sound intensity discrimination in the bushcricket Requena verticalis (Tettigoniidae, Orthoptera). Journal of Comparative Physiology, 182: 595-607. [ Links ]
Suga, N. (1966). Ultrasonic production and its reception in some neotropical Tettigoniidae. Journal of Insect Physiology, 12: 1039-1050. [ Links ]
1.School of Life Sciences, University of Lincoln, Riseholme Campus, LN2 2LG, UK; fmontealegrez@lincoln.ac.uk
2.School of Life Sciences, University of Lincoln, Riseholme Campus, LN2 2LG, UK; fsarria@lincoln.ac.uk
3.Departamento de Biología, Entomología, Universidad del Valle (Meléndez) Cali, Colombia; cleopim@gmail.com
4.Department Sciences Life, University of Toronto Scarborough, 1265 Military Trail, Scarborough, Ontario, Canada, M1C 1A4; amason@utsc.utoronto.ca
Recibido 18-X-2013. Corregido 20-XI-2013. Aceptado 19-XII-2013.














