Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.60 n.2 San José Jun. 2012
Impoundment effects in the population of Auchenipterus osteomystax (Siluriformes: Auchenipteridae): a Neotropical reservoir case
*Dirección para correspondencia:
Abstract
New impoundments provide opportunities to check whether species that present enough feeding flexibility in natural conditions may take advantage of this situation and, without reproductive restriction, can occupy the most conspicuous habitat in a large reservoir (open areas) and present higher success in the colonization of the new environment. We examined variations in the abundance and feeding of A. osteomystax in two environments, one natural (Sinhá Mariana floodplain lake) and one dammed (Manso Reservoir), during two periods: the first year after the filling phase and three years later. Our goal was to evaluate the occupation of the new hábitat (Manso Reservoir), by this species, as well as to test the hypothesis that in the reservoir, unlike the natural environment, there are remarkable changes in diet between the periods. Fish were sampled monthly in the floodplain lake and in the reservoir during two annual periods using gillnets. To evaluate the differences in abundance of A. osteomystax we employed the Kruskal -Wallis test, and the diet analysis was carried out using frequency of occurrence and volumetric methods. Temporal differences in the diet were tested by Kruskal-Wallis test using the scores from a detrended correspondence analysis. A. osteomystax was significantly more abundant in the floodplain lake, where the captures were higher than in the reservoir in almost all months analyzed, and significant variations in abundance between the two periods were not recorded in either the reservoir or the floodplain lake. The diet variation between the two periods, which had a time lag of three years between them, was much less pronounced in the natural environment, where the resource availability is essentially regulated by seasonality. Thus, our hypothesis was accepted; that is, the interannual variations in the diet of A. osteomystax are more relevant in an artificial environment than in a natural one. Rev. Biol. Trop. 60 (2): 699-708. Epub 2012 June 01.
Key words: pantanal, floodplain lake, feeding changes, insectivores.
Resumen
Los embalses nuevos ofrecen la oportunidad de comprobar si especies que presentan suficiente flexibilidad en la alimentacion en condiciones naturales pueden aprovechar esta situacion y, sin restricciones de reproduccion, ocupar la mayor parte del habitat visible en un gran embalse (espacios abiertos), ademas, presentar un alto exito en la colonizacion del nuevo entorno. Asimismo, examinamos variaciones en la abundancia y alimentacion de A. osteomystax, en dos ambientes, uno natural (Sinha Mariana floodplain lake) y otro alterado (Embalse Manso), durante dos periodos: el primer ano despues de la fase de llenado y tres anos mas tarde. Nuestro objetivo fue evaluar la ocupación del nuevo habitat (Embalse Manso) por esta especie, asi como probar la hipotesis de que en el embalse, a diferencia del ambiente natural, se producen cambios notables en la dieta entre los periodos. Los peces fueron muestreados mensualmente en el lago de la planicie de inundación y en el embalse durante dos periodos anuales con redes de enmalle. Para evaluar las diferencias en la abundancia de A. osteomystax empleamos la prueba de Kruskal-Wallis, y el analisis de la dieta se llevo a cabo con el uso de la frecuencia de ocurrencia y metodos volumetricos. Las diferencias temporales en la dieta fueron probadas con Kruskal-Wallis, se usaron los resultados a partir de un análisis de correspondencia sin tendencia. A. osteomystax fue significativamente mas abundante en el lago de la llanura de inundacion, donde las capturas fueron mas altas, que en el embalse en casi todos los meses analizados, y no se registraron variaciones significativas en la abundancia entre los dos periodos tanto en el embalse como en el lago de inundacion. La variacion en la dieta entre los dos periodos, en los cuales habia un desfase de tres anos entre ellos, fue mucho menos pronunciada en el entorno natural, donde la disponibilidad de recursos es esencialmente regulada por la estacionalidad. Por lo tanto, nuestra hipotesis fue aceptada, es decir, las variaciones interanuales en la dieta de A. osteomystax son mas relevantes en un ambiente artificial que en uno natural.
Palabras clave: pantanal, llanura de inundacion, cambios en la alimentacion, insectivoros.
Success in the colonization of new reservoirs by fish has been explained by morphological and behavioral pre-adaptations associated with the new habitat uses, diet, and reproduction (Fernando & Holcik 1991, Rodriguez-Ruiz 1998, Agostinho et al. 2008). The scarcity of pre-adapted species occupying the open areas in Neotropical reservoirs, which is certainly related to the absence of large lakes in the main river basins of South America, can explain the distribution of fish in such an environment, as they are concentrated essentially in marginal areas (floodplain lakes species), and fluvial zones (rheophilic and migratory species). In general, this success is assessed by comparisons between pre- and post-impoundment (Benedito-Cecilio et al. 1997, Marques et al. 2009).
The catfish Auchenipterus osteomystax (Miranda Ribeiro, 1918) belongs to the restricted group of Neotropical species with morphological pre-adaptations that enable them to occupy the pelagic areas of reservoirs (body shape, orientation of mouth and eyes; Agostinho et al. 1999). Abundance of the genus Auchenipterus in reservoirs during the first years has been reported (Ferreira 1984a, Benedito-Cecilio et al. 1997, Marques et al. 2009). Internal fertilization is a characteristic associated with its success in the occupation of impounded environments (Agostinho et al. 2008), besides its ability to incorporate, opportunistically, zooplankton in the diet (Mol et al. 2007). However, its abundance can be severely reduced after the heterotrophic period (Mol et al. 2007), suggesting that food availability is a key factor in this success.
Nevertheless, for a suitable understanding of the factors involved in this success we need to know the habitat and diet requirements in relation to the available resources in these environments. Reports about remarkable changes (temporal and spatial) in the diet of Neotropical fish have been frequently found in the literature (Araujo-Lima et al. 1995, Albrecht & Caramaschi 2003, Hahn et al. 2004), and they are related to the variations in the availability of food resources (Luz-Agostinho et al. 2006). These variations in natural aquatic systems are principally associated with the seasonality of hydrometeorological conditions (Kalk et al. 1979); consequently, the diet of fish in these environments is also highly seasonal (Welcomme 1979, Hahn et al. 2004). In natural environments, these changes are more or less predictable and gradual, allowing species to make better use of the resource thanks to their adaptations (Hahn & Fugi 2008). On the other hand, the formation of a reservoir changes the physical, chemical, and biological composition of the river, with several environmental effects (Mol et al. 2007, Agostinho et al. 2008), modifying or decreasing the seasonality to which the biota is adapted. Furthermore, the food availability changes on a plurianual scale in a reservoir as a consequence of alterations in nutrient concentrations (longitudinal and transversal stratification and exportation), productivity, biotic interaction, and dam operation. Thus, the formation of an impoundment provides excellent opportunity to test whether species that present enough feeding flexibility in natural conditions may take advantage of this situation and, without reproductive restriction, can occupy the most conspicuous habitat in a large reservoir (open areas) and present higher success in the colonization of the new environment.
In the present study, we examined the variations in abundance and feeding of A. osteomystax in two distinct environments: natural (Sinha Mariana floodplain lake) and dammed (Manso Reservoir), during two periods: the first year after the filling phase (Period I) and three years later (Period II). We intend to evaluate, through the variation in abundance, the occupation by this species of a new hábitat formed by the Manso Reservoir as well as to test the hypothesis that in the reservoir, unlike the natural environment, there are remarkable changes in diet between the periods. This hypothesis is based upon the fact that reservoirs present great temporal changes in the communities and resources during the colonization (Agostinho et al. 1999).
Material and methods
The study area is located in the watershed of Manso/Cuiaba River, Brazil, encompassing two lentic environments, an artificial one, Manso Reservoir (located in Manso River), and a natural one, the Sinha Mariana floodplain lake, situated in the complex area of lakes and wetlands of the Pantanal of Mato Grosso (Fig. 1). The Manso Reservoir (14°32’-15°40’ S and 54°40’-55°55’ W) was created in November 1999 by flooding an area of 427km2. About 80km downstream from the dam, the Manso River joins Cuiabazinho River, forming the Cuiaba River, which flows to lower regions, with a large catchment draining into the Pantanal of Mato Grosso, where the Sinha Mariana floodplain lake (area=11.2km2) is located (Fig. 1).
Fish were sampled monthly in the reservoir and in the lake floodplain during two annual periods: Period I between March 2000 and February 2001 (the first year after the filling phase), and Period II between March 2003 and February 2004 (three years later). Fishes were sampled by a set of gillnets 10m long with different mesh sizes (12 nets; 2.4, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14 and 16cm opposite knots). Nets were set simultaneously in open areas, littoral areas, and at the bottom of the reservoir and in open and littoral areas of the lake during 24hr, and fish were removed in the morning (0800h), in the evening (1600h), and at night (2200h). Immediately after the capture, the fish were identified and measured and the stomachs were removed. The stomachs with food (n=295) were preserved in 4% formaldehyde for diet analysis. Voucher specimens were deposited at the Museum of the Universidade Estadual de Maringa, Parana State, Nup. 928.
The abundance of A. osteomystax was expressed as the number of individuals captured by 1 000m2 of gillnet during 24hr (CPUE). In order to evaluate spatial (reservoir and floodplain lake) and temporal (Periods I and II) differences in the abundance, we employed the Kruskal-Wallis nonparametric test (Zar 1996), since the assumptions of normality and homogeneity were not reached. The evaluation of the A. osteomystax abundance at the surface (open areas), margin (littoral areas), and bottom was performed only for the reservoir, where the samplings were accomplished in the three habitats. The differences in captures were tested using the Kruskal-Wallis test, and when significant differences were detected, the a posteriori test (multiple comparisons of mean ranks for all groups) was used. The significance level adopted was p<0.05.
Stomach contents were examined, and to express the results on diet, volumetric (obtained by water displacement in graduated cylinders) and occurrence frequency methods (Hyslop 1980) were used, combined in the Alimentary Index (IAi) proposed by Kawakami & Vazzoler (1980) and described by the equation: IAi=(Fi*Vi/Σ Fi*Vi)*100, where: i=1, 2, ..., n food items; Fi=frequency of occurrence (%) of the item i, and Vi=percentage of volume of the item i.
With the aim of evaluating temporal differences in diet, we tested the scores of two detrended correspondence analyses carried out individually for the reservoir and floodplain lake (DCA-Hill & Gauch 1980). The matrix used in this ordination was based on the relative frequencies of the volume of food items. The scores from the DCA did not reach the assumptions of normality and homogeneity, and thus the diet differences were tested using the Kruskal-Wallis nonparametric test. The first axis of the DCAs was the only one retained for analysis, whose eigenvalues (0.65 to reservoir and 0.54 to floodplain lake) were greater than 0.20-Matthews 1998).
Results
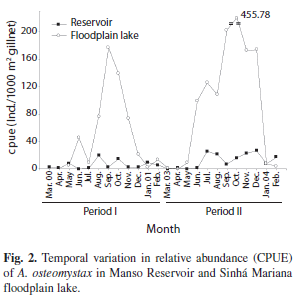
Auchenipterus osteomystax was significantly more abundant (H=5.97, p=0.014) in the floodplain lake, where the captures were higher than in the reservoir in almost all analyzed months (Fig. 2). We did not record significant variations in abundance between the two periods (I and II) in either the reservoir (H=1.6147, p=0.2038) or the floodplain lake (H=1.143, p=0.285). Nevertheless, a strong seasonal trend was observed in the floodplain lake, where A. osteomystax was more abundant in September and October in the two periods.
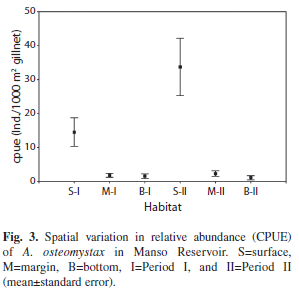
Significant differences in the abundance of A. osteomystax were observed between the habitats within the reservoir during both Period I and Period II (H=13.73, p=0.001 and H=15.827, p=0.0004, respectively) (Fig. 3). The abundance was significantly higher at the surface in both periods (Period I: S and B p=0.0039; S and M p=0.0046; Period II: S and M p=0.0004; S and B p=0.0005), while the abundance of A. osteomystax was not different between the margin and the bottom in either period (Period I: p=0.9973; Period II: p=0.9839).
The diet of A. osteomystax was basically compounded by insects during the two study periods in both locations. Meantime, when considering inferior taxonomic levels, the similarity between the periods was observed only for the population from the floodplain lake. In relation to the population from the reservoir, besides the remarkable variation between the periods, the diet was also different from those in the natural environment (Fig. 4).
The differences in the diet between the periods were significant in the reservoir (H=37.48, p=0.0001). During Period I, Chaoboridae larvae was the dominant food (IAi=76.1%), and in Period II, Chironomidae pupae, Hymenoptera, and Hemiptera were the most consumed ítems (IAi=42%, 23%, 12.4%, respectively) (Fig. 5A, B, C). In Period II, Coleoptera and Chaoboridae were consumed in small amounts, but presented relevant occurrence in the diet (Fig. 5A). In the floodplain lake, we did not register a significant difference in the diet of A. osteomystax between Periods I and II (H=0.513; p=0.4738), and Ephemeroptera was predominant in both (IAi=56.2 and 74.2%, respectively) (Fig. 5E, F). Besides Ephemeroptera, the Chaoboridae larvae were an important food in Period I, totaling 34.8% of the diet, whereas Chironomidae pupae and microcrustaceans were important only in relation to the occurrence (30.3% and 27.3%, respectively) (Fig. 5D). Regarding Period II, there was a decrease in the consumption of Chaoboridae larvae (IAi=3.6%) and an increase in the consumption of microcrustaceans (IAi=17.8%) (Fig. 5F). Chaoboridae larvae presented a lower quantitative participation in the diet during Period II; however, these organisms were an important food, occurring in 54.4% of the stomachs (Fig. 5D).
Discussion
The experimental fishing carried out in Manso Reservoir reveals that the density of A. osteomystax was low compared to the natural lentic environment (floodplain lake), with secondary importance in the reservoir (1.3% and 7.2% of the captures, respectively; A.A. Agostinho, unpublished). The preference of A. osteomystax for lentic habitats and its success in the colonization of reservoirs has been reported frequently in the literature. In the Itaipu and Rosana reservoirs, which are also in the Prata River basin, this species was one of the most abundant in the first years after the impoundment (Agostinho & Zalewski 1995, Benedito-Cecilio et al. 1997, Agostinho et al. 1999), exceeding its density in natural environments. This same result was observed for a congeneric species (A. nuchalis) in the reservoirs of Curua-Una (Ferreira 1984a) and Peixe-Angical (Marques et al. 2009). The colonization of a reservoir by the pre-existing ichthyofauna depends on both the pre-adaptations to the new environment (Fernando & Holcik 1991, Agostinho et al. 1999) and the number of individuals of each population in the environment that will be impounded (Agostinho et al. 2008). Thus, despite the absence of data on the abundance of A. osteomystax in the previous period, the result of low capture in the first months of the reservoir formation suggests that this species was not abundant in the dammed region. Although seasonality in the abundance of this species was evident in both locations, it was more pronounced in the floodplain lake, with high capture during the months of low water and beginning of flooding. The remarkable reduction in the water level during the drought and the displacement that this species performs towards more lotic environments in the tributaries for reproduction (Benedito-Cecilio & Agostinho 2000) may explain this seasonality. Indeed, this species spawns between November and April in lotic areas of Manso River basin (H.I. Suzuki, unpublished), period that coincides with that of Tocantins River (Medeiros et al. 2009).
Many of the fish species from Neotropical reservoirs occupy the margins of these environments (Araujo-Lima et al. 1995, Agostinho et al. 1999, Mol et al. 2007), while the open areas are less occupied, mainly due to the absence of species pre-adapted to lacustrine conditions (Agostinho et al. 1999). Nevertheless, A. osteomystax, which has morphological adaptations to displacement and feeding in pelagic regions (Freire & Agostinho 2000), principally occupied the reservoir surface. Furthermore, A. osteomystax is not considered an obligatory zooplanktivorous species since it feeds primarily on insects (mainly Ephemeroptera; Hahn et al. 1998), but it may be considered as facultative zooplanktivorous, that is, able to use the zooplankton that suddenly becomes abundant in newly formed reservoirs (Strictar-Pereira et al. 2010). Therefore, the predominance of the species in open areas of Manso Reservoir might have been favored by the ability to consume zooplanktonic organisms. A similar indication is given by the higher abundance of this species in surface water in the reservoir transition zone (Ferreira 1984a, Benedito-Cecilio et al. 1997), where the primary productivity and the zooplankton density are higher due to the balance between the light penetration and nutrient concentration (Kimmel et al. 1990, Marzolf 1990).
The success of zooplanktivorous species in new impoundments has been described (Ferreira 1984b, Agostinho et al. 1992, Cassemiro et al. 2003). Although a slight increase in the occurrence of microcrustaceans was observed in the diet of A. osteomystax during the second period in Manso Reservoir, this species may not be classified as typically zooplanktivorous, nor did it present the expected increase in abundance after the formation of Manso Reservoir. Mol et al. (2007) reported that the congeneric species A. nuchalis, which was abundant soon after the formation of Brokopondo Reservoir, was not present during the samplings accomplished 40 years later.
The diet of A. osteomystax was predominantly compounded by insects independently of the location and period. Meanwhile, we verified differences in the composition and abundance of insects consumed by this species, with a higher relevance of aquatic forms in the natural environment (floodplain lake). The main change in the diet observed in the Manso Reservoir three years after its formation was a decrease in the consumption of Chaoboridae. Although this reduction was partially compensated by an increase in the consumption of other aquatic organisms (Chironomidae, Ephemeroptera, and microcrustaceans), the terrestrial organisms Hemiptera, Hymenoptera, and Coleoptera presented more important increases, especially in occurrence. On the other hand, in the population at the floodplain lake, where the variations were less pronounced, the decrease in Chaoboridae was accompanied by a reduction in the values for terrestrial insects and increase in aquatic forms such as Ephemeroptera and microcrustaceans. The predominance of allochthonous resources, especially terrestrial insects, in the diet of Auchenipterus species has been discussed for other reservoirs (Merona et al. 2001, 2003), rivers (Tejerina-Garro & Merona 2010), and várzea lakes of the Amazon (Merona & Rankin-de-Merona 2004). However, the predominance of aquatic forms of insects in the diets of the same species of Auchenipterus is reported for the Itaipu reservoir (Hahn et al. 1998) and lakes of the Upper Parana River floodplain (Hahn et al. 2004).
The intense proliferation of zooplankton that characterizes new reservoirs (Rocha et al. 1999, Bonecker et al. 2001) was not directly exploited by A. osteomystax. Nevertheless, the initial diet of Chaoboridae larvae is based on flagellate protozoans and, during final stages, on microcrustaceans (Arcifa 2000). These larvae may belong to plankton or benthos depending on the time of day and instar (Arcifa 2000, Bezerra-Neto & Pinto-Coelho 2002). The fact that the final instars develop nocturnal migrations to the surface, coinciding with the location and time of higher activity of A. osteomystax (A.A. Agostinho, unpublished), makes the Chaoboridae a dominant prey in the diet. Research performed in the Curua-Una Reservoir, six years after its formation, indicates that where the congeneric species A. nuchalis was more abundant, the diet was clearly dominated by Chaoboridae or microcrustaceans (Ferreira 1984b).
Pupae of Chironomidae together with terrestrial insects compounded the basis of the diet of A. osteomystax three years after the formation of the reservoir. The Chironomidae larvae are among the principal benthic organisms (Higuti & Takeda 2002, Callisto et al. 2002) and in the present study their pupae must have been consumed at the surface during the period when these insects, whose adults are terrestrial, are emerging and may be found at the surface. Besides that, the consumption of Hymenoptera by fish is frequent (Goulding et al. 1988); these terrestrial organisms fall into the water and are mostly consumed by fish that feed at the surface. In this way, although the diet was changing between periods, A. osteomystax continued to consume food available at the surface of the water column, evidencing that this layer was the foraging location in the second period too.
In contrast to what was observed in the reservoir, at the floodplain lake the diet of A. osteomystax did not present significant temporal differences, and Ephemeroptera was the most consumed item during both periods. The high consumption of Ephemeroptera subimagos indicates that, as occurred in the reservoir, A. osteomystax feeds at the surface, since at this stage of development these insects emerge at the surface and become available for capture. In lakes of the Upper Parana River floodplain, the diet of this species was also composed of Ephemeroptera consumed at the surface (Hahn et al. 2004), as recorded for A. nuchalis in the Curua-Una River upstream of the reservoir with the same name (Ferreira 1984b). Meanwhile, in the impounded environment of Itaipu, Ephemeroptera was also the main food item of this species (Hahn et al. 1998).
Therefore, we conclude that the reservoir occupation by Auchenipteridae fish presents variable chance of success, with the most successful occupation being in the superficial layer of the water column in the transition zones of these environments, where the zooplankton density is higher. The seasonality observed in the abundance and the temporal coincidence with reproductive events suggests that the species show active displacements towards waters with higher turbulence. The diet is based on aquatic forms of insects that come to the surface to emerge (Chaoboridae, Diptera, and Ephemeroptera) or terrestrial forms that fall on the surface (Hymenoptera, Hemiptera, and Coleoptera). The variation in diet between the two periods, which had a time lag of three years between them, was much less pronounced in the natural environment (floodplain lake), where the resource availability is essentially regulated by the seasonality. The heterotrophic features of the processes during the first years of the reservoir, due to the flooding of biomass, and the unnatural fluctuations of the wáter level must abort successional processes that lead to biota adjustments, causing the variation in trends in food availability for fish to be random. Thus, our hypothesis was accepted; that is, the interannual variations in the diet of A. osteomystax are more relevant in an artificial environment than in a natural one.
Acknowledgments
We express our appreciation to Nupelia (Nucleo de Pesquisas em Limnologia, Ictiologia e Aquicultura) and Furnas Centrais Eletricas for their financial support and infrastructure, and to the Brazilian Council of Research (CNPq) for providing a grant. We thank Sidinei Magela Thomaz for correcting the English and for helpful comments on the manuscript.
References
Agostinho, A.A. & M. Zalewski. 1995. The dependence of fish community structure and dynamics on floodplain and riparian ecotone zone in Parana River, Brazil. Hydrobiologia 303: 141-148. [ Links ]
Agostinho, A.A., H.F. Julio-Junior & J.R. Borghetti. 1992. Consideracoes sobre os impactos dos represamentos na ictiofauna e medidas para sua atenuacao. Um estudo de caso: reservatorio de Itaipu. Rev. Unimar 14: 89-107. [ Links ]
Agostinho, A.A., L.E. Miranda, L.M. Bini, L.C. Gomes, S.M. Thomaz & H.I. Suzuki. 1999. Patterns of colonization in Neotropical reservoirs, and prognoses on aging, p. 227-265. In J.G. Tundisi & M.S. Straskraba (eds.). Theoretical reservoir ecology and its applications. IIE, Sao Carlos, Sao Paulo, Brazil. [ Links ]
Agostinho, A.A., F.M. Pelicice & L.C. Gomes. 2008. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz. J. Biol. 68: 1119-1132. [ Links ]
Albrecht, M.P. & E.P. Caramaschi. 2003. Feeding ecology of Leporinus friderici (Teleostei; Anostomidae) in the upper Tocantins River, Central Brazil, before and after installation of a hydroelectric plant. Stud. Neotrop. Fauna Environ. 38: 33-40. [ Links ]
Araujo-Lima, C.A.R.M., A.A. Agostinho & N.N. Fabre. 1995. Trophic aspects of fish communities in Brazilian Rivers and reservoirs, p. 105-136. In J.G.Tundisi, C.M.E. Bicudo & T. Matsumura-Tundisi (eds.). Limnology in Brazil. ABC/SBL, Rio de Janeiro, Rio de Janeiro, Brazil. [ Links ]
Arcifa, M.S. 2000. Feeding habits of Chaoboridae larvae in a tropical Brazilian reservoir. Rev. Bras. Biol. 60: 591-597. [ Links ]
Benedito-Cecilio, E. & A.A. Agostinho. 2000. Distribution, abundance and use of different environments by dominant ichthyofauna in the influence area of the Itaipu Reservoir. Acta Sci. Biol. Sci. 22: 429-437. [ Links ]
Benedito-Cecilio, E., A.A. Agostinho, H.F. Julio Jr & C.S. Pavanelli. 1997. Colonizacao ictiofaunistica do reservatorio de Itaipu e areas adjacentes. Rev. Bras. Zool. 14: 1-14. [ Links ]
Bezerra-Neto, J.F.B. & R.M. Pinto-Coelho. 2002. Migracao vertical das larvas de Chaoborus brasiliensis (Diptera: Chaoboridae) em um reservatorio tropical: lagoa do Nado, Belo Horizonte, Estado de Minas Gerais. Acta Scientiarum 24: 329-336. [ Links ]
Bonecker, C.C., F.A. Lansac-Toha, L.F.M. Velho & D.C. Rossa. 2001. The temporal distribution pattern of copepods in Corumba Reservoir, State of Goias, Brazil, p. 375-384. In R.M. Lopes, J.W. Reid & C.E.F. Rocha (eds.). Copepoda: developments in ecology, biology and systematics. Kluwer, London, England. [ Links ]
Callisto, M., V. Vono, F.A.R. Barbosa & S.M. Santeiro. 2002. Chironomidae as a food resource for Leporinus amblyrhynchus (Teleostei: Characiformes) and Pimelodus maculatus (Teleostei: Siluriformes) in a Brazilian reservoir. Lundiana 3: 67-73. [ Links ]
Cassemiro, F.A., N.S. Hahn & T.F.L.V.B. Rangel. 2003. Diet and trophic morphology of the silverside, Odontesthes bonariensis, of the Salto Caxias Reservoir, Rio Iguacu, Parana, Brazil. Neotrop. Ichthyol. 2: 127-131. [ Links ]
Fernando, C.H. & J. Holcik. 1991. Fish in reservoirs. Int. Rev. Gesamten Hydrobiol. 76: 149-167. [ Links ]
Ferreira, E.J. 1984a. A ictiofauna da represa hidreletrica de Curua-Una, Santarem, Para. I- Lista e distribuicao das especies. Amazoniana 8: 351-363. [ Links ]
Ferreira, E.J. 1984b. A ictiofauna da represa hidreletrica de Curua-Una, Santarem, Para. II- Alimentacao e habitos alimentares. Amazoniana 9: 1-16. [ Links ]
Freire, A.G. & A.A. Agostinho. 2000. Distribuicao espaco temporal de 8 especies dominantes da ictiofauna da bacia do alto rio Parana. Acta Limnol. Bras. 12: 105-120. [ Links ]
Goulding, M., M.L. Carvalho & E.G. Ferreira. 1988. Rio Negro, rich life in poor water: Amazonian diversity and food chain ecology as seen through fish communities. SPB, The Hague, Netherlands. [ Links ]
Hahn, N.S. & R. Fugi. 2008. Environmental changes, hábitat modifications and feeding ecology of freshwater fish, p. 35-65. In J.E.P. Cyrino, D.P. Bureau & B.G. Kapoor (eds.). Feeding and digestive functions of fishes. Science, New Hampshire, USA. [ Links ]
Hahn, N.S., A.A. Agostinho, L.C. Gomes & L.M. Bini. 1998. Estrutura trofica da ictiofauna do reservatorio de Itaipu (Parana-Brasil) nos primeiros anos de sua formacao. Interciencia 23: 299-305. [ Links ]
Hahn, N.S., R. Fugi & I.F. Andrian. 2004. Trophic ecology of the fish assemblages, p. 247-259. In S.M. Thomaz, A.A. Agostinho & N.S. Hahn (eds.). The upper Parana River and its floodplain physical aspects, ecology and conservation. Backhuys, Leiden, Netherlands. [ Links ]
Higuti, J. & A.M. Takeda. 2002. Spatial and temporal variations and densities of Chironomidae larvae (Diptera) in two lagoons and two tributaries of the upper Parana River floodplain, Brazil. Rev. Bras. Biol. 62: 807-818. [ Links ]
Hill, M.O. & H.G. Gauch. 1980. Detrended correspondence analysis: an improved ordination technique. Vegetatio 42: 47-58. [ Links ]
Hyslop, E.J. 1980. Stomach contents analysis review of methods and their applications. J. Fish Biol. 17: 411-429. [ Links ]
Kalk, M., A.J. MacLachlan & C. Howard-Willians. 1979. Lake Chilwa: studies of change in a tropical ecosystem. Dr. W. Junk, The Hague, Netherlands. [ Links ]
Kawakami, E. & G. Vazzoler. 1980. Metodo grafico e estimativa de indice alimentar no estudo de alimentacao de peixes. Bol. Inst. Oceanogr. 29: 205-207. [ Links ]
Kimmel, B.L., O.T. Lind & L.J. Paulson. 1990. Reservoir primary production, p. 133-194. In K.W. Thorton, B.L. Kimmel & F.E. Payne (eds.). Reservoir Limnology: ecological perspectives. Wiley, New York, New York, USA. [ Links ]
Luz-Agostinho, K.D.G., L.M. Bini, R. Fugi, A.A. Agostinho & H.F. Julio Jr. 2006. Food spectrum and trophic structure of the ichthyofauna of Corumbá reservoir, Paraná River Basin, Brazil. Neotrop. Ichthyol. 4: 61-68. [ Links ]
Marques, E.E., R.M. Silva & D.S. Silva. 2009. Variaçōes espaciais na estrutura das populaçōes de peixes antes e apos a formaçāo do reservatorio da UHE Peixe Angical, p. 51-57. In C.S. Agostinho, F.M. Pelicice & E.E. Marques (eds.). Reservatório de Peixe Angical: bases ecológicas para o manejo da ictiofauna. Rima, Sāo Carlos, Sāo Paulo, Brasil. [ Links ]
Marzolf, G.R. 1990. Reservoirs as environments for zooplankton, p. 195-208. In K.W. Thorton, B.L. Kimmel & F.E. Payne (eds.). Reservoir limnology: ecological perspectives. Wiley, New York, New York, USA. [ Links ]
Matthews, W.J. 1998. Patterns in freshwater fish ecology. Chapman & Hall, New York, New York, USA. [ Links ]
Medeiros, E.R., A.L. Neuberger & C.S. Agostinho. 2009. Variaçōes sazonais na atividade reprodutiva de peixes na área de influência do reservatorio de Peixe Angical, p. 59-68. In C.S. Agostinho, F.M. Pelicice & E.E. Marques (eds.). Reservatório de Peixe Angical: bases ecológicas para o manejo da ictiofauna. Rima, Sāo Carlos, Sāo Paulo, Brasil. [ Links ]
Mérona, B. & J. Rankin-de-Mérona. 2004. Food resource partitioning in a fish community of the central Amazon floodplain. Neotrop. Ichthyol. 2: 75-84. [ Links ]
Mérona, B., G.M. Santos & R.G. Almeida. 2001. Short term effects of Tucuruí Dam (Amazonia, Brazil) on the trophic organization of fish communities. Environ. Biol. Fishes 60: 375-392. [ Links ]
Mérona, B., R. Vigouroux & V. Horeau. 2003. Changes in food resources and their utilization by fish assemblages in a large tropical reservoir in South America Petit- Saut Dam, French Guiana. Acta Oecol. 24: 147-156. [ Links ]
Mol, J.H., B. Mérona, P.E. Ouboter & S. Sahdew. 2007. The fish fauna of Brokopondo Reservoir, Suriname, during 40 years of impoundment. Neotrop. Ichthyol. 5: 351-368. [ Links ]
Rocha, O., T. Matsumura-Tundisi, E.L.G. Espíndola, K.F. Roche & A.C. Rietzler. 1999. Ecological theory applied to reservoir zooplankton, p. 457-476. In J.G. Tundisi & M.S. Straskraba (eds.). Theoretical reservoir ecology and its applications. IIE, Sao Carlos, Sao Paulo, Brazil. [ Links ]
Rodriguez-Ruiz, A. 1998. Fish species composition before and after construction of a reservoir on the Guadalete River (SW Spain). Arch. Hydrobiol. 142: 353-369. [ Links ]
Strictar-Pereira, L., A.A. Agostinho & L.C. Gomes. 2010. Cage culture with tilapia induces alteration in the diet of natural fish populations: the case of Auchenipterus osteomystax. Braz. J. Biol. 70: 1021-1030. [ Links ]
Tejerina-Garro, F.L. & B. Mérona. 2010. Flow seasonality and fish assemblage in a tropical river, French Guiana, South America. Neotrop. ichthyol. 8: 145-154. [ Links ]
Welcomme, R.L. 1979. Fisheries ecology of floodplain Rivers. Longman, London, England. [ Links ]
Zar, J.H. 1996. Biostatistical Analysis. Prentice Hall, New Jersey, USA. [ Links ]
*Correspondencia a: Elcio Barili & Angelo Antonio Agostinho: Programa de Pos-Graduacao em Biologia Comparada, Universidade Estadual de Maringa. Av. Colombo 5790, Cep 87020-900, Maringa, PR, Brasil; elciobarili@yahoo.com.br
Rosemara Fugi, Gisele Caroline Novakowski & Angelo Antonio Agostinho: Nucleo de Pesquisas em Limnologia, Ictiologia e Aquicultura. Universidade Estadual de Maringa. Av. Colombo, 5790, Cep 87020-900, Maringa, PR, Brasil; rosemarafugi@gmail.com, gcnovakowski@yahoo.com.br, agostinhoaa@gmail.com
1. Programa de Pos-Graduacao em Biologia Comparada, Universidade Estadual de Maringa. Av. Colombo 5790, Cep 87020-900, Maringa, PR, Brasil; elciobarili@yahoo.com.br
2. Nucleo de Pesquisas em Limnologia, Ictiologia e Aqüicultura. Universidade Estadual de Maringa. Av. Colombo, 5790, Cep 87020-900, Maringa, PR, Brasil; rosemarafugi@gmail.com, gcnovakowski@yahoo.com.br, agostinhoaa@gmail.com
Received 02-VI-2011. Corrected 11-IX-2011. Accepted 13-X-2011.