Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.60 n.1 San José Mar. 2012
Successional pattern of phytoplankton (>55μm) in Lekki lagoon, Nigeria
*Dirección para correspondencia
Abstract
Lagoons are dominant features along large stretches of the West Africa coast. These freshwater environments are very valuable areas where phytoplankton constitute the basis of aquatic food webs. In order to know the effects of environmental variables on phytoplankton, a study of the successional pattern of phytoplankton in Lekki lagoon was carried out monthly for two years (June 2003-May 2005). Phytoplankton samples were collected from 12 stations using a plankton net of 55μm mesh, and samples preserved in 4% unbuffered formalin. Besides, surface water samples were taken for physico-chemical analysis. For each year, the seasonal distribution and succession of dominant phytoplankton followed different patterns. Phytoplankton abundance was higher during the dry season (November-April) for the two annual cycles. The diatoms (Aulacoseira granulate and A. granulata var angustissima) and blue green algaes, Microcystis aeruginosa, Merismopedia tennuissima and Trichodesmium lacustre showed this trend by being the abundant species in some of these months. For the rainy season, the green alga Mougeotia sp. dominated. The replacement of one form by another throughout seasonal cycles was probably controlled by the changes in environmental variables such as rainfall, nitratenitrogen and phosphate-phosphorus.
Keywords: phytoplankton, tropical lagoon, shallowness, Lekki lagoon, seasonal changes.
Resumen
La costa oeste de África se caracteriza por la dominancia de lagunas, como ambientes de agua dulce tienen un gran valor, en donde el fitoplancton constituye la base de las cadenas tróficas acuáticas. Con el objetivo de conocer el efecto de las variables ambientales en el fitoplancton, se llevó a cabo un estudio sucesional mensual del fitoplancton, en la laguna Lekki, durante dos años (junio 2003-mayo 2005). En 12 estaciones, se obtuvieron muestras de fitoplancton mediante una red de plancton que tiene una malla de 55μm, que posteriormente fueron preservadas en formalina al 4%. Además, se tomaron muestras de agua superficial, para análisis físico-químico. En cada año, la distribución estacional y la sucesión de fitoplancton dominante siguieron patrones diferentes. La abundancia de fitoplancton fue mayor durante la estación seca (noviembre-abril) durante los dos ciclos anuales. Esta misma tendencia la presentaron las diatomeas (Aulacoseira granulata y A. granulata var angustissima) y las algas verde azules, además de Microcystis aeruginosa, Merismopedia tennuissima y Trichodesmium lacustre, las mismas fueron especies abundantes en algunos de estos meses. En la temporada de lluvias, el alga verde Mougeotia sp. fue la que dominó. La sustitución de una forma por otra a lo largo de los ciclos estacionales fue controlada probablemente por los cambios en las variables ambientales como la lluvia, el nitrato y fósfato.
Palabras clave: fitoplancton, laguna tropical, aguas someras,Laguna Lekki, cambios estacionales.
According to Solarin (1998), Lagoons represent 15% of the world coastal zone and their productivity results from the interaction between oceanic and continental inputs, which enable them to play a considerable biological and economical role, far beyond their seemingly limited geographical extent. Lagoons are dominant features along large stretches of the West Africa coast. They are of utmost importance as natural harbours, nursery grounds for marine fishes and shrimps and often sustain significant fisheries.
Early studies on freshwater phytoplankton in Nigeria included those of Holden & Green (1960), Eaton (1966) investigated the plankton of parts of River Niger, while Imevbore (1967, 1968) studied plankton of Eleiyele reservoir, Ibadan. Imevbore et al. (1969) investigated plankton of Ikogosi warm spring; and Egborge (1973, 1974, 1979) described the phytoplankton of Osun river and subsequently its seasonal variation. Additionally, Dahlin et al. (1985) investigated the composition of the phytoplankton and zooplankton communities in the Niger Delta area, Kadiri (1999) on phytoplankton of some coastal waters in Nigeria, while Powell & Chindah (1986) reported variations in phytoplankton communities and densities along the Bonny/New Calabar estuary. Adeniji (1971) made preliminary investigations into the composition and seasonal variation of phytoplankton in Kainji lake Nigeria. On the South Western coast of Nigeria, algological data on the lagoons are limited to the 9 lagoons (Yewa, Badagry, Ologe, Kuramo, Iyagbe, Lagos, Epe, Lekki and Mahin). Out of these lagoons only Lagos has been subjected to extensive investigations (Hill & Webb 1958, Webb & Hill 1958, Nwankwo 1984, 1993, 1996, 2000). Some published works on Lekki lagoon include Kusemiju (1973, 1976) who studied catfishes of the Lekki lagoon with particular reference to distribution, reproduction and growth of catfish Chrysichthys walkeri (Gunther). Marc et al. (2006) studied species assemblage succession in the shallow tropical lake Guiers, Senegal. Hence this paper aims on the study of the successional pattern of phytoplankton of Lekki for environmental biological monitoring.
Materials and Methods
Study area: Lekki lagoon, a large expanse of shallow freshwater, is located in Lagos and Ogun States of Nigeria, where the alternation of the dry and wet seasons are phenomenal (Adesalu & Nwankwo 2009). It covers an area of nearly 247km2. A greater part of the lagoon is shallow (<3.0m), while some areas are up to 6.0m deep (Adesalu 2007). It lies between 06°25’-06°35’ N and 004°00’-004°13’ E. Two peaks of rainfall are associated with the Lekki lagoon, a major peak in July and a lesser peak in September. There are two peaks of sunshine hours which approximately correspond to the equinoxes. The environment is characterized by fringing macrophyte vegetations which are dominated by mangrove and freshwater types. The mangrove species are dominated by few genera: Rhizophora racemosa (Red mangrove), Avicennia nitida (White mangrove), Acrosticum aureum, Paspalum orbiculare and Dryopteris. The riparian vegetation is also dominated by Cocos nucifera and Terminalia cattapa trees. A number of floating macrophytes find their way through the numerous adjoining rivers, creeks and creeklets into this lagoon. Notably important is Eichhornia crassipes Solm (Mart.) that may disturb navigation by impeding traffic and fishing within the lagoon. Other observed macrophytes were Pistia stratiotes, Lemna pausicaudata and Vosia cuspidata. Associated with these plants are periphytic organisms such as algae and mollusks. The mainstay of human communities that live around this environment is artisanal fishing.
Collection of samples: A motorized boat and Global Positioning System (GPS) were used during the 24 months sampling period (June 2003-May 2005). Phytoplankton samples were collected monthly from 12 stations (Adesalu & Nwankwo 2009) chosen to reflect distance and confluence of rivers and creeks into the lagoon. All samples were taken using a standard plankton net of 55μm mesh size towed steadily for ten minutes at low speed, and samples preserved in 4% unbuffered formalin. For physico-chemical analysis, surface water samples were stored in 1L properly labeled plastic containers with screw caps and transported to the laboratory in ice-chests. In the laboratory, all water samples were stored in refrigera≤40C) for further analysis within 24h. All samples were collected during daylight hours of to minimize variations due to diurnal migration (Bainbridge 1972).
Physical and chemical analysis of water samples: The methods described by America Public Health Association (Apha 1998) were used for physical and chemical analysis. Air and surface water temperature were measured in situ with a simple mercury thermometer. The transparency was measured using a 20cm diameter Secchi disc. The depth was measured with a calibrated pole and the water Ph determined using a Phillips pH meter (Model PW950). The chemical factors determined include salinity, conductivity, dissolved oxygen (DO) and biological oxygen demand (BOD5). Salinity was determined using the Silver Nitrate Chromate titration method as described by Barnes (1980), while Dissolved oxygen content was determined using a Griffin oxygen meter. Biological Oxygen Demand was done after the dissolved oxygen had been measured using the standard method of biochemical consumption of oxygen in five days at 20°C, while conductivity was determined using the HANNA instrument (H18733) and values recorded as mS/cm at 25°C. Chemical oxygen demand and Chloride ion were determined using titrimetric and titration methods, respectively. Oil and grease values were determined using Soxhlet extraction method (APHA 1998). Copper and Iron analysis were done by Atomic absorption spectrophotometer (APHA 1998). The Federal Meteorological Department, Oshodi, Lagos kindly provided rainfall and sunshine hours data for the investigation period.
Nutrient determination: For nitrate determination the colorimetric method was applied (Apha 1998). Phosphate-phosphorus was determined by the ascorbic acid method, and the values obtained were recorded in milligrams per liter (mg/L).
Phytoplankton studies: Dilution factor was used for quantitative analysis of biological samples. From a known volume, a 0.1mL aliquot of the sample was placed in a Palmer- Maloney counting chamber using Eppendorf pipette and enumerated using Olympus BX51 photomicroscope. A total of 30 fields were observed for each sample throughout the analysis. In this study, the filamentous blue green were counted using 10μm of filament length, that represented one unit (unicells), and solitary cells were tallied as separate individuals. Since many algae are colonial and consist of a few to many cells, all colonial forms were counted by the individual cell that comprised that colony, except for Microcystis sp. in which the method used for filamentous algae was applied. For further analysis, one unit was equated as one cell. Calculations for individual and total numbers of organisms per millimeter were made using the following formula:
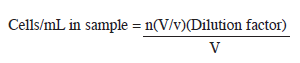
Calculation of number of cells per mL:
Where,
n=cells counted
V=original sample volume
v=volume counted, which is given by the following
calculation (Number of fields x volume of fields)
Volume of fields=One Palmer cell count field=00008954
Number of fields=30
(Number of fields x volume of fields)=(30 x .00008954)=0.0026862
Since specific identification of most species of diatoms could not be determined using high dry objective, samples were acid cleaned using concentrated Nitric acid (HN03). Taxonomic keys employed in the identification included Hustedt (1930, 1937, 1942 and 1971), Patrick & Reimer (1966, 1975), Prescott (1961, 1973 and 1982), Komarek & Fott (1983), Round (1981), John & Robert (2003), Krammer & Lange-Bertalot (1986, 1988, 1991a, 1991b, 2000), Komarek & Anagnostids (1998, 2005) and Wolowski & Hindak (2005).
Community structure: To obtain the estimate of species diversity, three community structure indices were used. The species richness given by the equation (Margalef 1970):
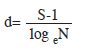
Shannon-Wiener diversity index using the following equation (Shannon & Wiener 1973):
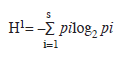
Species equitability with the following equation (Pielou 1975):
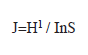
Correlation coefficient values (r): The coefficient of correlation values for the relationship between some physico-chemical parameters and different classes of phytoplankton in the study site were calculated using
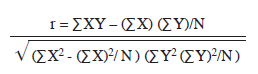
Where r=coefficient of correlation
X and Y=variables under consideration
N=Total number of individuals
Multidimensional Scaling (MDS): Multidimensional scaling (MDS) is specifically designed to graphically represent relationships between objects in multidimensional space. Analysis of similarities (ANOSIM) using Bray-Curtis dissimilarities was applied. The following equation was used:
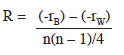
Principal Component Analysis (PCA): Principal Component Analysis (PCA) objects were plotted in multidimensional space with the distance between objects representing the biological dissimilarities. All multivariate plots were obtained using the PRIMER 5 computer program of Clarke & Gorley (2001).
Results
Physico-chemical parameters: The mean physico-chemical characteristics of Lekki lagoon water is shown in Table 1. Mean surface water temperature values ranged between 30.230C and 30.730C with the highest value recorded at station B. The surface water is essentially neutral as pH mean values ranged between 7.38-7.46 throughout the sampling period. Surface water salinity values were low throughout the study period (≤0.55mg/L) while phosphate-phosphorus content (mg/L) recorded mean values ranged from 2.44 to 2.96 in stations L and H respectively. High nitratenitrogen content (3.97mg/L) was recorded ata station L while the low value (2.73mg/L) was recorded in station A. Chloride ion values recorded throughout the study period was generally low with the mean highest value (10.00mg/L) recorded at Station D, and mean values for conductivity in the various stations ranged from 0.46 to 0.62mS/cm.
In general, higher dissolve oxygen content with mean value (4.25mg/L) was recorded at station F. The Biological oxygen demand value ranged from 0.22-0.31mg/L while chemical oxygen demand values ranged from 0.40- 0.36mg/L for all the stations. Oil and grease values were generally low; it ranged from 0.001 to 0.090mg/L throughout the sampling period.
Community structure: Throughout the sampling period, higher species richness (d) value (5.12) was recorded in March 2004 during the first annual cycle while the lowest value (0.18) was recorded in August 2004 of the second annual cycle. Shannon-Weiner (H1) index was generally low throughout the sampling period. The highest diversity (H=4.05) and lowest (H=0.04) values were recorded in August and October 2003 respectively throughout the 12 stations (Table 2). The HI during the wet season apparently was related to the introduction of numerous benthic forms in the phytoplankton. Highest (0.92) and lowest (0.01) equitability values were recorded during the wet season (August and October 2003) at stations I. Station L recorded highest (0.92) equitability value as station L. The Shannon-Weiner information value (H1) and species richness (d) followed almost same pattern with lowest value for species richness (2.09) corresponded with lowest value (2.52) for Shannon-Weiner index (Fig. 1).
Seasonal succession: The seasonal succession of phytoplankton which followed different pattern as shown in Table 3. The phytoplankton abundance was higher during the dry season (November-April) for the two annual cycles.
For example, diatoms (Aulacoseira granulata and A. granulata var angustissima); blue green algae, Microcystis aeruginosa, Merismopedia tennuissima and Trichodesmium lacustre showed this trend by being the abundant species in some of these months. In the rainy season, green alga, Mougeotia sp1 dominated this season in June-July 2003 with Chroococcus turgidus, Tetraspora gelatinosa and M. aeruginosa as the sub-dominant species in June while A. granulata, Oscillatoria germinata and A. islandica were sub-dominant species in July.
In corresponding year (June-July 2004) the diatom, A. granutala was recorded as the dominant species with Mougeotia sp. 11, A. islandica and Microcystis flos-aquae as some of the sub-dominant species. In August 2003 and 2004 A. granulata dominated and have Mougeotia sp. 11, Aphanocapsa delicatissima and Aulacoseira spp. as important members of the phytoplankton spectrum. M. aeruginosa remained dominant in September 2003 and 2004 while two members of the division Cyanophyta (Microcystis flos-aquae and C. minutus) were associated species in addition to Aulacoseira spp. October and November 2003 recorded Aulacoseira granulata as the dominant species while Aulacoseira spp. and Lyngbya hypolimnectica were recorded as the sub-dominant species.
In October 2004, M. aeruginosa took over the succession while T. lacustre for the first time was recorded as the dominant species in November 2004. December 2003 and 2004 also recorded different cyanophytes (Cyanosarcina huebeliorum and M. aeruginosa) as the dominant species. M. aeruginosa and A. granulata were recorded as dominant species in January 2004 and 2005, respectively. For the first time A. granulata maintained the dominance throughout February to April 2004 and 2005. Mougeotia sp. 11 was dominant in May 2004 while May 2005 recorded M. tennuisima.
Generally, A. granulata had variable patterns of abundance as it recorded its dominance both in dry and wet season.The correlation values between different classes of phytoplankton and some physico-chemical parameters showed inverse relationship between Bacillariophyceae, Chlorophyceae, Euglenophyceae and Xanthophyceae with phosphate-phosphorus (Table 4). Phytoplankton dynamics from different stations showed that is being controlled by the same factors and recorded almost same species (Figs. 2 and 3).These factors according to the result generated using Primer 5 software included Phosphate-phosphorus, Nitrate-nitrogen, rainfall, pH and depth.
Discussion
Dilution of lagoon water during the wet season and the degree of dilution and flushing depended on the amount of rainfall in the catchment areas and the attendant effects of flood waters. Flooding caused seasonal changes in the volume of water and the dilution of cations in the lagoon complex. Thomas (1966) and Biswas (1972) reported similar effects of dilution in some water bodies in Ghana. The low phytoplankton biomass in the rainy season may be due to flood water which diluted the physico-chemical environment, reduced retention time, reduced transparency and flushed out phytoplankton taxa. The present observation that diatoms dominate the phytoplankton community confirms earlier reports by Imevbore (1965, 1968) and Egborge (1979) who reported similar results for reservoirs of South Western Nigeria and Nwankwo (1984) for Lagos lagoon and adjacent sea. Centric diatoms were dominant almost all through each annual cycle with major floristic changes between the seasons. Centric diatoms dominance also conforms to Nwankwo (1984) who reported that Melosira was the dominant species in Lagos lagoon and the adjacent sea. The dominance of centric diatoms most especially Aulacoseira granulate could be an indication that Aulacoseira species needed low salinity level to thrive, since an increased salinity value especially in September 2003, favored the blue green algae during which highest relative abundance (96.7%) was recorded with Microcystis aeruginosa recording 95.5% out of the total (96.7%), this indicated that salinity range of 0.85-1.05mg/L probably favored M. aeruginosa and not Aulacoseira sp. In general, pH value recorded in this study highly favored A. granulata which remained dominant at most of the time and this supported Talling (1986) report, who by using data collected from some central African lakes suggested that the effects of pH and alkalinity in lake waters may be reflected in the occurrence of some species such as A. granulata.
The phytoplankton community and the physio-chemical parameters exhibited seasonal changes closely related to the pattern of rainfall. According to Egborge (1974) and Tezuka (1984), seasonal fluctuation in abundance of phytoplankton is influenced by changes in the physical and chemical properties of the water, which themselves can be dependent on rainfall. Differences in abundance of most species during the two seasonal cycles could be attributed to significant variations in the physicochemical variables and nutrients levels within these periods. This probably explained the successional pattern of the phytoplankton taxa, which showed that between September and December 2004, the blue green dominated the phytoplankton spectrum, living Aulacoseira spp. to be sub-dominant among the blue green that dominated the phytoplankton spectrum in one month; or the other during this study were, Cyanosarcina huebeliorum (December 2003) which is a new record for Lagos lagoon complex Merismopedia tennuissima (May 2005), Trichodesmium lacustre (November 2004) and M. aeruginosa that recorded its abundance in many occasions while Mougeotia sp. 1 dominated in June and July 2003 and May 2004. All the green and blue green algae showed dominance in the wet season, when the nitrate level was high, except for M. aeruginosa that appeared in both seasons but most abundantly in wet. The increase and decrease of phytoplankton populations and the replacement of one form by another throughout the season is controlled by the varying environmental parameters such as Phosphate-phosphorus, Nitrate-nitrogen and rainfall. Influence of the sea on phytoplankton of Lekki lagoon is not significant, because is fresh all year through and is not close to the sea as Lagos lagoon.
Acknowledgment
We acknowledge the Fulbright program for the scholarship awarded to Adesalu T.A. and Prof. Rex Lowe of Bowling Green State University Ohio, U.S.A for his assistance.
References
Adeniji, H.A. 1971. Preliminary investigation into composition and seasonal variation of plankton in the Kainji Lake, Nigeria. Abstract International Symposium on Manmade lakes. Ackermann, White and Worthington, 617-619. [ Links ]
Adesalu, T.A. 2007. Phytoplankton dynamics in relation to water quality indices in Lekkki lagoon, Lagos. Ph.D. Thesis, University of Lagos, Lagos, Nigeria. [ Links ]
Adesalu, T.A. & D.I. Nwankwo. 2009. A checklist of Lekki lagoon diatoms. Int. J. Bot. 5: 126-134. [ Links ]
APHA. 1998. Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington D.C., USA. [ Links ]
Bainbridge, R. 1972. The zooplankton of the Gulf of Guinea. Bull. Mar. Ecol. 8: 61-87. [ Links ]
Barnes, R.S.K. 1980. Coastal lagoons. Cambridge University, London, England. [ Links ]
Biswas, S. 1972. Ecology of phytoplankton of the Volta Lake. Hydrobiology 39: 277-288. [ Links ]
Clarke, K.R & R.N. Gorley. 2001. PRIMER v5: User manual/Tutorial. Primer-e, Plymouth, England. [ Links ]
Dahlin, J., S. Hess, P. Duncan & C.B. Powell. 1985. Composition of the Phytoplankton and zooplankton communities of the Niger Delta. Proc. International Seminar on the petroleum industry and the Nigerian environment, Kaduna, Nigeria. [ Links ]
Eaton, J.W. 1966. Algal investigations. The first scientific report of the Kainji Biological Research Team 8-16. White, Liverpool, England. [ Links ]
Egborge, A.B.M. 1973. A preliminary checklist of the phytoplankton of Oshun River, Nigeria. Freshwater Biol. 4: 569-572. [ Links ]
Egborge, A.B.M. 1974. The seasonal variation and distribution of phytoplankton in the Oshun River, Nigeria. Freshwater Biol. 4: 177-191. [ Links ]
Egborge, A.B.M. 1979. The effect of impoundment on the phytoplankton of the Oshun River, Nigeria. Nova Hedwigia 31: 401-417. [ Links ]
Hill, M.B. & J.E. Webb. 1958. The ecology of Lagos lagoon II. The topography and physical features of Lagos harbour and Lagos lagoon. Phil. Trans. R. Soc. Lond. 241: 319-417. [ Links ]
Holden, M.J. & J. Green. 1960. The hydrology and plankton of the River Sokoto. J. Anim. Ecol. 29: 65-84. [ Links ]
Hustedt, F. 1937. Die Kieselalgen Deutschlands, Österreichs und der Schweiz unter Berücksichtigung der übrigen Länder Europas sowie der angrenzendenn Meeresgebiete, p. 609-920. In L. Rabenhorst (ed.). Kryptogamen Flora von Deutschland, Österreich und der Schweiz, Fischer, Stuttgart, Germany. [ Links ]
Hustedt, F. 1942. Susswasser-Diatomeen des indomalayischen Archipels und der Hawaii-Inslen. Int. Rev. Hydrobiol. 42: 1-252. [ Links ]
Hustedt, F. 1971. Kryptogamen-flora von Deutschland, Österreichs un der Schweiz. A Kademiscehe Vergasell Schaft M.L.H., Leipzing, Germany. [ Links ]
Imevbore, A.M.A. 1965. A preliminary check-list of the planktonic organism of Eleiyele reservoir, Ibadan, Nigeria. J. West Afric. Sci. Assoc. 10: 156-160. [ Links ]
Imevbore, A.M.A. 1967. Hydrology and Plankton of Eleiyele reservoir, Ibadan, Nigeria. Hydrobiology 30: 154-176. [ Links ]
Imevbore, A.M.A. 1968. Planktonic algae of Eleiyele reservoir, Nigeria. J. Sci. 2: 85-90. [ Links ]
Imevbore, A.M.A., O.S. Adegoke & A.S. Rogers. 1969. Physical and chemical properties of the Ikogosi warm water spring, Western Nigeria. Nig. J. Mining Geol. 4: 69-81. [ Links ]
John, D.W & G.S. Robert. 2003. Freshwater algae of North America, ecology and classification. Academic, San Diego California, USA. [ Links ]
Kadiri, M.O. 1999. Phytoplankton distribution in some coastal waters of Nigeria. Nigerian J. Bot. 12: 51-62. [ Links ]
Komarek, J. & B. Fott. 1983. Chlorophyceae. Chlorococcales, p. 1044. In G. Huber-Pestalozzi (ed.). Das Phytoplankton de Süsswassers, Systematik u. Biologie, Teil.1 Stuttgart, Germany. [ Links ]
Komarek, J. & K. Anagnostidis. 1998. Cyanoprokaryota 1. Teil: Chroococcales, p. 548. In H. Ettl, G. Gärtner, H. Heynig & D. Mollenhauer (eds.). Süsswasserflora von Mitteleuropa 19/1. Fischer, Stuttgart, Germany. [ Links ]
Komarek, J. & K. Anagnostidis. 2005. Cyanoprokaryota 2. Teil/ 2nd Part: Oscillatoriales, p. 759. In B. Budel, L. Krienitz, G. Gärtner & M. Schagerl (eds.). Süsswasserflora von Mitteleuropa 19/2. Elsevier, Heidelberg, Germany. [ Links ]
Krammer, H. & H. Lange-Bertalot. 1986. Süßwasserflora von Mitteleuropa. Band 2. Bacillariophyceae. Teil 1. Naviculaceae. Fischer, Stuttgart, Germany. [ Links ]
Krammer, H. & H. Lange-Bertalot. 1988. Süßwasserflora von Mitteleuropa. Band 2. Bacillariophyceae. Teil 2. Bacillariaceae, Epithemiaceae, Surirellaceae. Fischer, Stuttgart, Germany. [ Links ]
Krammer, H. & H. Lange-Bertalot. 1991a. Süßwasserflora von Mitteleuropa. Band 2. Bacillariophyceae. Teil 3. Centrales, Fragilariaceae, Eunotiaceae. Fischer, Stuttgart, Germany. [ Links ]
Krammer, H. & H. Lange-Bertalot. 1991b. Süßwasserflora von Mitteleuropa. Band 2. Bacillariophyceae. Teil 4. Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) and Gomphonema. Fischer, Stuttgart, Germany. [ Links ]
Krammer, H. & H. Lange-Bertalot. 2000. Süßwasserflora von Mitteleuropa. Band 2/5. Bacillariophyceae. Part 5. English and French Translation of the Keys. Fischer, Stuttgart, Germany. [ Links ]
Kusemiju, K. 1973. A study of catfishes of Lekki lagoon with particular reference to species of Chrysichthys walkeri (Bagridae). Ph.D. Thesis University of Lagos, Lagos, Nigeria. [ Links ]
Kusemiju, K. 1976. Distribution, reproduction and growth of the catfish Chrysichthys walkeri (Gunther) in the Lekki lagoon, Nigeria. J. Fish. Biol. 8: 453-458. [ Links ]
Marc, B., B. Ngansoumana, K. Samba, S. Seyni, P. Marc & A. Robert. 2006. Phytoplankton community structure and species assemblage succession in a shallow tropical lake (Lake Guiers, Senegal). Aq. Microb. Ecol. 45: 147-161. [ Links ]
Margalef, D.R. 1970. Perspective in Ecological Theory. University of Chicago, Illinois, USA. [ Links ]
Nwankwo, D.I. 1984. Seasonal changes of phytoplankton of Lagos lagoon and the adjacent sea in relation to environmental factors. Ph.D. Thesis, University of Lagos, Lagos, Nigeria. [ Links ]
Nwankwo, D.I. 1993. Cyanobacteria bloom species in coastal waters of South-western Nigeria. Archiv. Hydrobiol. 90: 553-542. [ Links ]
Nwankwo, D.I. 1996. Phytoplankton diversity and succession in Lagos lagoon, Nigeria. Archiv. Hydrobiol. 135: 529-542. [ Links ]
Nwankwo, D.I. 2000. Seasonal variation in estuarine litoral diatom population in Lagos lagoon, Nigeria. J. Pure Appl. Sci. 3: 29-38. [ Links ]
Patrick, R. & C.W. Reimer. 1966. The diatoms of the United States exclusive of Alaska and Hawaii. Acad. Nat. Sci. Phil. [ Links ]
Patrick, R. & C.W. Reimer. 1975. The diatoms of the United States (exclusive of Alaska and Hawaii). Monographs. Acad. Nat. Sci. Phil. [ Links ]
Pielou, E.C. 1975. The measurement of diversity to different types of biological collections. J. Theor. Biol. 13: 131-144. [ Links ]
Powell, C.B. & A.C. Chindah. 1986. Variations in phytoplankton communities and densities along the middle and upper reaches of the Bonny/New Calabar estuary. Man and Biosphere Workshop on Wetlands in Niger Delta Port-Harcourt, Nigeria. [ Links ]
Prescott, G.W. 1961. Algae of the western Great Lakes area with an illustrated key to the genera of desmids and freshwater diatoms. Brown, Iowa, USA. [ Links ]
Prescott, G.W. 1973. Algae of the Western Great Lakes Area. Brown, Iowa, USA. [ Links ]
Prescott, G.W. 1982. Algae of the Western Great Lakes area with an illustrated key to the genera of desmids and freshwater diatoms. Brown, Iowa, USA. [ Links ]
Round, F.E. 1981. The ecology of algae. Cambridge University, London, England. [ Links ]
Shannon, C.E. & W. Weiner. 1973. The mathematical theory of communication. University of Illionois, Illinois, USA. [ Links ]
Solarin, B.B. 1998. The hydrobiology, fishes and fisheries of the Lagos lagoon, Nigeria. Ph.D. Thesis, University of Lagos, Lagos, Nigeria. [ Links ]
Talling, J.F. 1986. The seasonality of phytoplankton in African lagoons. (The Useu lakes in Morocco and South Africa). Hydrobiology 138: 139-160. [ Links ]
Tezuka, Y. 1984. Seasonal variations in the dominant phytoplankton. Chlorophyll a and nutrient levels in the pelagic regions of Lake Biwa. Jap. J. Limnol. 45: 126-137. [ Links ]
Thomas, J.D. 1966. Some preliminary observations on the fauna and flora of a small Man-made lake in the West Africa savannah. Bull. Inst. Fondam. Afrique Noire Sci. Nat. 28: 542-562. [ Links ]
Webb, J.E. & M.B. Hill.1958. The Ecology of Lagos Lagoon. IV. On the reactions of Branchiostoma nigeriense Webb to its environment. Phil. Trans. R. Soc. Lond. 241: 355-391. [ Links ]
Wolowski, K. & F. Hindak. 2005. Atlas of Euglenophytes. Slovak Acad. Sci., Cracow, Poland. [ Links ]
*Correspondencia a: Taofikat Abosede Adesalu: Department of Botany, University of Lagos, Nigeria; boseadesalu@yahoo.com
Dike Ikegwu Nwankwo: Department of Marine sciences, University of Lagos, Nigeria
1. Department of Botany, University of Lagos, Nigeria; boseadesalu@yahoo.com
2. Department of Marine sciences, University of Lagos, Nigeria
Received 21-IX-2010. Corrected 01-VIII-2011. Accepted 05-IX-2011.














