Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.58 n.4 San José Dec. 2010
Temporal variation of phytoplankton in a small tropical crater lake, Costa Rica
1. Centro de Investigación en Ciencias del Mar y Limnología (CIMAR), Universidad de Costa Rica, San José, Costa Rica; gerardo.umana@ucr.ac.cr
2. Escuela de Biología, Universidad de Costa Rica, San José, Costa Rica.
Dirección para correspondencia
Abstract
The temporal variation in lake’s phytoplankton is important to understand its general biodiversity. For tropical lakes, it has been hypothesized that they follow a similar pattern as temperate ones, on a much accelerated pace; nevertheless, few case studies have tried to elucidate this. Most studies in Costa Rica have used a monthly sampling scheme and failed in showing the expected changes. In this study, the phytoplankton of the small Barvas’s crater lake was followed for more than three years, first with monthly and later with weekly samplings, that covered almost two years. Additional information on temperature and oxygen vertical profiles was obtained on a monthly basis, and surface temperature was measured during weekly samplings around noon. Results showed that in spite of its shallow condition (max. depth: 7m) and low surface temperature (11 to 19°C), the lake stratifies at least for brief periods. The phytoplankton showed both, rapid change periods, and prolonged ones of relative stasis. The plankton composition fluctuated between three main phases, one characterized by the abundance of small sized desmids (Staurastrum paradoxum, Cosmarium asphaerosporum), a second phase dominated by equally small cryptomonads (Chryptochrysis minor, Chroomonas sp.) and a third phase dominated by the green alga Eutetramorus tetrasporus. Although data evidenced that monthly sampling could miss short term events, the temporal variation did not follow the typical dry and rainy seasons of the region, or any particular annual pattern. Year to year variation was high. As this small lake is located at the summit of Barva Volcano and receives the influence from both the Caribbean and the Pacific weather, seasonality at the lake is not clearly defined as in the rest of the country and short term variations in the local weather might have a stronger effect than broad seasonal trends. The occurrence of this short term changes in the phytoplankton of small tropical lakes in response to weather variations needs to be further explored in other lakes. Rev. Biol. Trop. 58 (4): 1405-1419. Epub 2010 December 01.
Keywords: Phytoplankton biodiversity, tropical lake, crater lake, temporal variation, community dynamics.
Resumen
Se ha especulado que la variación temporal del fitoplancton en lagos tropicales sigue un patrón similar al de lagos templados de forma más acelerada, pero pocos estudios de caso han abordado el tema. La mayoría de los estudios en lagos de Costa Rica se han basado en muestreos mensuales y no han logrado mostrar los cambios señalados. En este trabajo se estudió el fitoplancton del lago del volcán Barva por un periodo de más de tres años, al principio con un muestreo mensual y al final con un muestreo semanal por casi dos años. Mensualmente se hicieron perfiles verticales de temperatura y durante el periodo de muestreo semanal se registró la temperatura superficial cerca del medio día. A pesar de su condición somera (7m) y baja temperatura superficial, el lago se estratifica por periodos breves. El fitoplancton mostró tanto periodos de cambio rápido como periodos de estabilidad relativa. La composición fluctuó entre tres condiciones principales, la primera fase se caracteriza por la abundancia de desmidios (Staurastrum paradoxum, Cosmarium asphaerosporum), una segunda dominada por cryptomonadaceas (Chryptochrysis minor, Chroomonas sp.) y la tercera por Eutetramorus tetrasporus. Aunque fue evidente que los muestreos mensuales pueden obviar eventos de corta duración, la variación temporal no se ajustó a las estaciones de la zona. Dado que el lago se localiza en la cima del volcán Barva y recibe la influencia del clima tanto del Caribe como del Pacífico, la estacionalidad no está bien marcada en el sitio. Como resultado, las variaciones en el tiempo atmosférico local de corto plazo pueden tener un mayor efecto que los patrones estacionales del clima regional.
Palabras clave: biodiversidad del fitoplancton, lagos tropicales, lagos cratéricos, variación temporal, dinámica de comunidades.
Temporal variation of phytoplankton is one of the factors that explain the diversity of species of the plankton (Padisak 2003, Reynolds 2006), which was once considered as a paradox of theoretical ecology (Hutchinson 1961). This variation is well established and described for temperate lakes, and several models exist to explain the seasonal fluctuations in community composition (Reynolds 1984,2006, Sommer 1989). On the other hand, in tropical lakes this variation has been little studied, although there are a few hypotheses about how the plankton community changes along the year. Lewis (1978) proposed that tropical lake phytoplankton follows the same basic sequence that occurs in temperate lakes: it starts with a community dominated by diatoms and Cryptophyta during the turn-over event which is also the time of nutrient enrichment. Next, as the stratification develops green algae (Chlorophyta) first and then Cyanobacteria dominate the community, ending with dominance by dinoflagellates when stratification is fully established. However, in contrast to temperate zone lakes, Lewis (1978) suggested that this sequence occurs in short periods of time, following each circulation event, and subsequent re-stratification. Moreover, the succession is thought to be frequently interrupted by changes in the weather conditions which stops the process and sets it back to the beginning.
Lewis (1978, 1986) studies have concentrated in big tropical lakes such as Lake Lanao (Phillipines) (Lewis 1978) and Lake Valencia (Venezuela), respectively. These lakes stratify according to a marked seasonal pattern. Nevertheless, much smaller tropical lakes do not usually stratify in such a predictable and relatively long lasting pattern. Their circulation is more dependent on weather conditions which vary more frequently, as has been noted by Lewis himself (Lewis 1996). It is not clear whether the sequence in these shallower lakes follows the same pattern seen in large tropical lakes. For example, in studies carried in Costa Rica on lakes such as the Arenal Reservoir (Umaña & Collado 1990) and smaller lakes like Hule, Bonilla and Cote (Umaña 1988,1993,1997), the same species have been observed dominating the community with time. Frequent circulation could be the main reason why changes in the plankton community in Costa Rican lakes do not seem to follow the pattern predicted by Lewis (1978). There are other alternative explanations, however, one being the existence of alternative equilibrium states in lakes, especially in shallow ones (Scheffer 2004). Melack (1979) described shifts in the productivity of some tropical African lakes followed by periods of relative stability at the new conditions. More recently Scheffer (2004) suggested that planktonic communities are intrinsically chaotic, with fluctuations being the result of complex trophic and competitive interactions among the species. Nevertheless, a further alternative possibility is that the apparent discrepancy is an artifact of sampling frequency. Monthly sampling may be insufficient to detect the rapid succession events which may be characteristic of tropical lakes. Horn (1984), already noted that in temperate lakes, a more frequent sampling effort is necessary during the growing season, which resembles the tropical, year round conditions in terms of light and temperature.
Research on plankton in Costa Rican lakes has been based generally on monthly or quarterly sampling designs (Camacho & Charpentier 1989, Umaña & Collado 1990, Umaña 1993,1997). This has always left in doubts whether these low frequency samplings are really ignoring valuable information about temporal changes in phytoplankton. The main objective of the present work is to record the changes in the water column condition and the plankton community in a small tropical crater lake, and test whether short term changes in the phytoplankton follow the predicted pattern as proposed by Lewis (1978).
Materials and methods
Lake Barva is a small lake (0.77ha surface area, 7m deep), located at an old crater in Barva volcano, Costa Rica, at 1860m above sea level. In order to determine if there are phytoplankton fluctuations that are missed with quarterly samples, this study performed a weekly sampling period which may reflect particular successional chan Surface water samples of 100ml total volume were taken from surface water in Lake Barva from 1994 to 1998. In the first two years, sampling was made on a quarterly basis. In the last two years samples were taken weekly for several months intervals according to the availability of a national park ranger volunteer. Samples were preserved with acetic Lugol’s solution (Sournia 1978). Samples were settled for two days in 100ml cylinders and then the supernatant water was withdrawn with a U-shaped siphon, leaving the settled cells in the bottom. The concentrated sample was counted in a Palmer-Malloney chamber, according to the techniques described by Lund et al. (1959). Phytoplankton was counted in two diametric transects, stopping the count for those species that attained 100 counting units. Scanned area was noted for each species. Counting units (solitary cells, colonies or filaments according to the species morphology) were transformed into cell density per milliliter. Species identification of the plankton algae was performed according to (West & West 1904,1905,1908, 1912, 1923, Huber-Pestalozzi 1941, 1955, 1961, 1968, Prescott 1962, Whitford & Schumacher 1973, Kováčik 1975, Comas González & Permán 1978, Comas González 1980, Parra et al. 1981, Komárek 1983, Komárek & Anagnostidis 1986, Krammer & Lange-Bertalot 1986, Anagnostidis & Komárek 1988, Krammer & Lange-Bertalot 1988, Komárek & Anagnostidis 1989, Anagnostidis & Komárek 1990, Krammer & Lange-Bertalot 1991a, Krammer & Lange-Bertalot 1991b, González González & Mora-Osejo 1996, Wehr & Sheath 2003).
Along phytoplankton samples, surface temperature and Secchi disk transparency were registered on every occasion. For the first two years, and for selected dates later on, the vertical temperature profiles were determined at the middle of the lake with an YSI® oxygen meter (Model 57).
In order to observe the effect of sampling frequency, the variation with time of abundance of some species was plotted. Three plots were obtained. The first includes all the samples, the second includes samples separated by a month interval, and the third includes the samples taken every three months. The three plots were compared visually to determine if there were changes in the weekly sampling that did not appear in the less frequent sampling intervals. Additionally, the Shannon-Wiener index (H’) was used to estimate the variability of information accounted by all three plots per species, in a similar way, as it has been used for measuring niche breath (Krebs 1999). The resulting values were compared using a Kruskall-Wallis non parametric analysis of variance to test for differences in the uncertainty or variability among the plots.
The similarity among all samples from 1994 to 1999 was calculated using the Bray-Curtis Similarity Index, after eliminating the rare species according to a modification of the criteria of Olmstead-Tuckey (Sokal & Rohlf 1995), which classifies as rare those species with a frequency and total abundance lower than the median values for all species (López & Serna 1999). The resulting similarity matrix was used for a Cluster Analysis using the Average Grouping method. A Detrended correspondence analysis was also performed as a confirmatory analysis, using the Past 3.2 (Hammer et al. 2001) statistical package.
Results
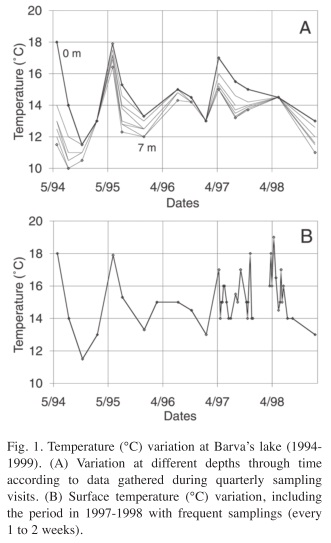
Temperature: Temperature showed an interesting variation over the year since it departs from the usual pattern expected for high altitude tropical lakes. Surface temperature was quite variable; it oscillated between 11 and 18°C. It can vary up to 4°C in a week (Fig. 1A, B). In addition, the lake shows stratification periods, with a temperature difference between surface and bottom greater than 1°C and up to 6.5°C (Fig. 1A). At the same time, bottom temperature also oscillates over a wide range, from 10 to 17°C, following closely the variation of surface temperature.
Phytoplankton: A total of 75 species of phytoplankton were observed in the study period (Table 1). The most diverse algal group was Chlorophyta (41 species), followed by the Bacillariophyceae (11 species). The number of species per sample varied from 12 to 27, with a mean of 19 species per sample. The most frequent 16 species are highlighted in Table 1. These 16 species were not necessarily highly abundant (for example Peridinium volzii and Arthrodesmus incus) but their high frequency and ease of identification allow their use in the analysis.
To examine the effect of sampling frequency, three plots were obtained for selected species. The first includes all the samples from May 10th, 1997 to July 15th, 1998, (Fig. 2), the second includes samples separated by a month interval (Fig. 3) and the third includes the samples taken every three months (Fig. 4). In most cases, there were duplicate samples per date. The Shannon-Weaver index calculated to describe the variability of each plot for each species showed a decrease from the weekly sampling (Median=3.38, Range=2.44-3.84), to monthly (Median=2.73, Range=1.91-3.07), to quarterly sampling (Median=1.59, Range=0.64- 2.02). These differences gave a significant result with the Kruskall-Wallis test (H=29.08, p=0.00). However, the amplitude of the species abundances variation was not significantly different among the three sampling frequencies (H=0.24, p=0.89), meaning that relative variation was similar in the three sampling regimes, although total information was much reduced as sampling frequency decreased.
As can be seen in figures 2, 3 and 4, variation in weekly samples is high. Nevertheless, since the overlap between values of one week and those of the next is often high, not all changes that occur from week to week reflect actual changes in the community composition. The high overlap denotes a lack of significant differences between adjacent weeks. The differences observed may well indicate estimation errors, which are fairly high for phytoplankton counts. Notwithstanding, there are cases where the observed variations are very marked, with population peaks that are established within a few weeks. Some of the peaks disappeared in a short time. These short term peaks can go unnoticed in a quarterly sampling program, as is the case for Eutetramorus tetrasporus, Scenedesmus bijuga and Cosmarium asphaerosporum. These peaks were observed in the monthly sampling interval, but as expected, the actual variation curve in monthly sampling does not reveal all the detail seen in the weekly sampling.
The analysis of the variation by major taxonomic groups during the whole study period does not show a clear cyclical variation (Fig. 5). At the beginning of the study period, Chlorophyta had a high density, mainly through the contribution of E. tetrasporus. There is a peak of Dinophyceae when the green algae are waning, but they did not attain dominance levels, followed by a small peak in the abundance of Cryptophyta. From then on, the Chlorophyta increased and dominated the community for the rest of the period, oscillating at high percentages of the total abundance (>80%), mainly due to the high abundance of C. asphaerosporum.
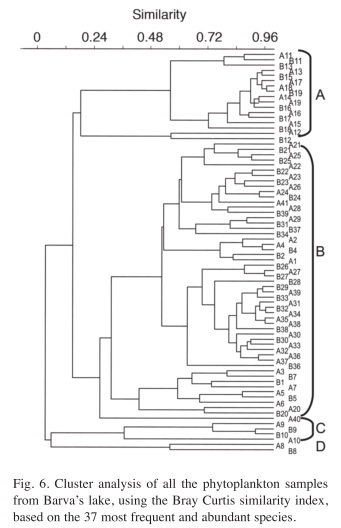
After eliminating the rare species, a total of 37 species remained in the analysis. The results of the Cluster Analysis show that the samples can be grouped in three main clusters (Fig. 6). The first one includes all samples from May 27th 1994 until March 22nd 1996 (Samples from A1 to B7 in Figs. 6 and 7) and again from August 29th 1997 until the end of the study period (Samples from A21 to A41). This group is characterized by the dominance of desmids, specially Staurastrum paradoxum and C. asphaerosporum. The second cluster includes the samples from August 10th 1996 (samples A8, B8) to February 14th 1997 (samples from A9 to B10). This second group was dominated by Cryptophytes (Chryptochrysis minor and Chroomonas sp.). The third group, from May 10th 1997 to July 28th 1997 (samples from A11 to B19) was dominated by the colonial green algae E. tetrasporus, which attained densities higher than any other dominant species in the whole study period, but only for a brief period. The Detrended correspondence analysis among all the dates based on the 16 most frequent phytoplankton species shows roughly the same pattern, with a grouping of the dates in three main clusters just described above (Fig. 7). Rare species or those that appear with low frequency do not really add more information to the present analysis.
Discussion
Temperature: The observed variation in lake temperature implies that it can cool down or warm up as a whole at any time during the year, in an irregular pattern, without a uniform minimum temperature at the bottom. According to its depth it can be considered as a discontinuous polymictic lake (Lewis 1996); however, according to Hutchinson & Löffler (1956) analysis, it was not expected to stratify even for brief periods of time, since it is located at a high elevation and in a windy region. An explanation for this is that the heat exchange at the lake surface does not impact down into the water column unless there is a period of very strong winds and lower temperatures due to high cloud cover. Another reason is related to the dark brown color of the water, which prevents the heating of deeper layers in the water column, as has been explained by Mazumder & Taylor (1994). Nevertheless, it is of interest to note that in some instances, the water column cools down without loosing its thermal gradient. Due to the lake’s location and its shallowness it cannot be excluded that the lake turns over very often, stratifying again after a few sunny days. According to the previous discussion, the lake can be classified as warm polymictic following Löffler’s terminology (Löffler 1973, Osborne 2000) or intermittent polymictic in Lewis (1996) terms.
During the study period an El Niño (ENSO) event took place, which coincided with the weekly sampling period. The ENSO period (1997 to 1998) does not show any particularly higher temperature, although a maximum of 18°C was recorded in April 1998. The main difference of this event from previous times was that the temperature never fell below 14°C as happened before 1997 and in February 1999.
Phytoplankton composition: The species composition at Barva Lake is different from other volcanic or high mountain lakes in Costa Rica. Botos Lake was dominated by dinoflagelates (Hargraves & Viquez 1981, Umaña 2001), Chato Lake was dominated by desmids (Arthodesmus bifidus) and Cryptophyta was not found in the samples (Umaña & Jiménez 1995). On a global scale, the phytoplankton composition of crater lakes in the tropics is variable, and mainly composed of cosmopolitan or pantropical species as discussed by Schabestsberger et al. (2009), with some common features such as the dominance of small, single-celled Chlorococcales and desmids, the presence of thin filamentous Cyanobacteria such as Lyngbya limnetica, of dinoflagellates, usually of the genus Peridinium, of small Cryptophyta and a few diatoms (Nyakoojo 2010, Nyakoojo & Byarujali 2008, Schabetsberger et al. 2004, Schabetsberger et al. 2009, Messyasz et al. 2007, Banderas-Tarabay 1997, Oliva et al. 2001), as has been found in Barva Lake. In comparison with a former description of the phytoplankton of Barva Lake (Umaña 1988), the present results showed that the assemblage changes back and forth between different compositions or phases, since at the beginning of the present study the composition was different from that in 1983, but later it returned to a similar condition as was previously observed.
Although the total number of species is high compared with some other tropical crater lakes, with 75 taxa identified, only a few species can be considered as common or frequent in the lake. The criteria used to separate rare species yielded a total of 38 rare species. This means that the overall similarity, taken as the mean species per sample relative to the total number species is 0.25, a low value, indicating a high turnover of species among the samples. Another way to look at this issue is that only 15% of the species were present in more than 75% of the sampling dates, and 52% of the species were present in less than 10% of the sampling dates. These rare species most probably are those with low populations that do not colonize successfully the site due to competition, unfavorable conditions and/or predation; they are maintained in the system at low densities or through repeated immigration, but going extinct soon after colonization, such as is explained by the island biogeographic model (MacArthur & Wilson 1967). Colonization occurs through the deposition of propagules in dust or rain, or from the activation of resting stages in the lake sediments. This high turnover of species adds to the species richness of the lake, but they may be viewed as not contributing to the main functioning of the lake as a whole. A similar idea to this comes from conservation ecology that talks of species that are "ecological extinct" (Primack 2002), so that they make little contribution to community processes such as competition, but are part of the whole diversity of the lake, which was considered as paradoxical by Hutchinson back in 1961.
Phytoplankton variation: It can be concluded that quarterly samplings did miss important variations in some species, and therefore that this sampling frequency does not give a good indication of the annual variation of phytoplanktonic populations. Monthly samples gave a better view of the variation, and in some cases enough; it was able to cover the main tendencies that were observed in the study period with the more frequent sampling program, but could miss some transient states where some species have a high abundance for short periods of time. More frequent samplings, in this case weekly, do give more information. Nevertheless since the uncertainty of phytoplankton counts is quite high, getting in the best case down to 20% (Venrick 1978), most of the minor variations that are detected with the more frequent sampling program can be attributed to measurement errors that do not necessarily reflect real population variations in the environment.
One possibility thus, is that species can show population peaks of short duration, most probably as a result to changing environmental conditions, and can disappear quickly. It follows that these peaks pass unnoticed by a monthly sampling routine. This is important since the "seasonal" or successional changes predicted by Lewis (1978) could be very rapid and be unobserved until now in Costa Rican lakes. It is also possible that the community goes through a period of rapid change as a response to environmental conditions, and then remains fairly constant for a long time without major shifts in species composition as long as conditions remain stable. A similar behavior has been observed for primary productivity in tropical lakes (Melack 1979).
This was observed in some cases in Barva’s lake, where rapid changes were followed by periods of relative stability, such as the case for Ankistrodesmus braunii, E. tetrasporus and C. minor. Scheffer (2004) speaks about a hysteresis in the fluctuation between alternative states in phytoplankton composition, which means that switching between states can be sudden after a period of slow decline of the dominant groups when a threshold point is reached.
Weekly sampling data covered almost two years, mainly in the period from April to September. The nine species examined more closely, showed a different pattern of variation each year, which didn’t support the idea of a seasonal variation pattern that repeats itself each year. From figures 2, 3 and 4, it is clear that E. tetrasporus presented a peak in 1997 but not in 1998. The same happened with A. braunii. The desmid C. asphaerosporum showed an overall increase during the study period as did S. paradoxum. On the other hand, C. minor had two peaks, each one by September each year. A similar pattern was observed for S. bijuga but during June and July. The dinoflagelate Peridinium inconspicuum also has a peak that is repeated at almost the same time both in 1997 and 1998, around August and September. A. incus, Desmactracum sp. and P. volzii showed low densities without a clear cyclical pattern. In the case of Cryptomonas spp. no clear pattern was observed, which could be the result of pooling the data for several species for the analysis. However, at the level of higher taxonomic groups, the general pattern of alternation of green algae and cryptophytes is more evident (Fig. 5). It seems to be a controversy about this issue, since some authors have claimed the existence of more or less defined successional sequences of species for a given lake (Reynolds 1984,2006), whereas others are more prone to view the process as less predictable and more chaotic (Sheffer 2004). In the Barva lake case, the variation seems to be of low predictability at the species level, although more data is needed to test if it can be considered chaotic.
The data also showed that the variation in the conditions resulting from the influence of weather changes from one year to the next is considerable. No periodicity or seasonality was detected in the study period which covered several years. Samples taken at about the same time year after year yielded different community compositions. From the present data, it seems that local minor variations during the day to day weather changes, especially fluctuations in wind strength, are more important in determining the water column behavior and changes in the plankton community, than the period of the year by it self. As already mentioned, the location of Lake Barva renders the weather less seasonal than in other parts of Costa Rica (Herrera 1986). This short term variation can preclude the establishment of a well defined successional variation in the plankton communities as stated by the general theory of plankton succession (Sommer 1989, Padisak 2003, Reynolds 2006). The frequent disturbances due to weather changes in light availability and turbulence can be an important factor in this respect. It will be necessary to have data on other small tropical lakes, with weekly sampling programs at least during critical periods of the year, and spanning several years of sampling, to get a better idea of seasonal variations and succession in the plankton communities.
Notwithstanding all the variations, the analysis of phytoplankton species composition at Barva’s lake allowed the identification of three main phases: one dominated by small desmids covering most part of the study period, a second characterized by the dominance of small Cryptophyta flagellates, which was similar to the composition of the phytoplankton observed in this lake in 1982 (Umaña 1988), and a third phase, in between the other two, when there was a short period dominated by the colonial green alga E. tetrasporus. This species was not detected prior to its dramatic increase, and remained at low abundances afterwards. It could represent a transient state or a response to unusual weather conditions due to the El Niño event that occurred.
Finally, the results support the notion that temporal variation, even in this small tropical lake, located in a region of low seasonal weather variation, enhances the global phytoplankton diversity of the lake, and explains in part, the coexistence of much larger number of species in a rather uniform environment such as the limnetic habitat of a small lake (Reynolds 2006). The importance of temporal variation in the conditions of the lake has been one of the main factors that explain this coexistence of a large number of species since Hutchinson (1961) proposed his seminal paper on the paradox of the plankton. Padisak (2003) also recognizes the importance of temporal variability for the phytoplankton species diversity maintenance in a lake. In this case the relevance of short term variations is supported, as there was a high turnover of species with time in the small tropical Barva Lake, even though there was only a small subset of species that were common throughout the study.
Acknowledgments
This work was possible through grants No. 808-94-278 and 808-97-282 from the Vicerrectoría de Investigación, Universidad de Costa Rica. I want to express my sincere thanks to Gerardo Obando, Carlos Jiménez, Esteban Estrada and Astrid Mitchells for their invaluable help. I’m also grateful to Richard Petersen who helped with initial versions of the manuscript and gave useful ideas. This is the … contribution of CIMAR.
References
Anagnostidis, K. & J. Komárek. 1988. Modern approach to the classification system of Cyanophytes. 3- Oscillatoriales. Arch. Hydrobiol. Suppl. 80: 327-472. [ Links ]
Anagnostidis, K. & J. Komárek. 1990. Modern approach to the classification system of Cyanophytes. 5- Stigonematales. Algological Studies 59: 1-73. [ Links ]
Banderas-Tarabay, A.G. 1997. Phycoflora of the tropical high-mountain lake El Sol, Central Mexico, and some biogeographical relationships. Hydrobiologia 354: 17-40. [ Links ]
Camacho V.L. & Charpentier E.C. 1989. Ciclo anual del fitoplancton en el lago de Río Cuarto, Costa Rica. Uniciencia 6: 17-22. [ Links ]
Comas González, A. & J. Permán. 1978. Review of the genus Dictyosphaerium (Chlorococcales). Arch. Hydrobiol. Suppl. 51: 233-297. [ Links ]
Comas González, A. 1980. Nuevas e interesantes Chlorococcales (Chlorophyceae) de Cuba. Acta Bot. Cub. 2: 1-17. [ Links ]
González González, L.E. & L.E. Mora-Osejo. 1996. Desmidioflorula de lagunas de páramo en Colombia. Caldasia 18: 165-210. [ Links ]
Hammer, Ø., Harper, D.A.T. & P.D. Ryan, 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Paleontología Electrónica 4: 9pp. http://palaeo-electronica.org/2001_1/past/issue1_01.htm [ Links ]
Hargraves, P.E. & R. Víquez. 1981. Dinoflagellate abundance in the Laguna Botos, Poas Volcano, Costa Rica. Rev. Biol. Trop. 29: 257-264. [ Links ]
Herrera, W. 1986. Clima de Costa Rica. Vol. 2. In L.D. Gómez & W. Herrera. 1986. Vegetación y clima de Costa Rica. UNED, San José, Costa Rica. [ Links ]
Horn, H. 1984. The effects of sampling intervals on phytoplankton growth and loss values derived from seasonl phytoplankton biomass variations in an artificial lake. Int. Revue der ges. Hydrobiologia 69: 111-119. [ Links ]
Huber-Pestalozzi, G. 1941. Das Phytoplankton de Süβwasseres. Systematik und Biologie. 2. Teil. Chrysophyceen. Farblose Flagellaten Heterokonten. E. Schweizerbart’sche, Stuttgart. [ Links ]
Huber-Pestalozzi, G. 1955. Das Phytoplankton de Süβwasseres. Systematik und Biologie. 4. Teil. Euglenophyceen. E. Schweizerbart’sche, Stuttgart. 606 p + CXIV plates. [ Links ]
Huber-Pestalozzi, G. 1961. Das Phytoplankton de Süβwasseres. Systematik und Biologie. 5. Teil. Chlorophyceae (Grünalgen) Ordnung: Volvocales. E. Schweizerbart’sche, Stuttgart. 743 p. + CLVIII plates. [ Links ]
Huber-Pestalozzi, G. 1968. Das Phytoplankton de Süβwasseres. Systematik und Biologie. 3. Teil. Cryptophyceae, Chloromonadophyceae, Dinophyceae. 2. Auflage E. Schweizerbart’sche, Stuttgart. [ Links ]
Hutchinson, G.E. & H. Löffler. 1956. The thermal classification of lakes. Proc. Nat. Acad. Sci. 42: 84-86. [ Links ]
Hutchinson, G.E. 1961. The paradox of the plankton. Am. Nat. 95: 137-147. [ Links ]
Komárek, J. & K. Anagnostidis. 1986. Modern approach to the classification system of Cyanophytes. 2- Chroococcales. Arch. Hydrobiol. Suppl. 73: 157-226. [ Links ]
Komárek, J. & K. Anagnostidis. 1989. Modern approach to the classification system of Cyanophytes. 4- Nostocales. Arch. Hydrobiol. Suppl. 82: 247-345. [ Links ]
Komárek, J. 1983. Contribution to the Chlorococcal algae of Cuba. Nova Hedwigia 37: 65-180. [ Links ]
Kováčik, L. 1975. Taxonomic review of the genus Tetraedron (Chlorococcales). Archiv. Hydrobiol. Suppl. 46: 354-391. [ Links ]
Krammer, K. & H. Lange-Bertalot. 1986. Süβwasserflora von Mitteleuropa. Band. 2/1. Baccillariophyceae. 1. Teil: Naviculaceae. Gustav Fischer, Stuttgart. [ Links ]
Krammer, K. & H. Lange-Bertalot. 1988. Süβwasserflora von Mitteleuropa. Band. 2/2. Baccillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. Gustav Fischer, Stuttgart. [ Links ]
Krammer, K. & H. Lange-Bertalot. 1991a. Süβwasserflora von Mitteleuropa. Band. 2/3. Baccillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. Gustav Fischer, Stuttgart. [ Links ]
Krammer, K. & H. Lange-Bertalot. 1991b. Süβwasserflora von Mitteleuropa. Band. 2/4. Baccillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. Gustav Fischer, Stuttgart. [ Links ]
Krebs, J.K. 1999. Ecological methodology. Addison Wesley Longman, San Franciso, California, USA. [ Links ]
Lewis, W.M. Jr. 1996. Tropical lakes: how altitude makes a difference, p. 43-64. In F. Schiemer & K.T. Boland (eds.). Perspectives in tropical limnology. SPB Academic, Amsterdam, The Netherland. [ Links ]
Lewis, W.M., Jr. 1978. Dynamics and succession of the phytoplankton in a tropical lake: Lake Lanao, Phillipines. J. Ecol. 66: 849-880. [ Links ]
Lewis, W.M., Jr. 1986. Phytoplankton succession in Lake Valencia, Venezuela. Hydrobiologia 138: 189-203. [ Links ]
Löffler, H. 1973. Tropical high mountain lakes of New Guinea and their zoogeographical relationships compared with other high mountain lakes. Arctic Alpine Res. 5: 193-198. [ Links ]
López-López, E. & J.A. Serna-Hernández. 1999. Variación estacional del zooplankton del embalse Ignacio Allende, Guanajuato, México y su relación con el fitoplancton y factores ambientales. Rev. Biol. Trop. 47: 643-657. [ Links ]
Lund, J.W.G., C. Kipling & E.D. Le Cren. 1959. The inverted microscope methods of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 11: 143-170. [ Links ]
MacArthur, R. & J.O. Wilson. 1967. Island biogeography. Princeton, New York, USA. [ Links ]
Mazumder, A. & W.D. Taylor. 1994. Thermal structure of lakes varying in size and water clarity. Limnol. Oceanogr. 39: 968-976. [ Links ]
Melack, J.M. 1979. Temporal variability of phytoplankton in tropical lakes. Oecologia 44: 1-7. [ Links ]
Messyasz, B., N. Maidana, C. Mayr & A. Lücke. 2007. Summer phytoplankton and the hydrochemistry of the crater lake Laguna Azul (Santa Cruz, Argentina). Internat. J. Oceanogr. Hydrobiol. 36: 95-105. [ Links ]
Nyakoojo, C. & S.M. Byarujali. 2008. Temporal distribution of phytoplankton in lake Nyamusingiri in the Albertine Rift Valley, Uganda. African J. Ecol. DOI: 10.1111/j.1365-2028.2010.01216.x [ Links ]
Nyakoojo, C. 2010. The composition and abundance of phytoplankton in Lake Bukoni, western Uganda. African J. Ecol. DOI: 10.1111/j.1365-2028.2010.01209.x [ Links ]
Oliva, M.G., A. Lugo, J. Alcocer, L. Peralta & M. del R. Sánchez. 2001. Phytoplankton dynamics in a deep, tropical, hyposaline lake. Hydrobiologia 466: 299-306. [ Links ]
Osborne, P.L. 2000. Tropical ecosystems and ecological concepts. Cambridge, Cambridge, United Kingdon. [ Links ]
Padisak, J. 2003. Phytoplankton, p. 251-308. In O’Sullivan, P.E. & C.S. Reynolds (eds.). The lakes handbook. Vol. I: limnology and limnetic ecology. Blackwell, Oxford, United Kingdon. [ Links ]
Parra, O.O., E. Ugarte & V. DelLarossa. 1981. Periodicidad estacional y asociaciones en el fitoplancton de tres cuerpos lénticos en la región de Concepción, Chile. Gayana 36: 1-35. [ Links ]
Prescott, G.W. 1962. Algae of the western Great Lakes Area. W.M.C. Brown Co. Dubuqye, Iowa, USA. [ Links ]
Primack, R.B. 2002. Essentials of conservation biology. Sinauer, Massachusets, USA. [ Links ]
Reynolds, C. S. 1984. The ecology of freshwater phytoplankton. Cambridge, Cambridge, United Kingdon. [ Links ]
Reynolds, C.S. 2006. Ecology of phytoplankton. Cambridge, Cambridge, United Kingdon. [ Links ]
Schabestberger, R., E. Rott, G. Friedl, G. Drozdowski, E. Razafindranaivo & C. Holmes. 2009. First Limnological Characterization of the Tropical Crater Lake Amparihibe in the Makira Protected Area, Madagascar. Eco. Mont 1: 35-43. [ Links ]
Schabetsberger, R., G. Drozdowski, I. Drozdowski, C.D. Jersabek & E. Rott. 2004. Limnological aspects of two tropical crater lakes (Lago Biao and Lago Loreto) on the island of Bioko (Equatorial Guinea). Hydrobiologia 524: 79-90. [ Links ]
Scheffer, M. 2004. Ecology of shallow lakes. Kluwer, The Netherland. [ Links ]
Sokal, R.R. & F.J. Rohlf. 1981. Biometry. Freeman, California, USA. [ Links ]
Sommer, U. (ed.). 1989. Plankton ecology. Springer, Berlin, Germany. [ Links ]
Sournia, A. 1978. Phytoplankton manual. UNESCO, Paris, France. [ Links ]
Umaña V.G. & C. Collado. 1990. Plaktonic association in the Arenal Reservoir, Costa Rica. Rev. Biol. Trop. 38: 311-321. [ Links ]
Umaña V.G. & C. Jiménez. 1995. The basic limnology of a low altitude tropical crater lake: Laguna del Cerro Chato, Costa Rica. Rev. Biol. Trop. 43: 131-138. [ Links ]
Umaña V.G. 1988. Fitoplancton de las lagunas Barba, Fraijanes y San Joaquín, Costa Rica. Rev. Biol. Trop. 36: 471-477. [ Links ]
Umaña V.G. 1993. The planktonic community of Laguna Hule, Costa Rica. Rev. Bio. Trop. 41: 163-171. [ Links ]
Umaña V.G. 1997. Basic limnology of Lago Bonilla, a Tropical lowland lake. Rev. Biol. Trop. 45: 1429-1437. [ Links ]
Umaña V.G. 2001. Limnology of Botos Lake. Rev. Biol. Trop. 49:1-10. [ Links ]
Venrick, E.L. 1978. How many cells to count?, p. 167- 180. In A. Sournia (ed.). Phytoplankton manual. UNESCO, Paris, France. [ Links ]
Wehr, J.D. & R.G. Sheath (eds.). 2003. Freshwater algae of North America. Ecology and classification. Academic, Amsterdam, The Nederlands. [ Links ]
West, W. & G.S. West. 1904. A monograph of the British Desmidiaceae. Vol. I. The Ray Society, London. (Reprinted by Johnson Reprint Corporation, 1971). [ Links ]
West, W. & G.S. West. 1905. A monograph of the British Desmidiaceae. Vol. II. The Ray Society, London. (Reprinted by Johnson Reprint Corporation, 1971). [ Links ]
West, W. & G.S. West. 1908. A monograph of the British Desmidiaceae. Vol. III. The Ray Society, London. (Reprinted by Johnson Reprint Corporation, 1971). [ Links ]
West, W. & G.S. West. 1912. A monograph of the British Desmidiaceae. Vol. IV. The Ray Society, London. (Reprinted by Johnson Reprint Corporation, 1971). [ Links ]
West, W. & G.S. West. 1923. A monograph of the British Desmidiaceae. Vol. V. The Ray Society, London. (Reprinted by Johnson Reprint Corporation, 1971). [ Links ]
Whitford, L.A. & G.J. Schumacher. 1973. A manual of fresh water algae. Sparks, Raleigh, USA. [ Links ]
Correspondencia a: Gerardo Umaña-Villalobos. Centro de Investigación en Ciencias del Mar y Limnología (CIMAR), Universidad de Costa Rica, San José, Costa Rica; gerardo.umana@ucr.ac.cr / Escuela de Biología, Universidad de Costa Rica, San José, Costa Rica.
Received 18-VII-2010. Corrected 30-VII-2010. Accepted 10-VIII-2010.