Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.58 n.4 San José Dec. 2010
Beach erosion and nest site selection by the leatherback sea turtle Dermochelys coriacea (Testudines: Dermochelyidae) and implications for management practices at Playa Gandoca, Costa Rica
1. Associated Colleges of the Midwest, Apartado 10265, San José, Costa Rica.
2. Department of Biology, The Colorado College, 902 N. Cascade Ave., Colorado Springs, CO, 80903 USA; mjspanier@hotmail.com
Dirección para correspondencia
Abstract
Leatherback sea turtles (Dermochelys coriacea) nest on dynamic, erosion-prone beaches. Erosive processes and resulting nest loss have long been presumed to be a hindrance to clutch survival. In order to better understand how leatherbacks cope with unstable nesting beaches, I investigated the role of beach erosion in leatherback nest site selection at Playa Gandoca, Costa Rica. I also examined the potential effect of nest relocation, a conservation strategy in place at Playa Gandoca to prevent nest loss to erosion, on the temperature of incubating clutches. I monitored changes in beach structure as a result of erosion at natural nest sites during the time the nest was laid, as well as in subsequent weeks. To investigate slope as a cue for nest site selection, I measured the slope of the beach where turtles ascended from the sea to nest, as well as the slopes at other random locations on the beach for comparison. I examined temperature differences between natural and relocated nest sites with thermocouples placed in the sand at depths typical of leatherback nests. Nests were distributed non-randomly in a clumped distribution along the length of the beach and laid at locations that were not undergoing erosion. The slope at nest sites was significantly different than at randomly chosen locations on the beach. The sand temperature at nest depths was significantly warmer at natural nest sites than at locations of relocated nests. The findings of this study suggest leatherbacks actively select nest sites that are not undergoing erosive processes, with slope potentially being used as a cue for site selection. The relocation of nests appears to be inadvertently cooling the nest environment. Due to the fact that leatherback clutches undergo temperaturedependent sex determination, the relocation of nests may be producing an unnatural male biasing of hatchlings. The results of this study suggest that the necessity of relocation practices, largely in place to protect nests from erosion, should be reevaluated to ensure the proper conservation of this critically endangered species. Rev. Biol. Trop. 58 (4): 1237-1246. Epub 2010 December 01.
Key words: Leatherback sea turtle, Dermochelys coriacea, nest site selection, sea turtle management, sea turtle hatcheries, sex ratios, beach erosion.
The leatherback sea turtle (Dermochelys coriacea), once among the most abundant of the world’s marine turtles (Pritchard 1982), has become listed as an IUCN critically endangered species (Sarti-Martínez 2000). Inhabiting the Indian, Mediterranean, Pacific and Atlantic Oceans, leatherbacks are one of the most widely distributed reptiles in the world (Reina et al. 2002). Eastern Pacific populations have experienced serious decline, and extinction is now a looming reality (Sarti-Martínez et al. 2007, Spotila et al. 2000). Atlantic populations, on the other hand, are more stable and in some cases appear to be increasing (Chacón & Eckert 2007, Dutton et al. 2005). The offshore threats to leatherback populations appear to come from incidental capture in ocean fisheries, ocean pollution primarily from plastics, and possible climate change driven alterations in ocean productivity (Saba et al. 2008, Sarti-Martínez 2000).
Onshore, leatherback nests are threatened by the illegal poaching of eggs (Sarti-Martínez 2000), which in recent decades has been widely curbed by conservation programs (Chacón & Eckert 2007, Spotila et al. 2000). Additionally, it has long been hypothesized that beach erosion poses a major risk to leatherback nest success (Chacón & Eckert 2007, Mrosovsky 1983). This threat stems from the dynamic, erosion-prone nature of leatherback nesting beaches (Eckert 1987), where erosion has been estimated to be responsible for 36-50% leatherback nest loss (Mrosovsky 1983). As a result, some management programs have implemented the practice of nest relocation, where leatherback eggs are physically relocated from natural nest sites to other areas of the beach deemed more stable.
Beach erosion will likely be exacerbated in the future as a result of climate change and rising sea levels. The International Panel on Climate Change forecasts a nearly 0.6m rise in global sea levels during the next century (IPCC 2007). Worldwide, increased beach erosion and loss has already been attributed to climate change (Feagin et al. 2005, Perkins 2000, Zhang et al. 2004). Therefore, a better understanding of how erosive processes may influence leatherback nest success could be valuable to management programs.
The main objective of my study was to test the hypothesis that beach erosion plays a significant role in the selection of nest sites by leatherback sea turtles. I carried out my research at Playa Gandoca, Costa Rica, a highly dynamic beach where coastal erosion, along with the illegal taking of eggs, coastal development, and taking of adults for meat, is thought to be one of the largest obstacles to nest success (Chacón & Eckert 2007). Specifically, I predicted that beach slope would be used by leatherbacks coming ashore as a cue for assessing nest site stability.
Another objective of my research was to examine whether current nest relocation practices at Playa Gandoca have the potential to affect sex ratios of hatchlings. Over 50% of naturally deposited nests at Playa Gandoca are relocated to other areas of the beach deemed more stable, largely in response to presumed threats of erosion, as part of current conservation protocols (Chacón & Eckert 2007, this study). All sea turtle species undergo temperature dependent sex determination (Standora & Spotila 1985) and relocating nests has been shown to influence the temperature and subsequent sex ratios in nests of the green sea turtle (Chelonia mydas) (Spotila et al. 1987) and leatherbacks (Dutton et al. 1985). To investigate whether relocation practices were having a similar effect at Playa Gandoca, I tested the hypothesis that nest relocation influences the temperature of the nest environment.
Materials and methods
Study Site: Playa Gandoca (9°59.972’ N-82°60.530’ W) is an 8.85km beach located within the Gandoca-Manzanillo Wildlife Refuge on the Southern Caribbean coast of Costa Rica (Fig. 1). A conservation program run by the Wider Caribbean Sea Turtle Conservation Network (WIDECAST) has been in place since 1990. Nightly patrols of the beach take place from 20:00-04:00 hours along the entire length of the beach, which is divided into three sectors (A=1950m, B=2850m and C=2900m). To aid in documenting the distribution of turtle nests, sequential markers run the length of the beach in 100m increments along the forest edge.
Upon encountering a nesting turtle, nightly patrols collect biometric data and record the location of the turtle on the beach relative to both tide line and sequential markers. Nests in areas of the beach deemed unsuitable by patrol leaders (i.e., below tide lines, high vulnerability to erosion, proximity to creeks or rivers that may flood and high traffic areas of the beach) are relocated to areas of the beach thought to be more stable and suitable for nests or to two hatcheries constructed midway through each nesting season.
I carried out research on a 4.2km stretch of Playa Gandoca (sectors A & B) over the course of 54 days in the early part (24Feb-19Apr) of the 2008 nesting season (February-July). Sector A consists of the Northernmost portion of the beach, running South from Black Creek at Punta Mona to Middle Creek. Sector B contains the portion of beach between Middle Creek and the Gandoca Lagoon. All nests encounter by nightly patrols laid within 100m of Middle Creek were relocated. Sector C, which this study was not conducted in, is the remainder of the beach running South from the Gandoca Lagoon to the Sixaola River on the Panamanian border.
Erosion and slope data collection: I visited all nest locations where a nesting turtle had attempted to deposit eggs (some nests were relocated) as recorded by nightly patrols within 14 hours of the nesting event. Since the physical presence of eggs at nest locations was not important for the research questions of this study, I took measurements at all nest sites regardless of whether the eggs had been relocated.
I recorded the slope of the beach using a Sokkia® No.8047-10 Abney level with clinometer (Sokkia Corp; Olathe, KS) in the tracks left by the turtle as it ascended the beach from the ocean. I sighted the clinometer from one stake located in the water up the beach to another stake, located on the high tide line (determined by a visible morphological change in beach slope), with a marking the same distance above the sand as the clinometer was above the ocean floor and recorded the angle. Measurements were never taken within two hours of a high tide to allow sufficient beach to be exposed for measurement. This method allowed me to accurately measure the overall angle of ascent in the spot where a nesting turtle came ashore, without being influenced by slight variation in beach slope between measuring stakes.
From the center of the body pit above each nest, I measured distance across the beach from the vegetation line to the high tide line to the nearest one tenth of a meter. I also triangulated the distance to each nest using the two closest sequential beach markers. In order to observe changes in beach structure at nest sites during the incubation period, I revisited the locations of nests every 14 days.
Additionally, I recorded all nest and sequential beach marker locations using a Magellan® SporTrak Map handheld GPS (Magellan Navigation Inc, San Dimas, CA; accuracy: 10m). To examine changes in the overall structure of the beach, I measured the beach width and slope from the water to the high tide line at all sequential markers four times (every 18 days) during the study.
Sand Temperature: To record sand temperatures, I used Omega® Type K thermocouples and an Omega® HH501AJK Types J, K thermometer, Omega Engineering Inc. Stamford, CT; accuracy: +/- (0.1% rdg+1°C). Prior to use I tested the accuracy of all thermocouples with a mercury thermometer and only accepted those units that varied <1°C from the mercury thermometer. The homogenous color of the sand at Playa Gandoca made unnecessary the consideration of sand color for temperature measurement comparisons.
To examine temperature difference in the nest environments of natural and relocated nests, I established four temperature-measuring sites, all located within 50m of both a natural and relocated nest, along a 0.7km length of beach. Two nest sites were located at areas of the beach where the vegetation line consisted of low-growing palms and the other two at areas characterized by high growing palms and other trees to take into account differences in beach shading. At each site, I placed three thermocouples in a single hole at depths of 0.7m, 0.6m and 0.5m [the typical depth of leatherback egg chambers (Chacón et al. 2007)] at both the vegetation line where current conservation protocols require relocated nests to be placed (to characterize the sand conditions around a relocated nest) and the mid-beach halfway between the high tide line and the vegetation line (the most common natural nest location for leatherbacks). I recorded the reading from each thermocouple, as well as the temperature 0.5m above the sand and weather conditions, twice daily between 05:30-06:30 and 14:00-15:30 local time.
Data Analysis: I performed all statistical analysis using Statgraphics® Plus for Windows 3.1 (StatPoint, Inc. Herdon, Virginia), with the exception of a simple chi-square goodness-offit test run with BIOM (Sokal & Rohlf 1981) to analyze nest distribution.
The variance in beach width between the 18-day periods was estimated using a one-way ANOVA (Sokal & Rohlf 1981) with a Tukey multiple comparison. To investigate the relationship between beach slope and change in beach width, I chose the model with the most significant p-value among those offered in Statgraphics. A one-way ANOVA was also run to estimate the variance between beach slope at nesting sites and the slope of other random locations on the beach.
I grouped the data on changes in beach width during two-week intervals at natural nest sites by the total time monitored (two, four or six weeks). I then grouped data from each interval period according to the overall change in beach width, which was characterized as positive (accretion), negative (erosion) or no change (less than a 0.1m) over the time period it was monitored. I ran a chi-squared goodnessof- fit test on all three periods individually and pooled.
To further investigate erosion and accretion beach processes at turtle nesting sites, I performed a regression analysis of beach width change at the sequential beach marker nearest a nesting location during the 18-day period the nest was laid and the number of nests near each marker.
Additionally, I categorized markers that were within 50m of a nesting site into three beach change categories: erosion, stable or accretion (deposition) and by nesting frequency (1-5 events) during a given 18-day period. I performed a two-way ANOVA on the number of beach markers within 50m of a natural nest as the variable classified by these two factors (beach change category and nesting frequency). Both the ANOVA and regression tests were run on data from all three 18-day periods combined and on the first two 18-day periods only with the third thrown out (after data analysis indicated an overall positive change in beach width) to negate the confounding effect of an overall increase in beach width.
I compared sand temperature at nest depths between mid-beach and vegetation line locations using a two-way ANOVA. Sand temperature was the variable characterized by beach position (mid-beach or vegetation line) and depth (0.7m, 0.6m or 0.5m). Data from all sample sites were pooled. P-values for all analyses were considered significant if ≥0.05. All means are presented as χ±ISD (standard deviation).
Results
Beach changes at sequential markers: Data collected from the 42 sequential beach markers in the study area showed no overall significant change in average beach width during the first two 18-day study periods. During the final 18-day period of the study the beach underwent an overall accretion process, significantly increasing the average beach width (F=9.92, df=2.123, p<0.001, Fig. 2). Greater beach slopes were correlated with erosive processes in subsequent 18-day periods and lesser slopes with accretive processes (F=20.78, df=1.124, p<0.001, Fig. 3).
Nest site selection and erosion: I studied a total of 60 nest sites, at which 11 (18.3%) the eggs were left in their natural locations, 35 (58.3%) eggs were relocated to other parts of the beach and 14 (23.3%) the eggs were moved to a hatchery. Nesting occurred in a non-random, clumped distribution along the length of the beach (x2=74.0, df=20, p<0.001, Fig. 3). The mean slope of the beach from waterline to high tide line at nesting sites was 7.6±1.8° (range=4-11, n=60). The mean beach width at nesting sites was 17.4±1.0m (range=0-38.4, n=60). A total of 55 nests (92%) were laid above the high tide line. Forty-two of the 55 nests above the high tide line (76%) were on the outer half of the beach closer to the high tide line than the vegetation line. The mean slope of the beach at nest sites was significantly shallower than that of other randomly chosen locations (F=4.22, df=1.118, p=0.042).
I measured beach width and slope changes in two-week intervals for 28 natural nest sites (n two weeks=14, n four weeks=10, n six weeks=4). Four of these nests were located below the high tide line and three were lost to erosion during the study period. Nest sites monitored for two weeks were characterized by a marginally significant overall increase in beach width (x2=5.76, df=2, p=0.056). Nest sites monitored for four weeks showed a trend of increasing beach width (x2=5.44, df=2, p=0.066). Nest sites monitored for six weeks displayed no significant change in beach width (x2=1.90, df=2, p=0.388). The three time intervals pooled showed an overall increase in beach width at nesting sites (x2=12.56, df=2, p=0.002, Fig. 4).
Nest sites occurred within 50m of half (21) of the beach markers in the study area. More nesting sites occurred near beach markers that were undergoing accretion processes, as opposed to erosion, during all three 18-day periods of the study (F=4.37, df=1.29, p=0.045, Fig. 4). Additionally, more nesting sites from the first two 18-day periods (when the beach was not undergoing an overall beach building process) were located near beach markers experiencing accretion (F=9.79, df=1.11, p=0.010, Fig. 4).
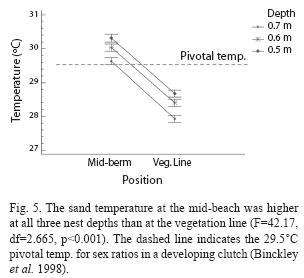
Temperature: A total of 689 temperature readings were taken over the course of 28 days. Temperatures were observed during clear, partly cloudy, mostly cloudy, overcast and raining weather conditions. Pooled data indicated that sand temperature at 0.7m, 0.6m and 0.5m depths was significantly higher at mid-beach locations than at the vegetation line (F=42.17, df=2.665, p<0.001, Fig. 5).
Discussion
Leatherbacks at Playa Gandoca appeared to selectively avoid erosion-prone areas of the beach when selecting nest sites. Nests were laid in a clumped distribution that placed most nests at locations on the beach that were not eroding. The shallower slopes measured at nest sites supports the prediction that beach slope is a potential means for leatherbacks to assess the stability of a nesting site. To the best of my knowledge, this study was the first direct test of the relationship of beach slope and erosion to nest site selection. This study indicates that leatherbacks appear to have a mechanism to maximize nesting success on erosion prone beaches.
Beach slope has been shown to be a cue for nest site selection in loggerhead (Caretta caretta) (Wood & Bjorndal 2000) and hawksbill (Eretmochelys imbricate) sea turtles (Horrocks & Scott 1991). I suggest that leatherbacks may also employ beach slope as a nest site selection cue, using it as a tool for selecting stable areas of the beach for their nests. The beach slope at nest sites was shallower than most areas of Playa Gandoca and the insertion of average nest site slope (7.6°) into the regression model correlating slope and beach width change revealed the angle of ascent selected by leatherbacks corresponds to a 1.6m increase in beach width per 18-day period. By having a range of acceptable beach slopes near the value found in this study, leatherbacks at Playa Gandoca may be avoiding nesting sites that are too low and risk being flooded as well as those that have beach slopes too steep to maintain beach stability and are also energetically unfavorable to ascend.
While this study indicates the role erosion and beach slope may play in nest site selection, additional factors are likely to also influence nesting behavior. Sea floor topography has been reported as one component, with nesting turtles preferentially following trenches in the sea floor running perpendicular to the beach (Clune et al. 2008). The strong current running parallel to Playa Gandoca immediately offshore (Cortés et al. 1998) likely keeps this aspect of nest site selection in a dynamic state. Offshore structures, such as reefs, are unnavigable to turtles as they approach the shore (Eckert 1987). The presence of a reef in the vicinity of Punta Mona at Playa Gandoca may explain why no turtles were observed nesting in the Northern 0.8km of the beach during this study, despite the fact that it appeared to meet ideal nest site criteria.
Some studies have suggested that leatherbacks engage in a scatter nesting strategy with nests randomly distributed along the length of a beach, which places at least a portion of nests at stable locations safe from erosion (Mrosovsky 1983, Tucker 1990). This is a seemingly maladaptive nesting strategy given the erosionprone nature of leatherback beaches, as it would still lead to a relatively high rate of nest loss to erosion (Eckert 1987). Other evidence has also been gathered contrary to the scatter nesting hypothesis. For example, at Grande Riviere Beach in Trinidad and Tobago a general East to West shift in nesting on the beach was observed as an apparent response to increasing erosion on the Eastern end (Lee Lum 2005).
My findings are consistent with those of Lee Lum (2005) and contradict the scatter nesting hypothesis in support of a nesting strategy that takes into account the threat posed by erosion on nesting beaches. This may be good news for the leatherbacks’ future, as nesting behavior that takes into account erosion may allow turtles to better adapt to the erosive effects of rising sea levels, rather than randomly losing more nests.
Conservation work at Playa Gandoca has had profound effects in helping maintain the vitality of the leatherback population in the rookery. Illegal poaching, which two decades ago affected nearly 100% of all eggs laid, now hovers around 1%-3% thanks to conservation efforts (Chacón & Eckert 2007). However, even if leatherbacks do not actively select stable areas of the beach, the results of this study indicate that they are nesting at stable locations. If this is truly the case, nest relocation practices should be carefully reconsidered.
Slightly over three quarters of the nests recorded in this study were laid near the high tide line on the outer portion of the beach, nesting behavior typical of the species (Chacón et al. 2007). This indicates that differences found in sand temperature at nest depths between natural, mid-beach locations and sites of relocated nests along the vegetation may be an accurate representation of a negative effect of current nest relocation practices. A one-half degree deviation from a 29.5°C pivotal incubation temperature can change the sex ratios of leatherback hatchlings to nearly 100% female (warmer temps) or male (cooler temps) (Binckley et al. 1998). Although nest relocation at Playa Gandoca has been shown not to affect hatching success (Furler 2005), nest relocation practices at Playa Gandoca may be skewing the sex ratios of leatherback hatchlings towards an unnatural male bias.
Relocating sea turtle nests has other drawbacks in addition to potentially skewing sex ratios. Eggs run the risk of being damaged when they are transported across beaches. Changing light conditions by moving nests closer to vegetation can disorient leatherback hatchlings (Mrosovsky 1983). Additionally, there appears to be a genetic component to nesting behavior (Kamel & Mrosovsky 2004), therefore relocating nests to safer areas may also affect natural selection against females that nest in inappropriate locations.
Even nests relocated to hatcheries at Playa Gandoca are not completely safe. During the 2008-2009 nesting season at Playa Gandoca, an entire hatchery full of nests was lost to erosion and it was not the first time (D. Chacón & C. Figgener, 2010 pers. comm.). Although nests relocated to hatcheries on Playa Gandoca have a slighter higher hatching success rate than natural or relocated nests (Furler 2005), it likely takes many nesting seasons of successful hatchery use to make up the detrimental effect of losing an entire hatchery. Nests located in hatcheries also experience different incubation temperatures than natural nests at Playa Gandoca (Furler 2005). In light of the stability of leatherback nest sites discovered in this study and given the potential risks, the necessity of hatcheries and the widespread practice of nest relocation at Playa Gandoca should be reevaluated.
Research into the natural leatherback sex ratios at Playa Gandoca, which includes the effects of global warming and the subsequent increase in nest temperature, is necessary for effective management of the species. The removal of old growth forests and the beach shading they provide may have already altered the natural temperatures of turtle nests. This study should serve as a springboard for future, more in-depth study of the findings of this research to ensure the proper conservation of the critically endangered leatherback sea turtle.
Acknowledgments
Many thanks are due to Didier Chacón, Michael McCoy, Judith Sénéchal, Christine Figgener, the supportive members of the Gandoca community, WIDECAST volunteers and Scott Pentzer along with the entire staff of the Associated Colleges of the Midwest Costa Rica program for their help in the planning, execution and writing of this research. I am also indebted to Brian Linkhart and Marc Snyder at The Colorado College for their advisory work and help with the extensive revisions of this manuscript".
References
Binckley, C.A., J.R. Spotila, K.S. Wilson & F.V. Paladino. 1998. Sex determination and sex ratios of pacific leatherback turtles, Dermochelys coriacea. Copeia 1998: 291-300. [ Links ]
Chacón, D., J. Sánchez, J. Joaquín & J. Ash. 2007. Manual para el manejo y la conservación de las tortugas marinas en Costa Rica; con énfasis en la operación de proyectos en playa y viveros. Sistema Nacional de Áreas de Conservación (SINAC), Ministerio de Ambiente y Energía (MINAE), San José, Costa Rica. [ Links ]
Chacón, D. & K.L. Eckert. 2007. Leatherback sea turtle nesting at Gandoca Beach in Caribbean Costa Rica: management recommendations from fifteen years of conservation. Chelonian Conservation and Biology 6: 101-110. [ Links ]
Clune, P., C. Williams, E. Flodin, J.R. Spotila & F.V. Paladino. 2008. Nest-site selection by leatherback sea turtles on Playa Grande based on bathymetric data of Tamarindo Bay. International Sea Turtle Society. 28th Annual Sea Turtle Symposium. (Downloaded: February 22, 2008, www.seaturtle.org/ists/). [ Links ]
Cortés, J., A.C. Fonceca, M. Barrantes & P. Denyer. 1998. Type, distribution, and origin of sediments of the Gandoca-Manzanillo National Wildlife Refuge, Limón, Costa Rica. Rev. Biol. Trop. 46: 251-256. [ Links ]
Dutton, D.L., P.H. Dutton, M. Chaloupka & R.H. Boulon. 2005. Increase of a Caribbean leatherback turtle Dermochelys coriacea nesting population linked to long-term nest protection. Biol. Conserv. 126: 186- 194. [ Links ]
Dutton, P.H., C.F. Whitmore & N. Mrosovsky. 1985. Masculinisation of leatherback sea turtle, Dermochelys coriacea, hatchlings from eggs incubated in Styrofoam boxes. Biol. Conserv. 31: 249-264. [ Links ]
Eckert, K.L. 1987. Environmental unpredictability and leatherback sea turtle (Dermochelys coriacea) nest loss. Herpetologica 43: 315-323. [ Links ]
Feagin, R.A., D.J. Sherman & W.E. Grant. 2005. Coastal erosion, global sea-level, and the loss of sand dune plant habitats. Frontiers in Ecology and the Environment 3: 359-364. [ Links ]
Furler, S. 2005. Hatching Success of the Leatherback Sea Turtle, Dermochelys coriacea, in Natural and Relocated Nests on Gandoca Beach, Costa Rica. M.Sc. Thesis, University of Basel, Basel, Switzerland. [ Links ]
Horrocks, J.A. & N.M. Scott. 1991. Nest site location and nest success in the hawksbill turtle (Eretmochelys imbricata) in Barbados, West Indies. Mar. Ecol. Prog. 69: 1-8. [ Links ]
Intergovernmental Panel on Climate Change (IPCC). 2007. Climate Change 2007: Synthesis Report (Downloaded: May 6, 2008, www.ipcc.ch). [ Links ]
Kamel, S.J. & N. Mrosovsky. 2004. Nest site selection in leatherbacks, Dermochelys coriacea: individual patterns and their consequences. Anim. Behav. 68: 357-366. [ Links ]
Lee Lum, L. 2005. Beach dynamics and nest distribution of the leatherback sea turtle (Dermochelys coriacea) at Grande Riviere Beach, Trinidad & Tobago. Rev. Biol. Trop. 53: 239-248. [ Links ]
Mrosovsky, N. 1983. Ecology and Nest-site Selection of Leatherback Turtles Dermochelys coriacea. Biol. Conserv. 26: 47-56. [ Links ]
Perkins, Sid. 2000. Enjoy the beach… while it’s still there. Science News 158: 20-21. [ Links ]
Pritchard, P.C.H. 1982. Nesting of the leatherback turtle, Dermochelys coriacea in Pacific Mexico, with a new estimate of the world population status. Copeia 1982: 741-747. [ Links ]
Reina, R.D., P.A. Mayor, J.R. Spotila, R. Piedra & F.V. Paladino. 2002. Nesting ecology of the leatherback turtle, Dermochelys coriacea, at Parque Nacional Marino Las Baulas, Costa Rica: 1988-1989 to 1999- 2000. Copeia 3: 653-664. [ Links ]
Saba, V.S., J.R. Spotila, F.P. Chavez & J.A. Musick. 2008. Bottom-up and climatic forcing on the worldwide population of leatherback turtles. Ecol. 89: 1414- 1427. [ Links ]
Sarti-Martínez, A.L. 2000. Dermochelys coriacea. In IUCN 2008. IUCN Red List of Threatened Species. Gland, Switzerland. (Downloaded: April 30, 2008, www.iucnredlist.org). [ Links ]
Sarti-Martínez, L., A.R. Barragán, D. García-Muñez, & N. García. 2007. Conservation and biology of the leatherback turtle in the Mexican Pacific. Chelonian Conservation and Biology 6: 70-78. [ Links ]
Sokal, R.R. & F.J. Rohlf. 1981. Biometry: the principals and practices of statistics in biological research. W.H. Freeman, New York, USA. [ Links ]
Spotila, J.R., E.A. Standora, S. J. Morreale & G.J. Ruiz. 1987. Temperature dependent sex determination in the green turtle (Chelonia mydas): effects on the sex ratio on a natural nesting beach. Herpetologica 43: 74-81. [ Links ]
Spotila, J.R., R.D. Reina, A.C. Steyermark, P.T. Plotkin & F.V. Paladino. 2000. Pacific leatherback turtles face extinction. Nature 405: 529-530. [ Links ]
Standora, E.A. & J.R. Spotila. 1985. Temperature Dependent Sex Determination in Sea Turtles. Copeia 3: 711-722. [ Links ]
Tucker, A.D. 1990. A test of the scatter-nesting hypothesis at a seasonally stable leatherback rookery. In T.H. Richardson, J.I. Richardson & M. Donelly (eds.). Proceedings of the 10th Annual Workshop on Sea Turtle Biology and Conservation. NOAA Tech. Memo. NMFS–SEFSC–278. Miami. USA. [ Links ]
Wood, D.W. & K.A. Bjorndal. 2000. Relation of temperature, moisture, salinity, and slope to nest site selection in loggerhead sea turtles. Copeia 2000: 119-128. [ Links ]
Zhang, K., B.C. Douglas & S.P. Leatherman. 2004. Global warming and coastal erosion. Climate Change 64: 41-58. [ Links ]
Correspondencia a: Matthew J. Spanier. Associated Colleges of the Midwest, Apartado 10265, San José, Costa Rica / Department of Biology, The Colorado College, 902 N. Cascade Ave., Colorado Springs, CO, 80903 USA; mjspanier@hotmail.com.
Received 02-VI-2009. Corrected 17-VI-2010. Accepted 19-VII-2010.