Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.57 suppl.1 San José Nov. 2009
Genitalic stridulation during copulation in a species of crane fly,Tipula (Bellardina) sp. (Diptera: Tipulidae)
William G. Eberhard1* & Jon K. Gelhaus2
1. Smithsonian Tropical Research Institute (STRI), and Escuela de Biología, Universidad de Costa Rica, 11501-2060,
Ciudad Universitaria, Costa Rica; william.eberhard@gmail.com
2. Department of Entomology, The Academy of Natural Sciences, 1900 Ben Franklin Parkway Philadelphia, PA,
USA 19103-1195; gelhaus@ansp.org
Correspondence.
Abstract: The male genitalia of many animal groups have elaborate and species-specific forms. One hypothesis to explain why this is so is that male genitalia function as stimulatory devices that are under sexual selection by cryptic female choice. This report is based on a videotaped observation of a single male of an unidentified species of Tipula (Bellarina) from
Males of related species have similar file and scraper structures on their genitalia, suggesting that they probably also stridulate during copulation. Rev. Biol. Trop. 57 (Suppl. 1): 251-256. Epub 2009 November 30.
Key words: copulatory courtship, genitalic evolution, sexual selection, cryptic female choice,
The male genitalia of many animal groups have elaborate and species-specific forms. One hypothesis to explain why this is so is that male genitalia function as stimulatory devices that are under sexual selection by cryptic femalechoice (Eberhard 1985). One prediction made by this hypothesis is that the male genitalia of some species will be found to move during copulation in ways that are appropriate to stimulate the female. One possible type of stimulation is vibration, and the structure of the males genitalia in two groups suggests that he stridulates (produces vibrations) during copulation, apatelodid moths (Forbes 1941, Franclemont 1973) and the vespid wasp Chartergellus (Richards 1978). No behavioral observations of genitalic stridulation have ever been made, however, and stridulation prior to copulation, as occurs in the pyralid moth Syntonarcha iriastis using the male genitalia (Gwynne & Edwards 1986),was not ruled out in these groups.
Behavior by male genitalia that Could stimulate the female, mainly involving rhythmic thrusting or squeezing, occurs in a variety of animals, is widespread, and has been observed in insects, spiders, mammals, pseudoscorpions, nematodes, reptiles and mites (Eberhard 1996). The functions of some of these behavior patterns are open to alternative interpretations, however. For example thrusting might provide the male with sensory information on female readiness to receive and accept his ejaculate, or help remove or immobilize the sperm from previous males (Waage 1984, Eberhard 1996). Thus further observations of apparent stimulatory movements in which such alternative interpretations can be ruled out are of interest. This note documents genitalic stridulation by the male genitalia of the tipulid fly Tipula (Bellardina) sp. It describes file and scraper structures in this and related species, and presents the first direct observations of the behavior of such a genital structure, confirming that the file and scraper are used to stridulate during copulation.
Materials and methods
On the relatively cool morning of 27 December
Results
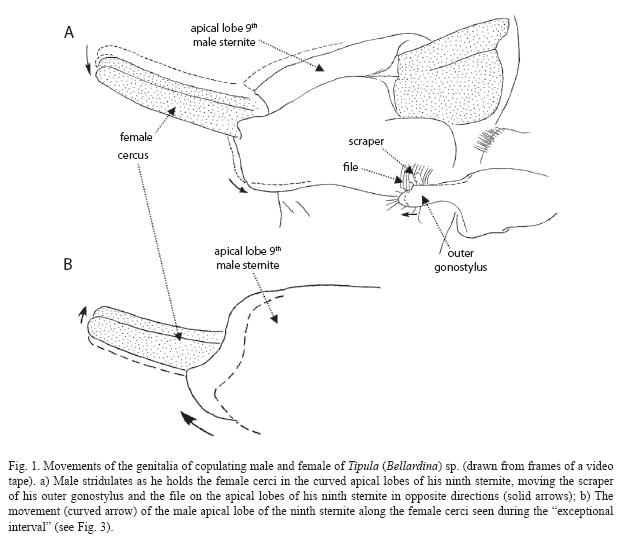
The females cerci were flexed sharply dorsally, and inserted through the cylinder formed by the males curved apical lobes of the ninth sternite; the tips of the female cerci protruded free in the air beyond (Fig. 1). For about 10-15min the pair was nearly completely immobile, except for one brief 2-3second burst of rhythmic genital movements. Then, however, the male abruptly began to vibrate his genitalia in bursts, and continued to do so nearly continually for more than 10min (just over 8.5min were taped). Each vibration consisted of two movements: movements of his ninth sternite apical lobes (and thus of the females cerci which they held) (two curved arrows at left of Fig. 1A); and a smaller, simultaneous movement of the outer gonostylus in approximately the opposite direction (arrow at right in Fig. 1A). The female cerci shifted little if at all in the grip of the male ninth sternite apical lobes during these movements.
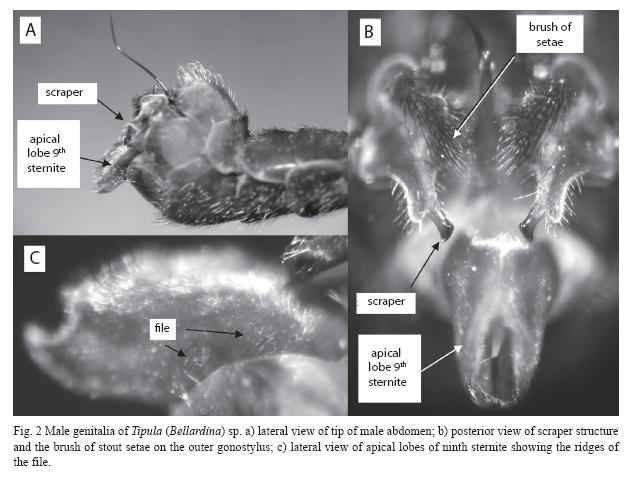
During each movement the tip of the strong, dark-tipped, flattened extension of the outer gonostylus (scraper in Fig. 1A, 2B) scraped across a striated region at the lateral base of the male apical lobes of the ninth sternite (file in Figs. 1A,
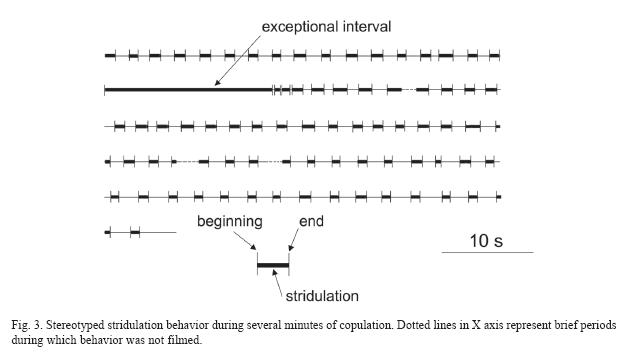
The rate of scrapes during a single burst of vibration was on the order of 15-30cycles/sec. The rhythm of bursts of scraping and pauses between them was very regular, except during one 33.9sec burst of scraping (Fig. 3). During the regular phase, the duration of a burst averaged 2.02+0.39sec (N=83), and bursts were separated by pauses that averaged 2.91+0.90sec (N=79). During the exceptional 33.9sec interval, both the rhythm of bursts of scraping and the movements themselves changed. Stridulatory movements were nearly continuous, but an additional movement was imposed, in which the males ninth sternite apical lobes periodically slid distally along the female cerci and also moved slightly dorsally (Fig. 1B), and then returned to the previous position. This movement was also very rhythmic, occurring on average once every 1.34+0.36sec.
The pair separated when an attempt was made to flash-freeze them with ethyl chloride spray, and they were placed in 80% ethyl alcohol. Any lingering doubt that the rhythmic movements just described were due to movements of the male rather than of the female genitalia was dispelled by the fact that the male outer gonostyli continued to execute the scraping movements in slow motion as the male expired.
Species identification: The subgenus Tipula (Bellardina) is an entirely New World group of 15 species distributed from the Central and Southwestern USA to the central
One of us (JKG) attempted to identify the species whose behavior was observed using the literature, and specimens, including types, in the Alexander Collection of the Smithsonian Institution and in The Academy of Natural Sciences. At least four species occur in
file morphology, three groups of species were identified:
The T. rupicola species group including at least T. cydippe (
The T. schizomera species group including at least T. flinti (
The T. theobromina species group includes only this single species (distributed in the Central to
Discussion
This is apparently the first direct observation of stridulatory behavior in male genitalia; previous examples of male genital stridulation were based only on observations of apparent file and scraper morphology in dead specimens. Although the observation described here concerns only a single pair, the sustained and highly stereotyped movements that caused the process of the outer gonostylus to scrape repeatedly across the low, closely spaced ridges on the ninth sternite apical lobes, and the appropriate designs of these two genitalic structures for stridulation leave little doubt that these portions of the males genitalia function to produce stridulation during copulation. It seems reasonable to conclude that the function of this behavior and of these structures is to stimulate the female, because stridulation behavior is ill-designed for any of the alternative functions that have been mentioned previously for genital movements, such as sensing the female, removing sperm, forcing open female passages, or penetrating more deeply.
An additional structure on the male genitalia of T. (Bellardina) sp. is the brush of robust pointed setae projecting medially from the males outer gonostylus (Fig. 2B). These setae probably press on the females 10th segment pleural membranous area, just basal to her cerci during copulation, and the setae may scrape against the female during male stridulatory movements. These setae may thus constitute
a second structure that produces copulatory stimulation of the female.
These behaviors are likely to occur in a number of other species related to the one studied here (see previous Identification section). The flat truncate scraper, strongly ridged file, and distinct brush of medially-directed setae all occur in at least five other species of T. (Bellardina). In addition, in six other species of T. (Bellardina), there is a spine-like scraper structure, and there is no file structure but instead a roughened or bumpy surface at midlength of the ninth sternite apical lobes, indicating either a lack of stridulation, or possibly a different mechanism. These species also lack a brush of setae on the mesal aspect of the outer gonostylus.
The evidence that favors a stridulation function for the male apical lobe of the ninth sternite (file) and outer gonostylus (scraper) of the species we studied also constitutes evidence against two other alternative hypotheses that have been proposed to explain the rapid divergent evolution of genitalic structures, species isolation by lock and key (Shapiro & Porter 1989), and sexually antagonistic coevolution (Chapman et al. 2003, Arnqvist & Rowe 2005.) There is no female lock with which the stridulatory structures mesh; and stridulation by the male exerts no appreciable coercive force on the female.
Acknowledgments
We thank STRI and UCR for financial support to WGE. We thank Wayne Mathis, curator, and Holly Williams, collection manager, for the loan of specimens from the Alexander Collection of Crane Flies at the Smithsonian Institution.
Resumen
Un macho de Tipula (Bellardina) sp. estriduló (produjo vibraciones) por medio de movimientos estereotipados de estructuras de sus órganos genitales durante varios minutos de la cópula. Los machos de algunas especies relacionadas poseen estructuras similares, lo cual sugiere que también estridulan durante la cópula.
Palabras clave: cortejo copulatorio, evolución órganos sexuales, selección sexual, selección críptica en hembras, Costa Rica.
Received 28-IX-2007. Corrected 05-VI-2009. Accepted 28-VII-2009.
References
Alexander, C.P. & G.W. Byers. 1981. Tipulidae, p. 153-
Arnqvist, G. & L. Rowe. 2005. Sexual conflict. PrincetonUniversity,
Chapman, T., G. Arnqvist, J. Bangham, & L. Rowe. 2003. Sexual conflict. Trends Ecol. Evol. 18: 41-47. [ Links ]
Contreras-Ramos, A. & J. Gelhaus. 2002. Tipulidae (Diptera), p. 583-
Eberhard, W.G. 1985. Sexual selection and animal genitalia.
Eberhard, W.G. 1996. Female control: sexual selection by cryptic female choice.
Forbes, W.T.M. 1941. Does he stridulate? (Lepidoptera: Eupteroptidae). Ent. News 52: 79-82. [ Links ]
Franclemont, J.C. 1973. The moths of America North of Mexico. Fasc.
E. W. Classey Ltd. and R. B. D. Publishers,
Gelhaus, J.K. 1986. Larvae of the crane fly genus Tipula in
Gelhaus, J.K. 2009. Tipulidae (Crane flies, Tipulidos), Chapter 8, pp. 193-
Gwynne, D.T. & E.D. Edwards. 1986. Ultrasound production by genital stridulation in Syntonarcha iriastis (Lepidoptera: Pyralidae): long-distance signaling by male moths? Zool. J. Linn. Soc. 88: 363-376. [ Links ]
Richards, O.W. 1978. The social wasps of the
Shapiro, A.M. & A.H. Porter. 1989. The lock-and-key hypothesis. Evolutionary and biosystematic interpretations
of insect genitalia. Ann. Rev. Entomol. 34: 231-245.
Waage, J. 1984. Sperm competition and the evolution of odonate mating systems. p. 251-
(ed.) Sperm competition and the evolution of animal mating systems.
Internet References
Oosterbroek, P. 2006. Catalogue of the Crane flies of the World (CCW). (http://ip30.eti.uva.nl/ccw/)Links ] font-family: Verdana;">