Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.57 n.4 San José Dec. 2009
Life history of the land snail Habroconus semenlini (Stylommatophora: Euconulidae) under laboratory conditions
Lidiane Silva1,2; Liliane Meireles1,2; Tércia Vargas1; Flávia O. Junqueira1 & Elisabeth C.A. Bessa1,2
1. Núcleo de Malacologia Prof. Maury Pinto de Oliveira, Universidade Federal de Juiz de Fora
2. Mestrado em Ciências Biológicas - Comportamento e Biologia Animal, Instituto de Ciências Biológicas, Universidade Federal de Juiz de Fora, Campus Universitário, 36036-330, Juiz de Fora, Minas Gerais, Brazil; lhybio@yahoo.com.br
Abstract: Habroconus semenlini is a micro-terrestrial gastropod native to South America. There are no previous studies on its biology. We studied its pattern of growth, fertility and lifespan under laboratory conditions. For this purpose, 80 snails were either grouped or kept isolated (40 animals in each condition) during their lifetime. Growth is indeterminate and the species is capable of self-fertilization with high reproductive success. Grouped snails had lower fecundity than the animals that were kept in isolation. This species has a short lifespan and only one reproductive period, which characterizes the occurrence of semelparity. Rev. Biol. Trop. 57 (4): 1217-1222. Epub 2009 December 01.
Key words: Growth, land snail, longevity, reproduction, self-fertilization.
Land snails are ecologically and economically important. Many species spread seeds and spores, which can become adhered to their mucus or be eliminated in their feces. In nature they recycle nutrients, especially calcium, and function as bioindicators, since they are sensitive to pollution (Simone 1999). Despite the relevance of the molluscan group, there are few studies on the physiology and biology (Almeida & Bessa 2001, Thomé et al. 2006, Meireles et al. 2008, Silva et al. 2008).
Habroconus semenlini (Moricand, 1846) is a land snail native to South America, with wide distribution in Brazil, Paraguay, Argentina and Uruguay (Simone 2006). They live in woods and forest areas, under rocks and in leaf litter (Veitenheimer- Mendes & Aguiar-Nunes 2001, Veitenheimer-Mendes & Postal 2003), and their life cycle has not yet been studied.
The goal of this study was to investigate the life history of H. semenlini its self- fertilization, fecundity, growth and lifespan under laboratory conditions.
Material and methods
A group of 50 adult individuals of H. semenlini, were collected in the field– Morro do Imperador (21°45’13"-21°46’13" S, 43°21’19"-43°22’15" W)– Juiz de Fora, State of Minas Gerais, Brazil, during summer (January 2008). These animals were reared in the Laboratory of Mollusk Biology, Museum of Malacology "Professor Maury Pinto de Oliveira" at the Federal University of Juiz de Fora. Some specimens were sent to the mollusk collection at the National Museum of Federal University of Rio de Janeiro, State of Rio de Janeiro, Brazil (register MNRJ 12855).
The life cycle of H. semenlini was studied from February to July 2008, using recently hatched animals only. This study was carried out at a temperature of 20°C± 2, relative humidity of 85% ± 2, during natural photoperiod, under laboratory conditions.
Four groups of animals (10 snails each) were kept in larger plastic terrariums (8cm diameter x 6cm deep) during their lifespan (Grouped). The substrate (mulch) was sterilized at 120°C for one hour and then humidified once a day during culture. Animals were fed commercial ration for broilers (SOMA INDÚSTRIA E COMÉRCIO DE ALIMENTOS LTDA ®), enriched with calcium carbonate at 3:1 (Bessa & Araújo 1995).
Shell diameter measurements were made, with the aid of a caliper (0.05mm precision), to each organism the first day of the study and every 15 days, so growth rates could be studied. The time range to the first reproduction was identified by the presence of hatched animals in the terrariums; throughout the experiment, terrariums were examined for daily hatched animals. In this case, all animals were counted and their day of hatch was scored.
In order to test self-fertilization, 40 recently hatched snails were kept individually isolated in small plastic terrariums (6 diameter x 4cm deep) during their lifespan (Isolated). This group was fed and observed, and reproduction was controlled as previously described, following Bessa & Araújo (1995). Mortality control was also considered daily through direct observation of Grouped and Isolated animals.
Results
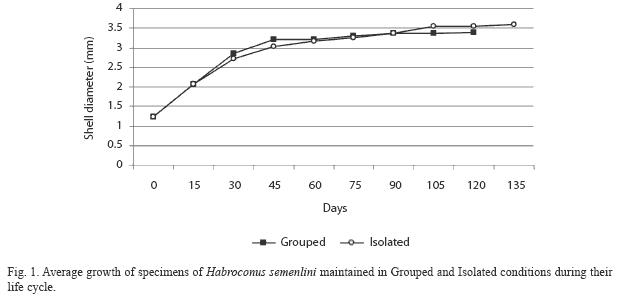
The average growth of Grouped and Isolated H. semenlini during their life cycle is shown in Fig. 1. There is a continuous growth after reproduction and for this species can be considered indeterminate.
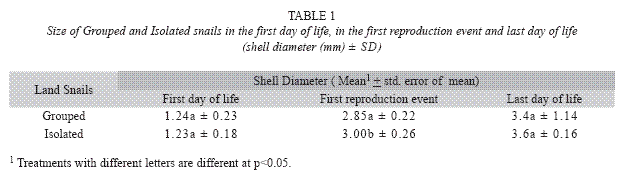
From the first reproduction event the average size of Grouped and Isolated snails was different (Mann-Whitney=3.62, p=0.0003), whereas no significant difference was measured in the first and last days of life (Mann-Whitney= 0.16; p=0.87 and Mann-Whitney=0.53, p=0.60, respectively) (Table 1).
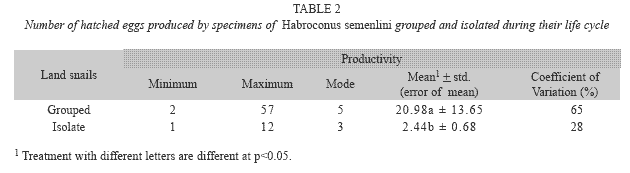
We observed that H. semenlini has a capacity of self-fertilization, evidenced by the fact that hatched snails appeared in isolation conditions (40 days±9). For this species only one reproductive period was observed during their life cycle. Isolated snails have produced a total of 1 332 hatched animals (33.3 hatched animals/snail). For animals kept in groups, reproduction was verified in 32 days±3, which was not significantly different in isolation conditions (Mann-Whitney=1.96, p=0.08). During the life cycle of Grouped animals a total of 1 280 hatched animals (32.0 hatched animals/ snail) appeared in all terrariums. However, the total number of hatched animals produced by Grouped snails was significantly lower than that produced by Isolated individuals (Mann- Whitney=9.59, p=0.0001). Table 2 shows the fecundity of H. semenlini.
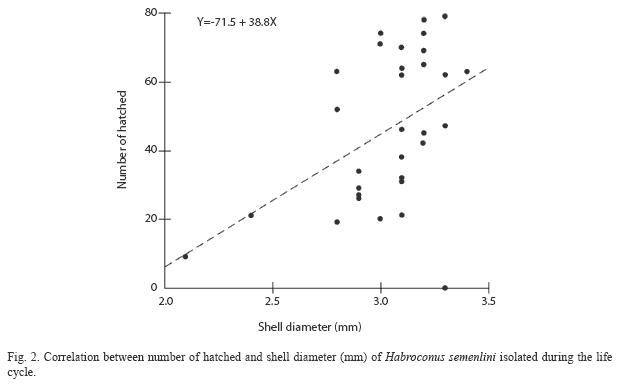
The test of simple linear regression showed significant correlation between shell diameter and number of hatched animals produced (Simple Linear Regression=8, p=0.0009; Fig. 2).
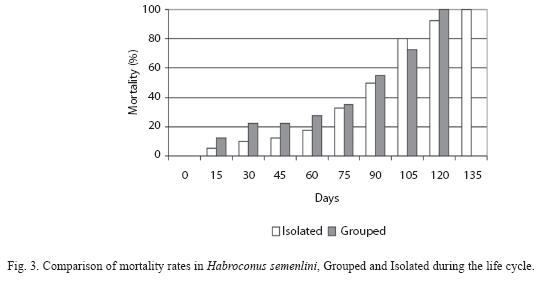
Isolated individuals lived longer than Grouped individuals (Fig. 3). Lifespan was 111.75 days ±6 for Isolated and 77.30 days ± 41.50 for Grouped, but with no statistical difference (Mann-Whitney=0.28, p=0.78).
Discussion
The pattern of indeterminate growth observed in this species has been reported for other tropical land snail species, such as Leptinaria unilamellata (d’ Orbigny, 1835) and Bulimulus tenuissimus (d’ Orbigny, 1835) (Almeida & Bessa 2001, Silva et al. 2008). Indeterminate growth is apparently optimal when productivity increases with shell size. Thus, growth after the initiation of egg-laying does not reduce reproductive success, since it is compensated by an increase in body size (Heller 2001).
For this species, there was a high correlation between body size and fecundity. Body weight, shell length and egg production are all correlated in land snails Subulina octona (Brugüière, 1789) (D’Ávila & Bessa 2005) and B. tenuissimus (Silva et al. 2008), which suggests that H. semenlini gain reproductive success by breeding when they have reached a larger size.
A relationship between body size and egg production is often assumed. Since eggs are energetically costly, larger individuals should have the capacity to produce more eggs (Ghiselin 1969). Body size can be an important factor in sex allocation since it is an important indicator of resources available and therefore of energy available for gamete production (Norton & Bronson 2006).
Habroconus semenlini was able to reproduce through self-fertilization. This is an important characteristic for snails, which inhabit forest areas that have been destroyed. Thus, the ability to self-fertilize can ensure survival of the species in cases of environmental disturbances (Heller 2001).
Unlike what has been observed for other terrestrial gastropods (Almeida & Bessa 2001, Meireles et al. 2008, Silva et al. 2008), H. semenlini displayed higher fecundity when kept in isolation. It has been reported that the frequency of self- fertilization can vary widely (Heller 1993, 2001). In B. tenuissimus there is a clear preference for cross-fertilization, and self-fertilization happens after a long period of isolation (Silva et al. 2008). For S. octona reproductive success was the same in both reproductive processes (Bessa & Araújo 1995). For slug Deroceras agrestis (Linnaeus, 1758) reproduction by cross-fertilization can be four times more successful than reproduction by self-fertilization (Foltz et al. 1982).
The frequency of self-fertilization may vary within a single genus, as reported for species of Arion (Férussac, 1819) some of which reproduce only by cross- fertilization, while others only by self-fertilization, and others use both processes (Heller 2001). Age may influence self-fertilization frequency, as seen for the land snail Partula taeniata (Mörch, 1876), whose rates of self-fertilization are reported to decrease their life cycle (Murray & Clark 1966, 1976). Self-fertilization is a costly process in terms of energy, due to the need to develop two reproductive apparatus. Thus, this process would be beneficial when reproductive success is higher than energy costs. The greater reproductive success of H. semenlini through selffertilization was compensated by an increase in the number of the hatched animals produced.
In H. semenlini, egg-laying was not observed, but the presence of hatched specimens in all terrariums was observed. This fact indicates that this species is viviparous or ovoviviparous. However, in order to establish the strategy adopted by this species, histological studies of the reproductive tract are required (Heller 2001).
The life cycle of H. semenlini was short. Johnson & Black (1991) suggest that smallsized land snails of tropical regions tend to be short-lived. Moreover, this species only has one reproductive period during their life cycle, which characterizes semelparity in this species. Land snails have two reproductive strategies, namely semelparity and iteroparity. In semelparity, animals only have one reproductive period during their life cycle, after which death occurs, as seen in this study with H. semenlini. In iteroparity, instead, animals have more than one reproductive period during their life cycle (Heller 2001). Semelparity has been reported for others land snails, such as Xeropicta vestalis (Pfeiffer, 1841) (Heller & Volokita 1981), Monacha cartusiana (Müller, 1774) (Staikou & Lazaridou-Dimitriadou 1990), Helicela itala (Linnaeus, 1758) (Lazaridou- Dimitriadou & Sgardelis 1995) and species of the genus Arion (South 1992). Semelparity and iteroparity were related to environmental conditions. Thus, semelparity would be favored in unstable environments, giving the animals a short life cycle, with maximum reproductive effort, as observed in this study for H. semenlini. It is believed that the life cycle characteristics seen in H. semenlini are related to habitat type and morphological characteristics of the species. Specimens of H. semenlini have been found in border areas (unpublished data) that make them susceptible to environmental variations throughout the day, such as light, temperature and humidity, common in these areas (Murcia 1995). Environmental variations and small body size cause water stress in these animals and facilitate desiccation. Thus, environmental and morphological conditions may have favored semelparity in this species.
Acknowledgment
We thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Pesquisa (CNPq) for the fellowship.
Resumen
Habroconus semenlini es un gastrópodo micro-terrestre nativo de América del Sur. Actualmente se carece de estudios sobre su biología. Este estudio tuvo como objetivo verificar el patrón de crecimiento, la fecundidad y la esperanza de vida de esta especie en condiciones de laboratorio. Con este fin, 80 caracoles fueron mantenidos aislados o agrupados (40 animales en cada condición) durante su ciclo de vida. La especie tiene crecimiento indeterminado y es capaz de auto-fertilización con alto grado de éxito reproductivo. Los caracoles agrupados tuvieron menor fecundidad que los que se mantuvieron en aislamiento. Esta especie tiene una vida útil corta, y sólo un período reproductivo, lo que caracteriza la semelparidad.
Key words: Crecimiento, caracoles de tierra, longevidad, reproducción, fecundación libre.
Received 01-II -2009. Corrected 17-IV-2009. Accepted 19-V-2009.
References
Almeida, M.N. & E.C.A. Bessa. 2001. Estudo do crescimento e da reprodução de Bradybaena similaris (Mollusca, Xanthonychidae) em laboratório. Rev. Bras. Zool. 18: 1115-1122. [ Links ]
D’Ávila, S. & E.C.A. Bessa. 2005. Influência do substrato sobre o crescimento de Subulina octona (Brugüière) (Mollusca, Subulinidae), sob condições de laboratório. Rev. Bras. Zool. 22: 205-211. [ Links ]
Bessa, E.C.A. & J.L.B. Araújo. 1995. Oviposição, tamanho de ovos e medida do comprimento da concha em diferentes fases do desenvolvimento de Subulina octona (Brugüière) (Pulmonata, Subulinidae) em condições de laboratório. Rev. Bras. Zool. 12: 647-654. [ Links ]
Foltz, D.W., H. Ochman, J.S. Jones, M. Evangelisti & R.K. Selander. 1982. Genetic population structure and breeding systems in arionid slugs (Mollusca, Pulmonata). Biol. J. Linnean Soc. 17: 225-242. [ Links ]
Ghiselin, M.T. 1969. The evolution of hermaphroditism among animals. Q. R. B. 44 :189-208. [ Links ]
Heller, J. 1993. Hermafroditism in molluscs. Biol. J. Linnean Soc. 48: 19-42. [ Links ]
Heller, J. 2001. Life History Strategies, p. 413-445. In G. M. Barker (ed.). The biology of terrestrial molluscs. CABI Publishing. Wallingford, New Zealand. [ Links ]
Heller, J. & M. Volokita. 1981. Gene regulation of shell in a land snail from Israel. Biol. J. Linnean Soc. 16: 261-277. [ Links ]
Johnson, M.S. & R. Black. 1991. Growth, survivorship, and population size in the land snail Rhagada convicta Cox, 1870 (Pulmonata: Camaenidae) from a semiarid environment in Western Australia. J. Molluscan Stud. 57: 367-374. [ Links ]
Lazaridou-Dimitriadou, M. & S. Sgardelis. 1995. Biological strategies and population dynamics of the Northern Greek terrestrial gastropods. In G. M. Barker (ed.). The biology of terrestrial molluscs. CABI Publishing. Wallingford, New Zealand. [ Links ]
Meireles, L.M.O., L.C. Silva, F.O. Junqueira & E.C.A. Bessa. 2008. The influence of diet and isolation on growth and survival in the land snail Bulimulus tenuissimus (Mollusca: Bulimulidae) in laboratory. Rev. Bras. Zool. 25: 224-227. [ Links ]
Murcia, C. 1995. Edge effects in fragmented forests: implications for conservation. Tree. 10: 58-62. [ Links ]
Murray, J. & B. Clark. 1966. The inheritance of polymorphic shell characters in Partula (Gastropoda), p. 413-445. In G. M. Barker (ed.). The biology of terrestrial molluscs. CABI Publishing. Wallingford, New Zealand. [ Links ]
Murray, J. & B. Clark .1976. Supergenes in polymorphic land snail Partula taeniata, p. 413-445. In G. M. Barker (ed.). The biology of terrestrial molluscs. CABI Publishing, Oxon, UK. [ Links ]
Norton, C. G. & J. M. Bronson. 2006. The relationship of body size and growth to egg production in the hermaphroditic freshwater snail, Helissoma tricolvis. J. Molluscan Stud. 72: 143-147. [ Links ]
Silva, L.C., L.M.O. Meireles, F.O. Junqueira & E.C.A. Bessa. 2008. Development and reproduction in Bulimulus tenuissimus (Mollusca, Bulimulidae) in laboratory. Rev. Bras. Zool. 25: 220-223. [ Links ]
Simone, L.R.L. 1999. Mollusca Terrestres, p. 5: 3-8. In C.R. Brandão & E.M. Cancello, (Org.). Biodiversidade do Estado de São Paulo, Brasil: síntese do conhecimento ao final do século XX: Invertebrados Terrestres. FAPESP Editora, São Paulo, São Paulo, Brasil. [ Links ]
South, H. 1992. A comparison of the life cicle of Deroceras reticulatum (Müller) and Arion intermedius Normand (Pulmonata: Stylommatophora) at different temperatures under laboratory conditions. J. Molluscan Stud. 48: 233-244. [ Links ]
Staikou, A. & M. Lazaridou-Dimitriadou. 1990. Aspects of the cycle, population dynamics, growth and secondary production of the snail Monacha cartusina (Müller, 1774) (Gastropoda, Pulmonata) in Greece. Malacol. 31: 353-362. [ Links ]
Thomé, J.W., S.R. Gomes & J.B. Picanço. 2006. Guia ilustrado: os caracóis e as lesmas dos nossos bosques e jardins, p. 1-124. Editora USEB, Pelotas, Rio Grande do Sul, Brasil. [ Links ]
Veitenheimer-Mendes, I. L. & J. Aguiar-Nunes. 2001. Moluscos, p. 48-57. In Mirapalhete, S. R. (Coord. E Org.). Flora e Fauna do Parque Natural do Morro do Osso. Secretaria Municipal do Meio Ambiente, Porto Alegre, Brasil. [ Links ]
Veitenheimer-Mendes, I. L. & M. Postal. 2003. Moluscos terrestres e límnicos registrados para a sub-bacia hidrográfica do arroio Itapuã, Viamão, Rio Grande do Sul, Brasil. R.B.B. 1: 55-68. [ Links ]