Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.56 n.4 San José Dec. 2008
Gastrointestinal Helminth Parasites of the fish Synodontis clarias (Siluriformes: Mochokidae) from Lekki lagoon, Lagos, Nigeria
B. Akinsanya1, A.A. Hassan2 & A.O. Adeogun2
1. Department of Zoology, Parasitology Unit, University of Lagos, Nigeria; akinbami2000@yahoo.com
2. Department of Zoology, University of Ibadan, Ibadan, Nigeria.
Abstract: A total of 362 specimens of the fish Synodontis clarias were randomly selected and subjected to parasitological examination for helminth parasites. They were collected over a period of one year from Lekki lagoon, Lagos, Nigeria. The prevalence of gastrointestinal infection was 38.7%. The helminth worms found include two cestodes, Proteocephalus spp., Wenyonia acuminata, and a nematode species, Raphidascaroides. Male specimens (196) presented a higher rate of infection (37.8%) than female specimens (166) which showed a rate of 23.5%. The overall worm burden was high (678) and it was independent of fish sex and fish size. Rev. Biol. Trop. 56 (4): 2021-2026. Epub 2008 December 12.
Key words: Synodontis clarias, helminth, parasites, Proteocephalus, laguna Lekki, Lagos, Nigeria.
In Nigeria the demand for fish exceeds supply and the proportion of animal protein in the diet is generally low. Parasitic diseases of fish seem to be one of the major problems confronting fish culturists. The Squeaker or upside-down catfish Synodontis clarias (Linnaeus, 1758) is a benthopelagic, potamodromous fresh water fish that inhabits water with a pH range of 6.5-9.5 (Reids 2004). The fish has been reported to present dioecism with external fertilization (Breeder and Rosen 1966).
Olaosebikan and Raji (1998) recorded a maximum size of 36.0 cm standard length for male/unsexed of the fish species. The fish is generally classified as omnivore, feeding mainly on insect larvae, mollusks and detritus (Willoughby 1974). Its native range covers Chad, Niger (including the Benoue River), Senegal, Gambia and the Volta basins, and the Nile (Paugy and Roberts 1992).
S. clarias is also a non-guarding, substratum and open water egg scatterer, oviparous, and distinct pairing during breeding (Breeder and Rosen 1966). This fish species has been reported to occur in different ecosystems such as Benue river, (Paugy and Roberts 1992), in the zoogeographic realm of Ethiopia (Gosse 1986), in Kainji Lake (Willoughby 1974), in Lake Chad (Gosse 1986), in Niger, (Paugy et al. 1994), and in the Nile river, Senegal and volta (Gosse 1986). The genus Synodontis belongs to the family Mochokidae and is the most common for commercial purposes (Reed et al. 1967). Twenty-one species of this genus have been reported in Nigerian inland waters and most of these have also been found within the Sudanean and Guinean zones (McConnell 1965, Reed et al. 1967).
This study was carried out in Lekki lagoon which serves as an important fishing zone to the inhabitants of Epe and Metropolitan Lagos at large.
Materials and methods
Study area: Lekki lagoon supports a major fishery in Nigeria. The lagoon is located in Lagos State, Nigeria (between 4°00 and 4°15 E; between 6o25 and 6°37 N). It has an extension of around 247 km2 with a maximum depth of 64 m, the greater part of the lagoon is shallow, and less than 3.0 m deep.
The Lekki lagoon is part of an intricate system of waterways made up of lagoons and creeks that are found along the coast of Southwestern Nigeria from the Dahomey border to the Niger Delta stretching over a distance of about 200 km. It is fed by the Oni river discharging into the North-eastern part, and by the rivers Oshun and Saga discharging into the North-western parts of the lagoon. Lekki lagoon experiences both dry and rainy seasons which are typical of the southern part of Nigeria.
The vegetation around the lagoon is characterized by shrub and raphia palms, Raphia sudanica and oil palms, Elaeis guineensis. Floating grass occur by the periphery of the lagoon while coconut palms, Cocos nucifera, are widespread in the surrounding villages.
The rich fish fauna of the lagoon includes Heterotis niloticus, Gymnarchus niloticus, Clarias gariepinus, Malapierurus electricus, Synodontis clarias, Chysichthys nigrodigitatus, Parachanna obscura; Mormyrus rume, Calabaricus calamoichthys, Tilapia zilli, Tilapia galilae, Hemichromis fasciatus and Sarotherodon melanotheron (Kusemiju 1981).
Collection and examination of specimens for parasites: From March 2003 to April 2004, a total of 362 randomly selected fresh specimens of S. clarias obtained from Lekki lagoon were purchased at Oluwo market, Epe, Lagos, Nigeria. The fresh specimens were immediately examined for gastrointestinal helminth parasites.
Weights, standard lengths and total lengths of the specimens were recorded. Fishes were dissected, the alimentary canals were removed, cut into parts and put into physiological saline for parasites recovery. The intestines were further carefully slit open longitudinally to aid the emergence of gastrointestinal helminth parasites. The recognition of the worms was enhanced by the wriggling movements when they were emerging.
Processing of parasites: The collected helminth parasites were fixed in 70% alcohol, counted and recorded, whole mounted histological preparations of them, stained with Haematoxylin and eosing, were prepared. Identification of the specimens to species level was undertaken and confirmed at the British Museum (Natural History), in the United Kingdom.
Results
Three hundred and sixty two specimens of S. clarias were examined for parasitic helminth fauna. All helminthic infections observed and recorded were restricted to the intestine. One hundred and forty specimens were found to be infected (38.2%).
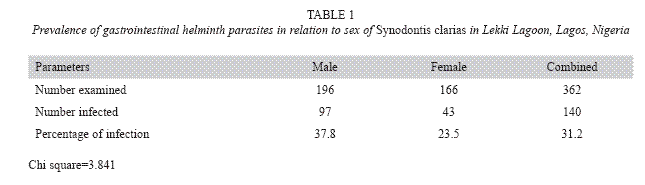
Table 1 shows the presence of gastrointestinal helminth infections in relation to sex of S. clarias in Lekki lagoon, Lagos, Nigeria. A total of 196 male specimens were examined and 97 were infected with helminth parasites, which shows an infection of 37.8% of the total sample. A total of 166 female specimens were examined and 43 were infected with helminth parasites, which shows an infection of 23.5% of the total sample.
Two kinds of helminth parasites collected included cestodes as Proteocephalus species (Weinland, 1858) (Proteocephalidae), Wenyonia acuminata (Woodland, 1923) (Caryophyllaeiidae), and a nematode Raphidascaroides species (Yamaguti, 1941) (Heterocheilidae). Neither acanthocephalan nor trematode infections were recorded in the examined specimens. Mixed infections with more than ten worms were observed. A total of 678 helminth worms was collected from the examined fish specimens.
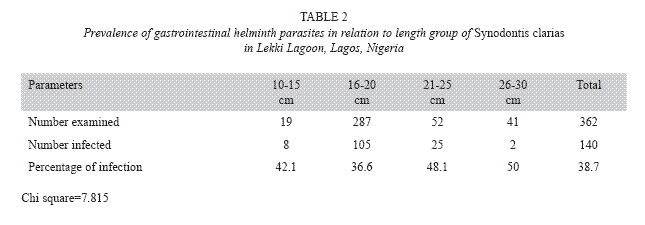
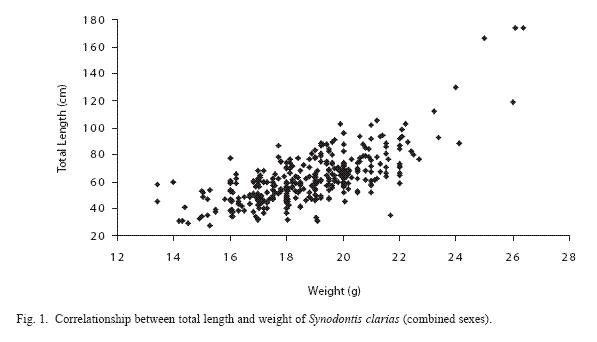
Table 2 shows intestinal helminth infections in relation to size of S. clarias. The group with a length between 10-15 cm showed 42.1% of infection, the group with a length between 16-20 cm showed 36.6% of infection, the group with a length between 21-25 cm showed 48.1% of infection, and the group with a length between 26-30 cm showed 50% of infection. The weight of the specimens examined ranged between 29.0 and 174.10 g. There was no relationship between sex and size in relation to gastrointestinal helminth infections in S. clarias. See the scatter diagram of male, female and combined sexes of S. clarias (Figs 1-3).
Discussion
According to the host-parasite checklist of Khalil and Polling (1997) none of the collected parasites described above have been recorded for S. clarias. Therefore, this study is the first scientific record of these helminth parasites in Synodontis clarias. The parasite W. acuminata has only been documented to infect S. membranaceus in Sudan.
Akinsanya and Otubanjo (2006) also found W. acuminata from Clarias gariepinus collected from Lekki lagoon. The prevalence of W. acuminata in different fish species could be an indication that there is no strict host specificity by this parasite. Other species of Wenyonia however have been found in some Synodontis species: W. longicauda (Woodland, 1937) occurred in Synodontis gambiensis, Wenyonia minuta (Woodland, 1923) in Chrysichthys auratus, and Wenyonia virilis (Woodland, 1923) in Svnodontis batensoda, Synodontis schall and Synodontis clarias (Khalil and Polling, 1997). Wenyonia species has therefore been known to infect members of the family Mochokidae.
Species of the genera Proteocephalus have also been documented in some Synodontis species, for instance Proteocephalus beauchampi occurred in Synodontis schall, and Proteocephalus synodontis in Synodontis batensoda and in S. schall.
Nematode species of the genera Raphidascaroides have not been documented to infect any species of Synodontis. Akinsanya et al. (2007), in a comparative study of the parasitic helminth fauna of two fish species, collected species of the nematode genus Raphidascaroides from the stomach of Gymnarchus niloticus collected from Lekki lagoon, Lagos, Nigeria.
The high worm burden in the intestine of the fish may be due to its omnivore nature. Khalil (1971), van As and Basson (1984), reported that a variety of adult stage tapeworms occur in native African fish especially the Caryophyllaeidae as well as one amphilinid representative, the segmented pseudophyllideans, and Proteocephalidae. These findings coincide with the present study which also isolated Caryophyllaeid and Proteocephalid cestodes from S. clarias.
Scholtz (1991) reported that the first intermediate host of Caryophyllaeid cestodes are the oligochaete worms Tubifex and allied gender. They emphasized that eggs contained within, or released from evacuated dead worms, are ingested and hatched within the digestive tract of the tubificid worm. The definitive host therefore becomes infected when consuming infected Oligochaetes. However, Hoffman (1967) reported that the first intermediate hosts of segmented tapeworms like Pseudophyllideans and Proteocephalids are copepods. He reported that a second larval stage of Pleurocercoids, develops in fish species that are not compatible as definitive hosts in Proteocephalus.
The high prevalence of gastrointestinal helminthic infections may then be attributable to the omnivorous feeding habits of the fish species.
Banhawy et al. (1975) also collected W. virilis from the intestine of S. schall. These authors observed degenerative changes in gut wall, liver and pancreas.
The prevalence of the nematode species of the genera Raphidascaroides is the first scientific reports in Synodontis clarias of Lekki lagoon. Rajyalakshmi (1995) also collected a new species of this nematode genera from the intestine of hammer-headed shark, Sphyrna zygaena (Linnaeus) in Sisakhapatnam, and this new species did not coincide with the description of already known species.
Moravec (1975) found that the larvae of Procamallanus laevionchus, anisakids (Heterocheilhdae) Dujar dinascaris, and of Raphidascaroides, when ingested by wrong piscine hosts, often survive as waiting stages (fourth stage larvae) in the gut or other tissues for a variable period of time and continue their development into the adult stage, if their carrier host (paratenic) is predated by a compatible host. Petter et al. (1989) has also demonstrated this in species of Anguillicola.
The gastrointestinal helminth infections of different length categories were also recorded. The length group 10-15 cm recorded an prevalence of 42.1% while the length group 16-20 cm recorded the lowest prevalence of 36.6%. This may be due to random selection of the specimens and to probable high level of immunity built up in the fish specimens. The other length groups, 21-25 and 26-30 cm, recorded an prevalence of 48.1 and 50%, respectively. The highest rate of infection recorded in the male specimens (37.8%) could also be attributable to the random selection of the specimens from Lekki lagoon. Further study is still required to carry out the genomic characterization of the gastrointestinal helminths and also to investigate the different intermediate hosts of the helminth parasites.
Resumen
Se muestrearon aleatoriamente un total de 362 especímenes de Synodontis clarias, los cuales fueron sometidos a análisis parasitológicos. Los especimenes fueron recolectados durante un período de un año del lago Lekki, Nigeria. La existencia de infecciones gastrointestinales fue de un 38.7% del total de especímenes examinados, lo cual representó 114 especímenes infectados con parásitos helmintos. Los gusanos helmintos encontrados incluyen dos céstodos, especies de Proteocephalus, Wenyonia acuminata, y una especie de nemátodo, Raphidascaroides sp. Los especímenes machos (196) presentaron una tasa de infección mayor (37.8%) que la presentada por hembras (23.5%). La cantidad total de gusanos parásitos fue alta (678) y fue independiente del sexo y la talla del pez.
Key words: Synodontis clarias, helmintos, parásitos, Proteocephalus, laguna Lekki, Lagos, Nigeria.
Received 07-VI-2007. Corrected 30-VI-2008. Accepted 31-VII-2008.
References
Akinsanya, B. & O.A. Otubanjo. 2006. Helminth Parasites of Clarias gariepinus (Clariidae) in Lekki Lagoon, Lagos, Nigeria. Rev. Biol. Trop. 54: 93-99. [ Links ]
Akinsanya, B., A.A. Hassan & O.A. Otubanjo. 2006. A comparative study of the parasitic helminth fauna of Gymnarchus niloticus (Gymnarchidae) and Heterotis niloticus (Osteoglossidae) from Lekki Lagoon, Lagos, Nigeria. Pak. J. Biol. Sci. 10: 427-432. [ Links ]
Banhawy, M.A., M.FA. Saoud, I.M. Anwar & M.K. El-Naffar. 1975. The histopathological effects of tine parasitic tapeworm Wenyonia virilis on the ileum and liver of the silurid fish Synodontis schall. Ann. Zool. 11: 83-101. [ Links ]
Breder, C.M. & D.E. Rosen. 1966. Modes of reproduction in fishes. T.F.H. Publications, Neptune City, New Jersey, USA. [ Links ]
Gosse, J.P. 1986. Mochokidae. P105 – 152. In J.Daget, J.P. Gosse and D.F.E. Thys. van den Audenaerde (eds.) Checklist of the freshwater fishes of Africa (CLOFFA). vol 2. ORSTOM, Paris. [ Links ]
Hoffman, G.L. 1967. Parasites of North American Freshwater Fishes. University of California, Berkeley and Los Angeles, CA, EEUU. 486 p. [ Links ]
Khalil, L.F. 1971. Checklist of the helminth parasites of African freshwater fishes. Tech. Comm. 42, Comm. Inst. Helm., C.A.B. England, England. 80 p. [ Links ]
Khalil, L.F. & L. Polling. 1997. Checklist of the helminth parasites of African freshwater fishes. University of the North Republic of South Africa, South Africa. 161 p. [ Links ]
Kusemiju, K. 1981. The Hydrobiology and fishes of Lekki Lagoon, Nigeria. Nig. J. Nat. Sci. 3: 135-146. [ Links ]
McConnell, R.H. 1965. Field Identification of Freshwater Fishes Likely to Occur in the Area above the Kainji Dam on the River Niger, p. 43-64. In E. White (ed.). The First Scientific Report of the Kainji Biological Research Team, Nigeria. [ Links ]
Moravec, F. 1975. The development of Procamallanus laevionchus (Wedl, 1862) (Nematoda, Camallanidae). vest. Cs. Spol. Zool. 39: 23-38. [ Links ]
Olaosebikan, B.D. & A. Raji. 1998. Field guide to Nigerian freshwater fishes. Federal College of Freshwater Fisheries Technology, New Bussa, Nigeria. 106p. [ Links ]
Paugy, D. & T.R. Roberts. 1992. Mochokidae, p. 500-563. In C. Leveque, D. Paugy & G.G. Teugels (eds.). Faune des Poissons deaux douces et saumatres d Afrique de Touest Tome. 2. Coll. Faune Tropicale n 0 28. Musee Royal de lAfrique Centrale, Tervuren, Belgique and O.R.S.T.O.M., Paris, France. [ Links ]
Paugy, D., K. Traore & P.S. Diouf. 1994. Faune chthyologique des eaux douces dAfrique de lQuest. P. 35-66. In G.G. Teugels, J.F. Guegan and J.J. Albaret (des.) Biological diversity of African fresh and brackish water. Fishes, Geographical overviews presented at the PARADI Symposium, Senegal, 15 – 20 November 1993. Ann. Mus. R. Afr. Centr., Sci. Zool. 275: 1-177 [ Links ]
Petter, A.J., Y.A. Frontaine & N. Ise Belle. 1989. Edute du development larvaire de Anguinicola Crassus (Dracunculoidea, Nematoda) Chez un cyclopidae de laregion parisienne. Ann. Parasit. 64: 347-355. [ Links ]
Rajyalakshmi, I. 1995. Description of a new species of the genus Raphidascaroides, Yarnaguti, 1941 (Nematoda: Heterocheihdae) (from the Shark sphyrna Ztgaena (Linnaeus) at viscakhapatnam, India. Bol. Cloil Parasitol. 50: 24-27. [ Links ]
Reed, W., J. Burchard, AJ. Hopson, J. Jennes & I. Yaro. 1967. Fish and fisheries of Northern Nigeria. Publ. M.A.N.R., Nigeria. 22 p. [ Links ]
Riede, K., 2004. Global register of migratory species from global to regional scales. Final Report of R & D-Projekt 80805081. Federal Agency for Nature conservation, Bonn, Germany. 329 p. [ Links ]
Scholtz, T. 1991. Early development of Khawia sinensis HSU, 1935 (Cestoda: Caryophyllidea). A carp parasite. Folia Parasitol. 38: 133-142. [ Links ]
Van As, J.G. & L. Basson. 1984. Checklist of freshwater fish parasites from Southern Africa. S. Afr. J. Wildl. 14: 49-61. [ Links ]
Willoughby, N.G. 1974. The ecology of the genus Synodontis (Pisces: Siluriodei) in Lake Kainji, Nigeria. Ph.D. Thesis, University of Southampton, U.K. 288 p. [ Links ]