Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.54 suppl.3 San José Dec. 2006
Morphology offers no clues to asexual vs. sexual origin of small Acropora cervicornis (Scleractinia: Acroporidae) colonies
D. E. Williams1 & M. W. Miller2
1 (*) University of Miami, Cooperative Institute for Marine and Atmospheric Sciences, RSMAS, 4600 Rickenbacker Cswy. Miami, Florida 33149 USA, tel: 305-361-4569, fax: 305-361-4478; dana.williams@noaa.gov
2 NOAA Fisheries, Southeast Fisheries Science Center, 75 Virginia Beach Dr. Miami, Florida 33149 USA.
Received 05-VI-2006. Corrected 02-X-2006. Accepted 13-X-2006.
Abstract: Sexual recruitment of the staghorn coral, Acropora cervicornis, is accepted to be very rare. Instead, these branching corals proliferate through fragmentation leading to dense mono-specific and possibly monoclonal stands. For acroporid corals, which have suffered drastic population declines, dominance of asexual reproduction results in low levels of genotypic diversity and limited ability to re-colonize extirpated areas. Small colonies with a single encrusting, symmetrical base, and few incipient branches are frequently presumed to be the result of a settled planula (i.e. sexual reproduction). Here, we show that colonies fitting this description (i.e., presumed sexual recruits) can result from asexual fragmentation. Acropora cervicornis colonies (~20 cm diameter) were tagged and observed over eighteen months. In several cases, colony offshoots fused with the adjacent substrate forming secondary disc-like attachment points. Following natural fragmentation, these discs of tissue became separated from the original colony, and were observed to heal and give rise to smaller colonies with striking similarity to the expected morphology of a sexual recruit. Thus, presuming a colony is a sexual recruit based on appearance is unreliable and may lead to inflated expectations of genetic diversity among populations. The accurate assessment of recruitment and genetic diversity is crucial to predicting the recovery potential of these imperiled and ecologically irreplaceable reef corals. Rev. Biol. Trop. 54 (Suppl. 3): 145-151. Epub 2007 Jan. 15.
Key words: recruitment, fragmentation, staghorn coral, clonal, colony, Acropora cervicornis.
Acropora cervicornis (Caribbean staghorn coral; Lamark 1816) proliferates largely through fragmentation leading to dense monospecific (e.g. Gilmore and Hall 1976, Tunnicliffe 1981) and likely mono-clonal stands. Once a colony is broken, reattachment of the loose fragment generally occurs when a growing (apical) tip encounters the surrounding substrate (Gilmore and Hall 1976, pers. obs.) but may also occur along the branch when the polyps/corallites fuse with the substrate (Gilmore and Hall 1976, Tunnicliffe 1981). Acropora cervicornis can rapidly dominate local environments through this form of asexual reproduction. Alternatively, sexual recruitment is needed to colonize distant areas and introduce genetic diversity.
Acropora cervicornis reproduces sexually by releasing egg-sperm bundles in a mass spawning event (Vargas-Angel and Thomas 2002) that presumably occurs annually. With one exception (van Moorsel 1989), recruitment studies in the Caribbean employing artificial substrates have found settled acroporid planulae (from here referred to as sexual recruits) to be rare (Sammarco 1980, Rylaarsdam 1983, Ruiz-Zárate and Arias-González 2005, Quinn and Kojis 2005). Rylaarsdam (1983) describes a new recruit as asmall patch on the substratum with projecting corallites, one of which gives rise to a single branch based on the one sexual recruit that appeared on marked substrates in that study. This description (eg. Fig. 1a) certainly fits our expectation of how a settled planula might grow into a small colony; however, direct observations of development from settled planula to a branching colony are lacking.
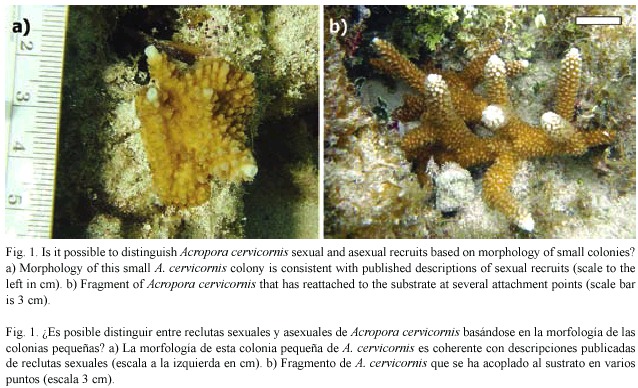
Several field studies report finding juvenile A. cervicornis that are presumed, based on their size, morphology or placement, to have originated from settled planulae (Gilmore and Hall 1976, Tunnicliffe 1981, Rylaarsdam 1983, Knowlton et al. 1990). Given that re-attached fragments are generally easily recognized (Fig. 1b) it seems reasonable to conclude that small sexual recruits should be discernable based on these criteria. Colonies situated on vertical or sloped surfaces could particularly be reasoned to be sexual recruits since loose fragments are unlikely to be retained in such locations, gravitating instead to flat surfaces. Here, we document a scenario whereby fragmentation can result in a small colony morphologically consistent with a sexual recruit.
Materials and methods
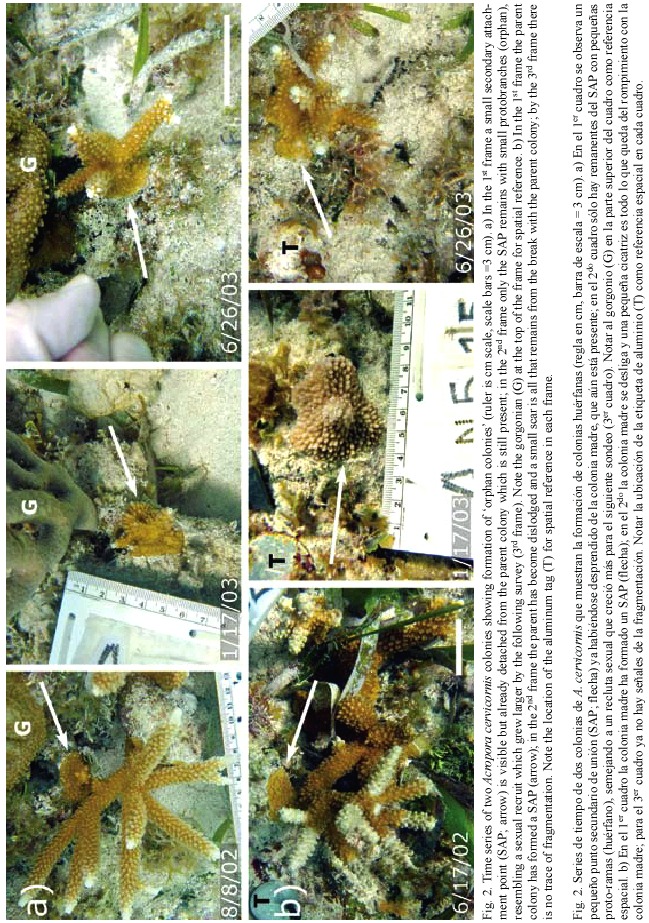
A monitoring study of A.cervicornis was initiated in summer 2002 at three sites in the Florida Keys (USA) including Anniversary Reef (25º23.293N, 80º09.869W, ANR), Elkhorn Reef (25º21.714N, 80º09.937W, ELR) and White Bank Dry Rocks (25º02.595N, 80º22.156W, WBDR).The ANR site is shallow (3 m) back reef/hard bottom habitat, while the ELR site is a slightly deeper (4-5 m) weakly zoned fore reef habitat. WBDR is a well-defined patch reef with a flat topside at 2-3 m depth. Both ANR and ELR differed notably from WBDR in that their foundations were unconsolidated reef rubble over sand with scattered Thalassia testudinum, while the topside of WBDR was generally consolidated limestone with relatively little loose rubble or sand.
The Acropora cervicornis population at all three sites consisted of isolated (non-thicket) colonies scattered among gorgonians, sponges and small head corals. Individual colonies were haphazardly chosen, measured (gross colony dimensions of length, width, height), photographed, and a numbered tag was nailed to the adjacent substrate to allow re-identification of the same colony over time (Table 1). Colonies were surveyed approximately every four months between June 2002 and November 2003 (see Williams and Miller 2006) for detailed explanation of methods and overall report of monitoring results). During these periodic surveys, each colony was located, measured, and photographed from several angles.
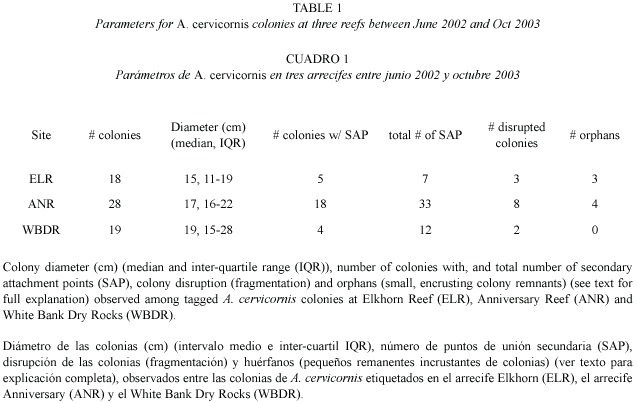
Time series of monitoring photographs for individual colonies were examined to specifically investigate the frequency of fragmentation events leading to small, encrusting remnants of A. cervicornis tissue. We quantified secondary attachment points, colony disruption, and resulting orphan colonies. Secondary attachment points (SAP) were classified as encrusting skeletal growths occurring where a live branch (usually the tip) grew into contact with the surrounding substrate, but not including the primary basal attachment. Colony disruption was defined as a subset of the observed fragmentation events whereby a substantial break (e. g. to the basal attachment of the colony) resulted in the colony being dislodged. Orphan colonies were identified as living encrusting patches of tissue that, although formerly observed as SAP of a parent colony, were subsequently observed disconnected from the parent colony following a colony disruption event. This analysis documents orphan colony formation and growth into a psuedo-sexual recruit, and characterizes the frequency of such events within the monitored populations.
Results and discussion
We documented the formation of several orphans (Table 1, Fig. 2) over the 18 month monitoring period. For each observed orphan colony, a SAP was observed on the parent colony at one survey, and at the following survey the parent colony was either entirely gone or dislodged and resting nearby. The remnant SAP remained attached to the substrate resulting in an orphan colony. Orphan colonies ranged in size from 2 - 5 cm diameter when they were first observed. The orphans later produced branches and grew in a symmetrical manner that resembled a sexual recruit, with no apparent signs that it resulted from fragmentation (i.e. a pseudo-sexual recruit). Furthermore, several orphans were formed on a sloping surface, further disguising their asexual origin.
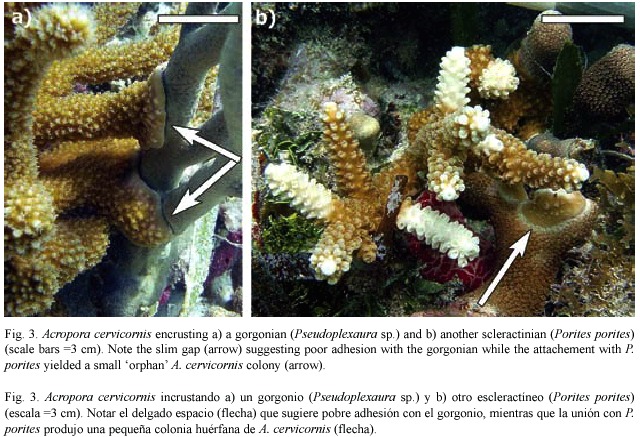
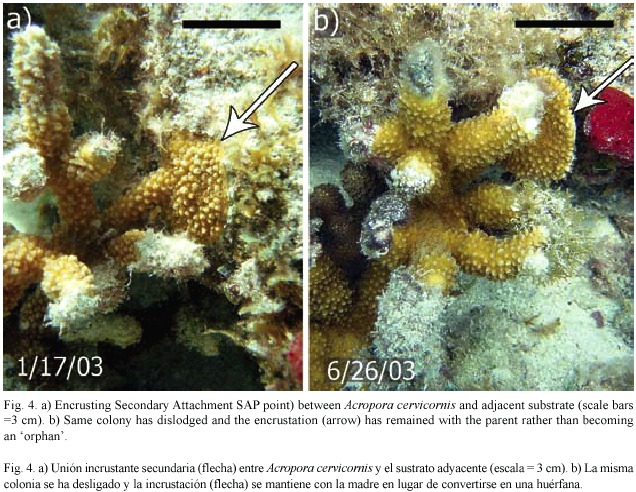
Colonies observed forming SAPs ranged in size from 9 - 31 cm in diameter (median=12 cm), suggesting that SAP formation is more a function of substrate proximity to growing branch tips (Gilmore and Hall 1976) rather than a function of colony size. In addition to reef substrate, SAPs formed with surrounding organisms including gorgonians, sponges and other species of hard corals including Porites porites (Pallas, 1766; Fig. 3) and Siderastrea siderea (Ellis and Solander, 1786). SAPs that were formed on hard corals resulted in orphans while those on gorgonians or sponges usually failed to adhere. In cases where the SAP failed to adhere to the substrate, it remained intact with the dislodged parent colony, (Fig. 4) indicating that not all SAPs become orphans. Based on these observations, the scenario for orphan formation involves three elements. First, A. cervicornis must form a SAP with the substrate; then the parent colony must be dislodged, and finally, the SAP must adhere to the substrate through the fragmentation event.
We observed the formation of three orphans from 18 (17%) monitored colonies at ELR, and four from 28 (14%) monitored colonies at ANR (Table 1) over the 18 month study period. SAPs were observed at all three sites (Table 1), and in many cases a single colony formed multiple (up to six) attachments. However, orphans were only found at ELR and ANR. The substrate at these two sites was relatively unconsolidated compared to WBDR. At these two sites colony attachments (including basal attachments) were often on unstable substrate. While we did not observe orphans among our tagged colonies at WBDR, all the necessary elements (SAP and colony disruption) were present, though not in the same colony, suggesting that they could occur there. A major disease outbreak at WBDR resulted in substantial mortality of A. cervicornis (Williams and Miller 2005) by April 2003, which likely compromised formation and/or detection of attachment points.
The orphan colonies we observed were notably consistent in morphology with sexual recruits, however they were indisputably of asexual origin and hence could be termed pseudo-sexual recruits. Since our monitoring program was not originally designed to detect this phenomenon, we cannot provide a robust estimate of orphan frequency among A. cervicornis populations. Nonetheless, we provide clear evidence that orphan formation does occur, and present a plausible, natural mechanism for the occurence of pseudo-sexual recruits.
In May 2006 Acropora cervicornis was listed as a Threatened Species under the U. S. Endangered Species Act (Anonymous 2006) due to its Caribbean-wide demise that began in the 1980s (e.g. Bruckner 2003). In highly clonal species such as A. cervicornis, individual colonies often do not represent genetically unique individuals (Baums et al. in press) meaning that the effective population size is likely to be substantially smaller than colony counts alone would suggest. Recovery of A. cervicornis in the future is likely more dependent on successful sexual recruitment than were historical populations (Bruckner 2003) since widespread mortality has drastically reduced fragment sources in many areas.
The morphological similarity between sexual recruits and some asexual recruits can hamper estimates of genotypic diversity, dispersal and thus the evaluation of recovery potential of this imperiled species.
Acknowledgements
This work was supported by the Southeast Fisheries Science Center under the NOAA Coral Reef Conservation Program and conducted under permit # BISC-2002-SCI-0038 from Biscayne National Park and # FKNMS- 2002-032 from the Florida Keys National Marine Sanctuary. We are particularly grateful for logistical support provided by Biscayne National Park.
Resumen
Se ha aceptado que el reclutamiento sexual del coral asta de venado, Acropora cervicornis, es muy raro. Por el contrario, estos corales ramificados proliferan a través de fragmentación, generando densas bases monoespecíficas e incluso monoclonales. Para corales acropóridos, los cuales han sufrido disminuciones de población drásticas, la dominancia de reproducción asexual resulta en bajos niveles de diversidad genotípica y abilidad limitada para recolonizar áreas de donde han sido erradicados. Frecuentemente se presume que las colonias pequeñas con una sola base incrustante simétrica y unas pocas ramas incipientes, son el resultado del asentamiento de una plánula (reproducción sexual). Aquí, nosotros demostramos que algunas colonias que calzan con esta descripción (supuesta reproducción sexual) pueden resultar de fragmentación asexual. Se etiquetaron y observaron colonias de Acropora cervicornis (~20 cm de diámetro) durante 18 meses. En muchos casos, los retoños de la colonia se fusionaron con el sustrato adyacente formando puntos de acoplamiento con forma de disco. Siguiendo con la fragmentación natural, estos discos de tejido se separaron de la colonia original, cicatrizaron y dieron paso a pequeñas colonias con tremenda similitud a la morfología esperada para un recluta sexual. Por lo tanto, asumir que una colonia es un recluta de origen sexual basándose en apariencia es poco fiable y puede generar expectativas infladas de diversidad genética entre poblaciones. La evaluación certera del reclutamiento y la diversidad genética es crucial para predecir la recuperación potencial de estos arrecifes de coral, los cuales están en peligro y son irremplazables.
Palabras clave: reclutamiento, fragmentación, coral asta de venado, clonal, colonia, Acropora cervicornis.
References
Anonymous. 2006. Endangered and Threatened Species: Final Listing Determinations for Elkhorn Coral and Staghorn Coral. Federal Register 71: 26852-26872. [ Links ]
Baums, I. B., M. W. Miller & M. E. Hellberg. 2006. Geographic variation in clonal structure of the Caribbean reef-building coral Acropora palmata. Ecology. Mono. 76(4): 503-519. [ Links ]
Bruckner, A. W. 2003. Proceedings of the Caribbean Acropora Workshop: Potential Application of the U.S. Endangered Species Act as a Conservation Strategy. NOAA Tech. Memo NMFS-OPR-24. 184 p. [ Links ]
Gilmore, M.D. & B. R. Hall. 1976. Life history, growth habits and constructional roles of Acropora cervicornis in the patch reef environment. J. Sed. Petr. 46: 519-522. [ Links ]
Knowlton, N., J. C. Lang & B. D. Keller. 1990. Case study of natural population collapse post-hurricane predation on Jamaican staghorn corals. Smiths. Contrib. Mar. Sci. 31: 1-25. [ Links ]
Quinn, N. J. & B. L. Kojis. 2005. Patterns of sexual recruitment of acroporid coral populations on the West Fore Reef at Discovery Bay, Jamaica. Rev. Biol. Trop. 53 (Suppl. 1): 83-89. [ Links ]
Ruiz-Zárate, M. A.& J. E. Arias-González. 2005. Recruitment of Acropora, Millepora and Stylaster roseus on an experimental substratum. Coral Reefs 24: 56. [ Links ]
Rylaarsdam, K. W. 1983. Life histories and abundance patterns of colonial corals on Jamaican reefs. Mar. Ecol. Prog. Ser. 13: 249-260. [ Links ]
Sammarco, P. W 1980. Diadema and its relationship to coral spat mortality: grazing, competition and biological disturbance. J. Exp. Mar. Biol. Ecol. 45: 245-272. [ Links ]
Tunnicliffe, V. 1981. Breakage and propagation of the stony coral Acropora cervicornis. Proc. Natl. Acad. Sci. 78: 2427-2431. [ Links ]
Van Moorsel, G. W. N. M. 1989. Settlement of stony corals (Scleractinia) on artificial substratum on a Caribbean reef: spatial aspects. Ph.D. Diss, Rijksuniversiteit, Groningen. 104 p. [ Links ]
Vargas-Angel, B. & J. D. Thomas. 2002. Sexual reproduction of Acropora cervicornis in near-shore waters off Ft. Lauderdale, Florida (USA). Coral Reefs 21: 25-26. [ Links ]
Williams, D. E.& M. W. Miller. 2005. Coral disease outbreak: pattern, prevalence, and transmission in Acropora cervicornis. Mar. Ecol. Prog. Ser. 301: 119-128. [ Links ]
Williams, D. E., & M. W. Miller 2006. Importance of disease and predation to the growth and survivorship of juvenile Acropora palmata and Acropora cervicornis: a demographic approach. Proc. 10th Int. Coral Reef Symp. 1096-1104. [ Links ]














