Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.54 suppl.3 San José Dec. 2006
Relationship between anthropogenic impacts and bleaching-associated tissue mortality of corals in Curaçao (Netherlands Antilles)
I. Nagelkerken
Department of Animal Ecology and Ecophysiology, Institute for Water and Wetland Research, Faculty of Science, Radboud University, Toernooiveld 1, 6525 ED Nijmegen, The Netherlands, Tel.: +31-24-3652471, Fax: +31-24-3652409; i.nagelkerken@science.ru.nl
Received 01-VI-2006. Corrected 02-X-2006. Accepted 13-X-2006.
Abstract: Chronic anthropogenic impacts can have a negative effect on coral health and on coral energy budgets needed for regeneration of lesions. I therefore hypothesise that during massive bleaching events, the degree of corals showing bleaching-related tissue mortality is higher in areas subject to chronic anthropogenic impacts than in relatively pristine areas. In the present study, the degree of bleaching and bleaching-related tissue mortality was estimated for eight abundant coral species in Curaçao, at the onset of a massive Caribbean bleaching event in 1995, and three months afterwards. To study the possible effects of anthropogenic disturbances, the study was done at four unpolluted control sites, two polluted sites (sedimentation, sewage), and four sites at the mouth of lagoons with outflow of nutrient-rich, warm and turbid seawater. No pattern of an overall difference in bleaching between impacted and control sites was found for the degree of bleaching. However, the percentage of corals showing bleaching-related tissue mortality was higher at the impacted sites than at the control sites for the total number of corals and for corals with < 50% of their surface area bleached. Highest and most significant values of tissue mortality were found at a reef site experiencing chronic pollution by raw sewage. The data thus suggest that unfavourable conditions caused by anthropogenic influences, such as increased sedimentation, eutrophication and seawater temperature, have an additional negative effect on the tissue survival of coral colonies during bleaching episodes. Rev. Biol. Trop. 54 (Suppl. 3): 31-43. Epub 2007 Jan. 15.
Key words: Stony corals, bleaching, tissue mortality, anthropogenic impacts, Caribbean.
Coral bleaching, defined as the loss of symbiotic zooxanthellae leading to a whitening of corals, can result from a variety of environmental stressors, but massive bleaching events have mainly been related to increased seawater temperature and solar radiation (Brown 1997, Fitt et al. 2001). Studies have indicated that the frequency of coral bleaching and the area affected has been increasing in the last decades (Goreau 1992, Glynn 1993, Goreau and Hayes 1994, Brown 1997). This trend has also been observed for coral diseases and both may be related to an increased background stress from global warming and/or a higher susceptibility of colonies due to a lower resistance (Brown 1997, Harvell et al. 1999).
Coral bleaching can have a significant effect on the survival of coral colonies (Harriott 1985), and massive coral bleaching events have resulted world-wide in large-scale loss of coral cover (Glynn 1984, Aronson et al. 2000, Goreau et al. 2000, McClanahan et al. 2001, Riegl 2002). Aside from natural stressors, coral reefs are also subject to many anthropogenic influences such as overfishing, nutrient enrichment and sedimentation, and together they can cause major phase shifts in coral reef communities (Hughes 1994, Shulman and Robertson 1996).
Extensive bleaching can result in partial tissue mortality of the coral (Quinn and Kojis 1999, Baird and Marshall 2002, Shenkar et al. 2005). Corals have a limited energy budget which is partitioned over important physiological processes such as regeneration, respiration, growth and reproduction (Bak 1983, Spencer Davies 1991). A trade-off in energy exists in favour of regeneration and to the detriment of growth (Bak 1983, Meesters et al. 1994) and fecundity (van Veghel and Bak 1994), which indicates the importance of this process. During bleaching events, the zooxanthellae which are the main providers of carbon for their coral host are expelled for an extended period of time, and energy budgets of corals may be even more limited. This is probably the cause for a slower growth (Goreau and Macfarlane 1990), reproduction (Szmant and Gassman 1990) and regeneration (Meesters and Bak 1993, Mascarelli and Bunkley-Williams 1999) in bleached colonies.
Bleaching often causes considerable lesions in the coral tissue. This means that during bleaching, corals are confronted with a lower energy budget (due to loss of zooxanthellae) and a higher surface area of tissue (i.e. on top of lesions due to other causes) to be regenerated. This may inhibit colony regeneration and survival, because a large lesion perimeter length is related to a slower regeneration, and large lesions are colonised more often by algae, and do not regenerate completely (Mascarelli and Bunkley-Williams 1999, Meesters et al. 1997a).
Anthropogenic stressors on top of natural disturbances can further lower the resistance, health and energy budgets of corals, especially when these impacts are chronic (Kuntz et al. 2005). I therefore hypothesise that during massive bleaching events, the degree of corals showing bleaching-related tissue mortality is higher in areas subject to chronic anthropogenic impacts than in relatively pristine areas. In the present study this hypothesis is tested on the island of Curaçao during the massive Caribbean bleaching event of 1995 (CARICOMP 1997). Coral reef sites influenced by anthropogenic stressors which are known to affect coral health were selected: sedimentation (Bak and Elgershuizen 1976, Bak 1978), eutrophication (Pastorok and Bilyard 1985, Nordemar et al. 2003), and water effluents with an elevated temperature (Jokiel and Coles 1974, Meesters and Bak 1993).
Materials and methods
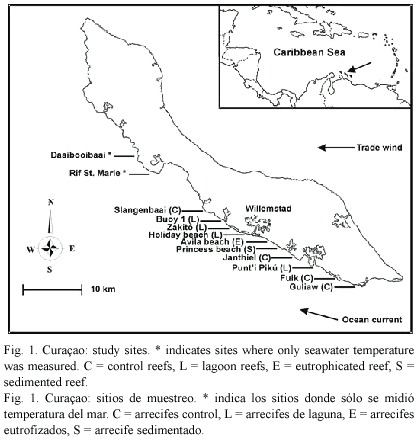
Study area: Fringing coral reefs run along the entire sheltered south-western coast of Curaçao. From the shore, a submarine terrace gradually slopes to a drop-off at approximately 7 - 11 m depth. At the drop-ooff, the reef slopes off steeply, sometimes interrupted by a small terrace at 50 to 60 m (Bak 1975). For a detailed description of the reef structure and distribution of corals on the reefs of Curaçao see Bak (1975).
Two directly impacted,four indirectly impacted, and four relatively pristine (control) reef sites were selected for the study (Fig. 1). Two control sites were located up-current of all coastal developments on the island (Fuik, Guliaw) and were located in the Curaçao Underwater Park. These pristine reefs receive fresh oceanic waters, are not influenced by any coastal impacts (no development whatsoever from Fuik south-eastwards) and are very well-developed in terms of coral cover (van Duyl et al. 2002). A third control site was a very well-developed reef at Slangenbaai, situated down-ccurrent of an area of approx. 3.5 km in length without any coastal development. The fourth control site was a well-developed reef at Janthiel, situated in an area with very little up-current coastal development (van Duyl et al. 2002).
The four indirectly impacted sites consisted of reefs located near the mouths of large semi-enclosed bays. Punti Pikú is located at the mouth of Spanish Water Bay, which is a bay with a relatively high degree of coastal development consisting of residential development (lacking sewage control), marinas, fishing harbours and water-sport facilities, as a result of which some areas in the bay have elevated levels of phosphate, heavy metals and human faeces (Anonymous 1987, Djohani and Klok 1988, Anonymous 1991). Likely as a result of eutrophication caused by indirect or direct sewage pollution from residential development along the shoreline of the bay, the coral populations in the bay have declined significantly during the last three decades and have been heavily overgrown by algae (Debrot et al. 1998).The bay water has an increased temperature, salinity and turbidity (Kuenen and Debrot 1995), which flows out onto the adjacent reef during outgoing tides, which can be visually seen as a thick brown, sediment-rich, warm water layer flowing out of the bay onto the reef, predominantly at the lower part of the water column (pers.obs.). Holiday beach is located near the mouth of the St. Anna Bay, which is a bay used entirely for industrial activities (harbour, container terminal, oil refinery, dry dock, etc.). The sediments are polluted by heavy metals, petroleum and other organic compounds (Debrot and Sybesma 2000), while the water is highly polluted by sewage and industrial waste-water (Buth and Ras 1992). The polluted bay water flows out of the bay during low tides, and corals are largely absent at the first kilometre down-current of the mouth of the bay (Gast et al. 1999). Zakitó is located at the mouth of a lagoon which harbours the local desalination plant, which releases its cooling water and salt-water effluents into the lagoon (Buth and Ras 1992). The surface seawater in front of the mouth of the lagoon has an elevated temperature (range 4.3 -7.9 ºC) and salinity (range 1.8 -2.0 ppt) compared to that of a control up-current reef site, whereas concentrations of copper in the sediment on the reef were four times higher than at the control site (Hoppe 1981). Buoy 1 is located near the mouth of Piscadera Bay, which is contaminated with sewage effluent overflow from the municipal sewage treatment plant at Klein Hofje (Buth and Ras 1992). E. coli measurements at the mouth of Piscaderabaai during 2004 ranged from 230 to beyond 2000 per cc (A. Debrot, pers. comm.). Djohani and Klok (1988) further showed elevated heavy metals contamination in sediments for two stations in the bay. A channel has been dredged in the mouth of the bay in 1972, as a result of which sediment and nutrient-rich water flows out onto the adjacent reef during outgoing tides (Bak 1978, pers. obs.).
The two directly impacted sites consisted of a coral reef located just downstream of a 340 m long artificial sandy beach causing chronic sedimentation (Princess Beach) and a coral reef near a sewage outlet with chronic eutrophication (Avila beach). The artificial beach was constructed during 1987-1990 and is protected from the waves by breakwaters placed parallel to the coastline. Continuous beach erosion causes sediment to be deposited on the adjacent reefs, as a result of which the beach needs to be replenished with sand periodically as sand retention is poor (Debrot and Sybesma 2000). In 1991, long-term (1 yr) sedimentation at 5 m depth was significantly higher at the studied reef site (40 g m-2 d-1) than at an up-current control reef (27 g m-2 d-1) (Meesters et al. 1992). Next to Princess Beach (up-current) a second, 500 m long, artificial beach (Seaquarium Beach) is located, causing additional sedimentation. At Avila beach, the water is polluted by sewage being discharged into the sea by three pipes, at a rate of approx. 1000 m3 raw sewage per day (Gast et al. 1999). During 1994/1995, concentrations of PO43-, NH4+, NO2- and NO3- were much higher here than at the control site Fuik Bay, and mainly originated from untreated sewage (Gast et al. 1999, van Duyl et al. 2002).
Differences between sites in physical variables are expected to be smaller than for the anthropogenic stressors. The surface ocean currents run parallel to the coast line (see Fig. 1), and differences in flushing of the study sites is therefore likely to be minimal. Difference in seawater temperature was negligible between sites (see Fig. 2). Wave exposure did not differ much between the sites. Seven of the ten sites had a wave energy index of level 4 (average wave height 0.5 - 1.0 m; see van Duyl 1985); Holiday beach and Guliaw had an index of level 3 (1.0 - 1.5 m), and Princess beach had an index of level 5 (0.3 - 0.5 m).
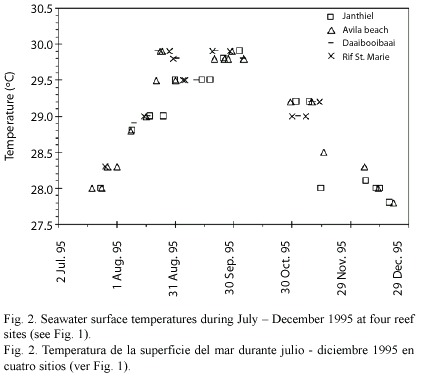
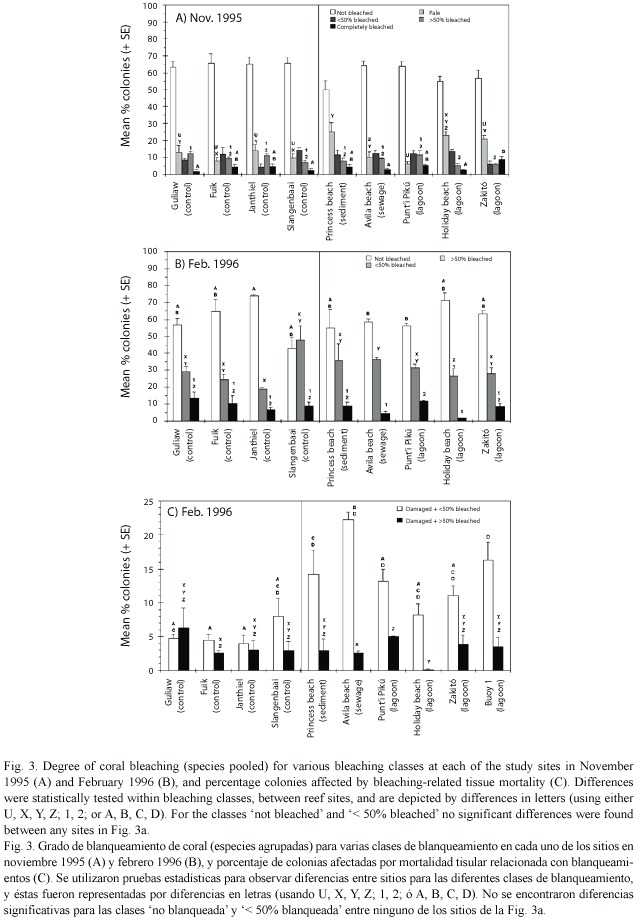
Sampling design: At each reef site, three permanent transects of 2 x 15 m were established on the reef terrace between 6 and 8 m depth. The replicate transects were placed in areas with highest coral cover and were separated from each other by an average distance of 15 m. Eight common coral species were selected for the study, viz. Colpophyllia natans (Houttuyn, 1772), Diploria strigosa (Dana, 1848), Meandrina meandrites (Linnaeus, 1758), Millepora complanata (Linnaeus, 1758), Montastrea annularis (Ellis and Solander, 1786) (consisting for 77% of the columnar morph and 23% of the massive morph),M. cavernosa (Linnaeus, 1767), Porites astreoides (Lamarck, 1816) and Siderastrea siderea (Ellis and Solander, 1786). Of these species, all coral colonies > 5 cm in diameter were counted within the transects. For each colony, bleaching status was determined following Gleason (1993): not bleached, pale (colonies slightly discoloured but not yet bleached white), and bleached (i.e. white) colonies further subdivided into three categories: 5 - 50% bleached (referred to as < 50% bleached), 50 -95% bleached (referred to as > 50% bleached), and completely bleached (100% bleached). Presence of lesions (> 1 cm) on the coral surface was noted for each colony. Highest sea surface temperature at four reef sites occurred during September 1995 (Fig. 2), with coral bleaching starting afterwards. The sampling took place during November 1995, at the onset of the massive coral bleaching event.
The study was repeated in the permanent transects after three months (February 1996) at the peak of the bleaching event. The bleaching class pale was no longer present (i.e., all pale corals had turned white and were thus classified as bleached colonies). Lagoon site Buoy 1 was included as an additional site (Fig. 1) and had not been surveyed during November 1995. Bleaching-related tissue mortality was noted for each colony, and was classified as either < 25% or > 25% of the colony surface area affected. The latter was only observed for 38 of the 6 030 studied colonies and was therefore not analysed separately. The percent damaged colonies thus refers to the number of colonies with one or more lesions, expressed as the percentage of the total number of colonies studied. Bleaching-related tissue mortality was distinguished as lesions of various sizes, almost always located at the part of the colony with most bleached tissue. Lesions were always located within the bleached parts of the colony. Tissue mortality was mostly represented by tissue sloughing off the colony, with parts of the lesion already devoid of tissue and showing the clean white coral calices. On basis of the above, the lesions could be readily distinguished from parrotfish-associated lesions which have a sharp edge along the living tissue, are devoid of coral calices, show biting grooves, and are often < 5% of the total surface area in size. Furthermore, lesions caused by non-bleaching associated processes are mostly < 4 cm2 (lesion Type I, Meesters et al. 1997b). Tissue mortality due to coral diseases is often identified by either a sharp line between dead and living tis- sue or a disease band with a different colour than that of coral bleaching. The most common diseases in the coral species investigated at the time of study were yellow band, black band and dark-spot disease, all of which are differently coloured or shaped than bleached tissue (Goreau et al. 1998). Also, since these diseases progress relatively slow (Goreau et al. 1998, Cervino et al. 2001), the dead coral calices close to the disease band are often overgrown by filamentous algae. White plague which proceeds much faster and also shows clean coral calices where the coral tissue has died was not yet apparent in 1995 (pers. obs.).
Data analysis: For the pooled data (see Fig. 3), mean percent of colonies affected was calculated as follows. First the percent colonies affected was calculated separately for the various classes of bleaching, within each transect, for each species. Then the average percentage of colonies affected (per bleaching class) was calculated per transect based on the values of the eight species. This was done to avoid that the pooled averages per transects were influenced by the abundance of coral colonies within a transect. Afterwards, the averages (pooled for species) of the three replicate transects were used to calculate an average and standard error for each reef site, in this way depicting the variability between transects within sites.
Reef sites were statistically compared using a one-way ANOVA followed by a Tukey HSD (for homogeneous variances), or a Kruskal-Wallis test followed by a Games-Howell (for non-homogeneous variances) post-hoc test (Field 2002). A nested design was not used because although the six (in)directly impacted sites were all affected, they were affected differently and thus did not represent the same type of replicate. For example, Princess beach and Avila beach were both highly affected by anthropogenic influences, but a reef suffering from sedimentation cannot serve as a replicate for a reef being affected by sewage pollution. The results showed that the impacted sites had a higher percentage of damaged colonies than the control sites. To account for the effect of non-bleaching related lesions in determining this difference, the percentage of all damaged colonies in November (i. e., non-bleaching related), when the onset of bleaching had not yet resulted in partial tissue mortality, was compared between affected and control sites using a Students t- test. This was also done for the data of February. Significant differences were accepted at 
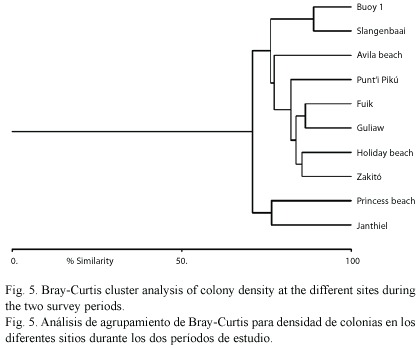
Cluster analysis (Bray-Curtis similarity) was done on colony density of the eight different coral species at the different sites (mean density of the two survey periods).
Results
In November 1995, no significant difference (one-way ANOVA, p>0.132) was found between the various reef sites for the percent colonies not bleached (Fig. 3a). This was also the case at species level (one-way ANOVA, p>0.292, data not shown), except for D. strigosa which showed a lower value at Princess beach than at Avila beach, Fuik, Guliaw, Holiday beach, PuntiPikú and Slangenbaai (Tukey HSD post-hoc test, p<0.047), and for M. cavernosa which showed a lower value at Holiday beach than at Janthiel, Fuik and Slangenbaai (p<0.043).
There were some small differences between reef sites for the pale colonies and the three bleaching categories, but without a consistent pattern for the impacted versus control reefs. The most obvious difference at the level of individual sites was the significantly highest percentage of pale colonies at the sedimented site (Princess beach), and the significantly highest percentage of completely bleached colonies at the lagoon site with outflow of hot seawater (Zakitó) (Fig. 3a).Three months later, the percentage of colonies not bleached was still similar between all reef sites, except for one lagoon site (PuntI Pikú) and one eutrophicated site (Avila beach) showing a significantly lower percentage of colonies not bleached than one control site (Janthiel) (Fig. 3b). Also at species level no difference was found between impacted and control sites for the percentage of colonies not bleached (Kruskal-Wallis test, p>0.073, data not shown); the only differences found were lower values of not bleached corals at Zakitó than at Holiday beach for M. cavernosa (Games-Howell post-hoc test, p=0.019), and for S. siderea at Buoy 1 than at Avila beach and Guliaw (p<0.017) and at Slangenbaai than Guliaw (p=0.047). For the two bleaching classes (< 50% and > 50%) no obvious trends in degree of bleaching could be detected between the reef sites (Fig. 3b).
The percentage of damaged colonies was much higher at the impacted sites than at the control sites, for corals with < 50% surface area bleached (Fig. 3c). A significantly higher percentage of colonies with lesions was found at the sedimented site (Princess beach), the eutrophicated site (Avila beach) and one lagoon site (Buoy 1) than at several of the control sites. For corals with > 50% surface area bleached, no obvious trend could be discerned between the various reef sites (Fig. 3c).
The difference in bleaching-related tissue damage between impacted and control sites could not be attributed to other causes than bleaching. In November, when bleaching had not yet resulted in partial tissue mortality, the occurrence of natural lesions was equal between impacted and control sites (mean % colonies with lesions ± SD: 0.6 ± 0.5 vs 0.7 ± 0.6, respectively, t -test, p=0.725), whereas in February when many colonies were bleached and suffered from partial mortality the percentage of corals with lesions was much higher at impacted than at control sites (24.44 ± 8.9 vs 11.7 ± 3.5, respectively, t -test, p=0.033).
At species level, the percentage of damaged colonies with < 50% surface area bleached was higher at the impacted sites than at most of the control sites for D. strigosa , M. meandrites, M. complanata, M. cavernosa, P. astreoides and S. siderea (Fig. 4). Probably due to the low numbers of replicate transects (N = 3) and coral colonies per site, significant differences were only found between the eutrophicated site (Avila beach) and control sites, for several species (M. meandrites, M. complanata, S. siderea ). For M. annularis, the percent of damaged colonies was similar between sites, except at the eutrophicated site (Avila beach) where it was significantly higher (Fig. 4e). C. natans did not show a clear difference between the impacted and control sites (Fig. 4a).
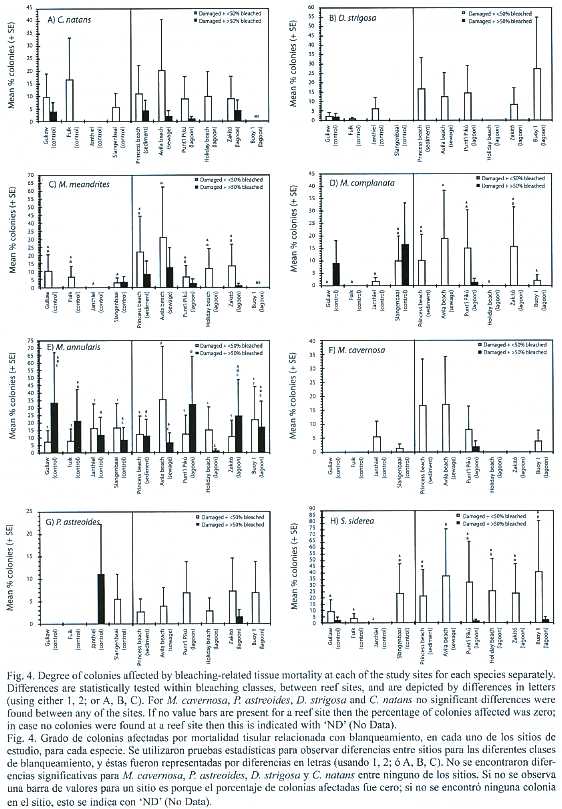
For the percentage of damaged colonies with > 50% surface area bleached, no clear trend in percent colonies damaged was observed between the impacted and control sites (Fig. 4).
The overall absence of a difference in bleaching or presence of a difference in bleaching-associated tissue mortality between impacted and control sites could not be attributed to differences in colony density of bleaching-resistant or mortality-susceptible species at the various sites, since cluster analysis showed a high similarity between coral community structure at impacted versus control sites (Fig. 5).
Discussion
Spatial variation in bleaching: The percentage of bleached coral colonies was similar at all reef sites along the sheltered coast of Curaçao, suggesting that various environmental variables (other than seawater temperature) and anthropogenic impacts may have had relatively little effect on the process of coral bleaching. Nevertheless, there were some minor indications of a positive relationship between coral bleaching and environmental impacts: the percentage of pale colonies and completely bleached colonies in November 1995 and the percentage of bleached colonies in February 1996 were all highest at an impacted site. However, because there was no consistent and clear pattern present, I conclude that in the present study no single relationship is present between various types of anthropogenic impacts and the degree of coral bleaching. This is supported by studies in the South Pacific showing that coral bleaching was not related to any of 15 environmental variables (including pollution, terrestrial mud and fleshy algae cover) except water temperature (Goreau and Hayes 1996, Goreau et al. 1997). Meesters and Bak (1993) showed an effect of the elevated seawater temperature at Zakitó lagoon on the degree of bleaching. When considering this reef site alone, the latter observation is partly supported by the present study, since the degree of completely bleached colonies was highest at the Zakitó lagoon.
Spatial variation in tissue mortality: The current data suggest a positive relationship between bleaching-related tissue damage and some types of anthropogenic impacts. For pooled species, as well as at species level (for six out of eight coral species), the percent coral colonies with lesions resulting from coral bleaching was much higher at the impacted sites than at the control sites. This was visible for total number of colonies and for colonies with < 50% of their surface area bleached (which applied to the greater majority of the colonies); no explanation can be provided why colonies with > 50% of surface area bleached did not show such a pattern. The lack of a relationship between impacts and bleaching-related tissue mortality in M. annularis can possibly be explained by the fact that this species was the most sensitive to bleaching (Meesters and Bak 1993; this study, 97% of all colonies), resulting in significant mortality also at control sites. Also bleaching-related tissue mortality has been reported to be highest in M. annularis compared to other coral species (Williams and Bunkley-Williams 1990).
Although bleaching-related mortality was higher at impacted reef sites, this did not apply to each individual site. In almost all cases the eutrophicated site Avila beach showed the (significantly) highest degree of damaged colonies, suggesting that direct pollution by sewage effluents negatively affects coral tissue integrity of bleached colonies. Chronic stress on corals at this eutrophicated site has probably caused a higher vulnerability of the colonies, either resulting in faster tissue mortality and/or a lower degree of tissue regeneration (Meesters and Bak 1993, Kuntz et al. 2005). This is supported by a study of Nordemar et al. (2003), which showed that nutrient enrichment at elevated water temperatures had a stronger negative effect on coral health than elevated temperature alone.
The impacted reef sites other than Avila beach, in most cases also showed a higher degree of damaged colonies related to bleaching (although not as high as Avila beach) than the control sites, but no hierarchy could be detected between the various types of anthropogenic impacts. Apparently, eutrophication, sedimentation and elevated seawater temperature can all, depending on the reef area, have an effect on bleaching-related tissue damage in corals, either directly, or indirectly through outflow of nutrient/sediment-rich water of elevated temperature from lagoons.
Colony size was not measured in this study, and this factor may be related to degree of bleaching (Bena and van Woesik 2004, Shenkar et al. 2005). In 1996, Meesters et al. (2001) compared the size-frequency distributions of 13 coral species (including 7 of the species studied here) between two polluted sites (including Avila beach) and two pristine sites (just up-current of Princess beach), but found no significant differences in colony size between the pristine and impacted areas. Although the size-distribution of all sites has not been compared, the study of Meesters et al. (2001) suggests that there is no general pattern in smaller or larger colonies at the impacted sites, and differences in colony size could not have played the main role in the degree of bleaching and the related tissue mortality.
In conclusion, degree of coral bleaching showed little relationship with various types of anthropogenic impacts on coral reefs, whereas bleaching-related tissue mortality was related to anthropogenic impacts, especially to eutrophication by sewage.
Acknowledgments
I thank G. A. M. T. Wiltjer, L. P. J. J. Pors and L. A. M. Aerts for logistic support in the field, and the latter two persons also for providing the seawater temperature data. I also thank A. O. Debrot for providing information on the pollution of Piscadera Bay. This is Centre for Wetland publication # 409.
Resumen
Los impactos antropogénicos crónicos pueden tener efectos negativos en la salud y en las cantidades de energía necesarias para la regeneración de lesiones en los corales. Mi hipótesis fue que durante los casos de blanqueamiento masivo, el grado en que los corales muestren mortalidades de tejido relacionadas con el blanqueamiento, será mayor en áreas sujetas a impactos antropogénicos crónicos que en áreas relativamente prístinas. Estimé los grados de blanqueamiento y mortalidad tisular en ocho especies de coral abundantes en Curaçao, durante el comienzo de un de blanqueamiento masivo en el Caribe en 1995 y tres meses después. El estudio se realizó en cuatro sitios control no contaminados, dos sitios contaminados (sedimentación, aguas residuales), y cuatro sitios en la boca de lagunas con aguas tibias, ricas en nutrientes y turbias. En general, no se encontró ningún patrónx de diferencias en el grado de blanqueamiento entre sitios. Sin embargo, el porcentaje de corales que mostraron mortalidad tisular relacionada con el blanqueamiento fue mayor en los sitios impactados que en los controles, tanto en el número total de corales como en corales con <50% de su superficie blanquedada. Los valores más altos y más significativos de mortalidad tisular se encontraron en un arrecife que experimentaba contaminación crónica por aguas residuales crudas. La información sugiere que las condiciones desfavorables causadas por la influencia antropogénica, como el incremento en la sedimentación, eutrofización y la temperatura del agua, tienen un efecto negativo adicional en la supervivencia del tejido de las colonias de coral, durante el blanqueamiento.
Palabras clave: corales pétreos, blanqueamiento, mortalidad tisular, impactos antropogénicos, Caribe.
References
Anonymous. 1987. Waterkwaliteitsonderzoek Spaanse Water. Environmental Service, Curaçao, Netherlands Antilles. 19 p. [ Links ]
Anonymous. 1991. Improvement of the environment and infrastructure of Spanish Waters and Caracasbay. Grabowsky and Poort International Consulting Engineers, Curaçao, Netherlands Antilles. 6 p. [ Links ]
Aronson, R. B., W. F Precht, I. G. Macintyre & T. J. Murdoch. 2000. Coral bleach-out in Belize. Nature 405: 36. [ Links ]
Baird, A. H. & P. A. Marshall. 2002. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 237: 133-141. [ Links ]
Bak, R. P. M. 1975. Ecological aspects of the distribution of reef corals in the Netherlands Antilles. Bijdr. Dierk. 45: 181-190. [ Links ]
Bak, R. P. M. 1978. Lethal and sublethal effects of dredging on reef corals. Mar. Pollut. Bull. 9: 14-116.
Bak, R. P. M. 1983. Neoplasia, regeneration and growth in the reef-building coral Acropora palmata. Mar. Biol. 77: 221-227. [ Links ]
Bak, R. P. M. & H. B. W. Elgershuizen. 1976. Patterns of oil-sediment rejection in corals. Mar. Biol. 37: 105-113. [ Links ]
Bena, C. & R. van Woesik 2004. The impact of two bleaching events on the survival of small coral colonies (Okinawa, Japan). Bull. Mar. Sci. 75: 115-1125.
Brown, B. E. 1997. Coral bleaching: causes and consequences. Coral Reefs 16: S129- S138. [ Links ]
Buth, L. & J. Ras. 1992. Inventory of the land-based sources of marine pollution, Netherlands Antilles. RZZ (Council for Sea Research and Sea Activities), Curaçao, Netherlands Antilles. 22 p. [ Links ]
CARICOMP. 1997. Studies on Caribbean coral bleaching, 1995-996. Proc. 8 th Int. Coral Reef Symp., Panama 1: 673-678.
Cervino, J., T. J. Goreau, I. Nagelkerken, G. W. Smith & R. Hayes. 2001. Yellow band and dark spot syndromes in Caribbean corals: distribution, rate of spread, cytology, and effects on abundance and division rate of zooxanthellae. Hydrobiol. 460: 53-663.
Debrot, A. O. & J. Sybesma. 2000. The Dutch Antilles, p. 595-614. In C. R. C. Sheppard (ed.). Seas at the Millennium: An Environmental Evaluation. Vol. I, Regional Chapters: Europe, the Americas and West Africa. Pergamon, Amsterdam, Holland. [ Links ]
Debrot, A. O., M. M. C. E. Kuenen & K. Dekker. 1998. Recent declines in the coral fauna of the Spaanse Water, Curaçao, Netherlands Antilles. Bull. Mar. Sci. 63: 571-580. [ Links ]
Djohani, R. H. & C. Klok. 1988. Een onderzoek naar de waterkwaliteit van enkele baaien van Curaçao op basis van biologische en abiotische parameters. Carmabi Foundation, Curaçao, Netherlands Antilles. M. Sc. thesis, Univ. Amsterdam, Amsterdam. 59 p. [ Links ]
Field, A. 2002. Discovering Statistics Using SPPP for Windows. Sage Publ., London. 496 p. [ Links ]
Fitt, W. K., B. E. Brown, M. E. Warner & R. P. Dunne. 2001. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 20: 51-665.
Gast, G. J., P. J. Jonkers, F. van Duyl F & R. P. M. Bak. 1999. Bacteria, flagellates and nutrients in island fringing coral reef waters: influence of the ocean, the reef and eutrophication. Bull. Mar. Sci. 65: 523-538. [ Links ]
Gleason, M. G. 1993. Effects of disturbance on coral communities: bleaching in Moorea, French Polynesia. Coral Reefs 12: 193-201. [ Links ]
Glynn, P. W 1984. Widespread coral mortality and the 1982-1983 El Niño warming event. Environ. Conserv. 10: 149-154. [ Links ]
Glynn, P. W 1993. Coral reef bleaching: ecological perspectives. Coral Reefs 12: 1-17. [ Links ]
Goreau, T. J. 1992. Bleaching and reef community change in Jamaica: 1951-1991. Amer. Zool. 32: 683-695. [ Links ]
Goreau, T. J. & R. L. Hayes. 1994. Coral bleaching and ocean "hot spots". Ambio 23: 176-180. [ Links ]
Goreau, T. J. & R. L. Hayes. 1996. A survey of coral reef bleaching in the South Central Pacific during 1994: Report to the International Coral Reef Initiative. Global Coral Reef Alliance, Chappaqua, New York, New York, USA. 201 p. [ Links ]
Goreau, T. J. & A. H. Macfarlane. 1990. Reduced growth rate of Montastrea annularis following the 1987- 1988 coral bleaching event. Coral Reefs 8: 211-215. [ Links ]
Goreau, T. J., R.L. Hayes & A. E. Strong. 1997. Tracking South Pacific coral reef bleaching by satellite and field observations. Proc. 8th Int Coral Reef Symp., Panama 2: 1491-11494.
Goreau, T. J., J. Cervino, M. Goreau, R. Hayes., M. Hayes, L. Richardson, G. Smith, K. DeMeyer, I. Nagelkerken, J. Garzon-Ferreira, D. Gil, E. C. Peters, G. Garrison, E. H. Williams, L. Bunkley-Williams, C. Quirolo, K. Patterson, J. W. Porter & K. Porter. 1998. Rapid spread of diseases in Caribbean coral reefs. Rev. Biol. Trop. 46 (Suppl. 5): 157-171. [ Links ]
Goreau, T. J., T. McClanahan, R. Hayes & A. Strong. 2000. Conservation of coral reefs after the 1998 global bleaching event. Conserv. Biol. 14: 5-15. [ Links ]
Harriott, V. J. 1985. Mortality rates of scleractinian corals before and during a mass bleaching event. Mar. Ecol. Prog. Ser. 21: 81-888 [ Links ].
Harvell, C. D., K. Kim, J. M. Burkholder, R. R. Colwell, P. R Epstein, D. J. Grimes, E. E. Hofmann, E. K. Lipp, A. D. M. E. Osterhaus, R. M. Overstreet, J. W. Porter, G. W. Smith & G. R. Vasta. 1999. Emerging marine diseases – climate links and anthropogenic factors. Science 285: 150. [ Links ]
Hoppe, W. 1981. Invloed van vervuiling op het koraalrif van Curacao met speciale referentie naar de KAE en COT. M. Sc. thesis, Univ. Nijmegen, Nijmegen. 98 p. [ Links ]
Hughes, T. P 1994. Catastrophes, phase-shifts, and large-scale degradation of a Caribbean coral-reef. Science 265: 1547-11551.
Jokiel, P. L. & S. L. Coles. 1974. Effects of heated effluent on hermatypic corals at Kahe Point, Oahu. Pac. Sci. 28: 1-18. [ Links ]
Kuenen, M. M. C. E. & A. O. Debrot. 1995. A quantitative study of the seagrass and algal meadows of the Spaanse Water, Curaçao, The Netherlands Antilles. Aquat. Bot. 51: 291-310. [ Links ]
Kuntz, N. M., D. I. Kline, S. A. Sandin & F. Rohwer. 2005. Pathologies and mortality rates caused by organic carbon and nutrient stressors in three Caribbean coral species. Mar. Ecol. Prog. Ser. 294: 173-180. [ Links ]
Mascarelli, P. E. & L. Bunkley-Williams. 1999. An experimental field evaluation of healing in damaged, unbleached and artificially bleached star coral, Montastrea annularis. Bull. Mar. Sci. 65: 577-586. [ Links ]
McClanahan, T. R., N. A. Muthiga & S. Mangi. 2001. Coral and algal changes after the 1998 coral bleaching: interaction with reef management and herbivores on Kenyan reefs. Coral Reefs 19: 380-391. [ Links ]
Meesters, E. H. & R. P. M. Bak. 1993. Effects of coral bleaching on tissue regeneration potential and colony survival. Mar. Ecol. Prog. Ser. 96: 189-198. [ Links ]
Meesters, E. H., A. Bos & G. J. Gast. 1992. Effects of sedimentation and lesion position on coral tissue regeneration. Proc. 7 th Int. Coral Reef Symp., Guam 2: 681-688. [ Links ]
Meesters, E. H., M. Noorderloos & R. P. M. Bak. 1994. Damage and regeneration: links to growth in the reef-building coral Montastrea annularis. Mar. Ecol. Prog. Ser. 112: 119-128. [ Links ]
Meesters, E. H., W. Pauchli & R. P. M. Bak. 1997a. Predicting regeneration of physical damage on a reef-building coral by regeneration capacity and lesion shape. Mar. Ecol. Prog. Ser. 146: 91-99. [ Links ]
Meesters, E. H., I. Wesseling & R. P. M. Bak. 1997b. Coral colony tissue damage in six species of reef-building corals: partial mortality in relation with depth and surface area. J. Sea Res. 37: 131-144. [ Links ]
Meesters, E. H., M. Hilterman, E. Kardinaal, M. Keetman, M. de Vries & R. P. M. Bak. 2001. Colony size-frequency distributions of scleractinians coral populations: spatial and interspecific variation. Mar. Ecol. Prog. Ser. 209: 73-54. [ Links ]
Nordemar, I., M. Nyström & R. Dizon. 2003. Effects of elevated seawater temperature and nitrate enrichment on the branching coral Porites cylindrica in the absence of particulate food. Mar. Biol. 142: 669-677. [ Links ]
Pastorok, R. A. & G. R. Bilyard. 1985. Effects of sewage pollution on coral-reef communities. Mar. Ecol. Prog. Ser. 21: 175-189. [ Links ]
Quinn, N. J. & B. L. Kojis. 1999. Subsurface seawater temperature variation and the recovery of corals from the 1993 coral bleaching event in waters off St. Thomas, US Virgin Islands. Bull. Mar. Sci. 65: 201-214. [ Links ]
Riegl, B. 2002. Effects of the 1996 and 1998 positive sea-surface temperature anomalies on corals, coral diseases and fish in the Arabian Gulf (Dubai, UAE). Mar. Biol. 140: 29-40. [ Links ]
Shenkar, N., M. Fine & Y. Loya. 2005. Size matters: bleaching dynamics of the coral Oculina patagonica. Mar. Ecol. Prog. Ser. 294: 181-188. [ Links ]
Shulman, M. J. & D. R. Robertson. 1996. Changes in the coral reefs of San Blas, Caribbean Panama: 1983 to 1990. Coral Reefs 15: 231-236. [ Links ]
Spencer Davies, P. 1991. Effect of daylight variations on the energy budgets of shallow-water corals. Mar. Biol. 108: 137-144. [ Links ]
Szmant, A. M. & N. J. Gassman. 1990. The effects of prolonged bleaching on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs 8: 217-224. [ Links ]
van Veghel, M. L. J. & R. P. M. Bak. 1994. Reproductive characteristics of the polymorphic Caribbean reef building coral Montastrea annularis. III. Reproduction in damaged and regenerating colonies. Mar. Ecol. Prog. Ser. 109: 229-233. [ Links ]
Williams, E. H. & L. Bunkley-Williams. 1990. The world-wide coral reef bleaching cycle and related sources of coral reef mortality. Atoll Res. Bull. 335: 1-71. [ Links ]
van Duyl, F. C. 1985. Atlas of the living reefs of Curaçao and Bonaire (Netherlands Antilles). Uitgaven Natuurwetenschappelijke Studiekring Suriname en de Nederlandse Antillen No. 117, Utrecht. 37 p. [ Links ]
van Duyl, F. C., G. J. Gast, W. Steinhoff, S. Kloff, M. J. W. Veldhuis & R. P. M. Bak. 2002. Factors influencing the short-term variation in phytoplankton composition and biomass in coral reef waters. Coral Reefs 21:293-306. [ Links ]