Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.54 n.3 San José Sep. 2006
Fitting together: copulatory linking in some Neotropical Chrysomeloidea
R. Wills Flowers1 & William G. Eberhard2
1 Center for Biological Control, Florida A&M University, Tallahassee, FL 32307 USA; rflowers@mail.istal.com
2 Escuela de Biología, Universidad de Costa Rica, 2060 San José, Costa Rica; archisepsis@biologia.ucr.ac.cr
Received 21-VII-2004. Corrected 12-VIII-2005. Accepted 29-III-2006.
Abstract: Copulatory linking of male and female genitalic structures in 11 Neotropical species of Chrysomelidae and one species of Megalopodidae was studied by freezing and then dissecting pairs of beetles in copula. In Megalopus armatus (Megalopodidae) the male has a long endophallus with complex membranous protuberances and a terminal flagellum that probably reaches the spermatheca. In the subfamily Eumolpinae the females have telescoping ovipositors through which the male endophalli pass, reaching to or near the mouth of the spermathecal duct. A long thin flagellum is probably inserted into the spermathecal duct. The male endophalli are braced inside the female using various structures, including two pairs of lateral appendages and apical appendages (both lateral pairs sclerotized in Colaspis sanjoseana and only the basal pair in Brachypnoea irazuensis), a pair of membranous swellings (in Metaxyonycha amasia), and apical microspicules on the endophallus (in Xanthonia). In the subfamily Galerucinae, males of Metrioidea and Diabrotica (tribe Galerucini) have relatively short endophalli ornamented with sclerotized hooks, spines and needles. In Metrioidea elongata the long needle-like endophallic spines of the male were erected inside the female and penetrated the wall of her bursa. In the tribe Alticini, the male endophallus is very short and does not enter the female in two species, Alagoasa gemmata and Walterianella sp. Instead, the apical parts of the female bursae were everted, and were clamped by sclerites of the male median lobe. The male genitalia of Plectrotetra hirsuta reached deep into the female, to the median oviduct, far beyond the mouth of the spermathecal duct. Both rigid and membranous structures meshed in complex ways. When these observations are combined with published descriptions of mating in other Chrysomelidae, the great diversity of copulatory morphology in these families comes into sharper focus. Rev. Biol. Trop. 54 (3): 829-842. Epub 2006 Sept. 29.
Key words: genitalia, sexual selection, copulation, Chrysomelidae, Megalopodidae.
One of the most sweeping trends in animal evolution is for male genitalia to diverge especially rapidly, so that they differ even in closely related species (Eberhard 1985, 1996, Shapiro and Porter 1989). This trend has made them especially useful characters for taxonomists in distinguishing closely related species, and drawings of genitalia are commonplace in taxonomic papers on many different groups of animals with internal insemination. This is often true for chrysomelid beetles (reviewed in Flowers 1999). But the genitalic descriptions of male chrysomelids (and many other beetles) are generally limited to the hollow chitinized tube, the median lobe. This tube houses the folded endophallus (internal sac), which often bears one or more sclerites which are more or less visible through the wall of the median lobe. Their form, as well as that of the endophallus itself, has been studied by pulling the sac free or inflating it artificially (Mann and Crowson 1996, Flowers 1999). In some cases artificial inflation of the endophallus is not sufficient to determine the shape (or shapes) of the endophallus during copulation, (Eberhard 1996, but see Flowers 1999 on Colaspis spp.). During copulation, the endophallus is inflated inside the female, and it has a complex form in the few species in which it has been fixed in this position (Eberhard and Kariko 1996, Konstantinov 1998, Flowers 1999, Crudgington and Siva-Jothy 2000). This report more than doubles the previous sample of naturally inflated endophalli describing 11 species in 10 genera in two subfamilies of Chrysomelidae, and one species in the closely related family Megalopodidae.
Materials and methods
Copulating pairs found opportunistically in the field at undetermined stages of copulation were frozen with ethyl chloride spray and immediately preserved in 70 % ethyl alcohol at ambient temperatures. After at least 24 hrs in alcohol, the united genitalia were dissected from the beetles and were drawn or photographed. Some genitalia were then placed for a short time in a hot solution of 8 % KOH, followed by further dissection to reveal positions of the male and female structures. Clearing and dissecting were repeated if necessary until all genitalic structures were revealed. Specimens were prepared for the scanning electron microscope using standard techniques. Terminology in Eumolpinae follows Flowers (1999), and in Alticini it follows Duckett (1999) and Konstantinov (1998). We limit ourselves to descriptive terms when we are unsure of homologies (e.g. "medial process" of Plectrotetra hirsuta Jacoby). The classification of Alticini follows Bechyné (1997). Orientations are described in terms of the female in copulating pairs.
Results
Megalopodidae
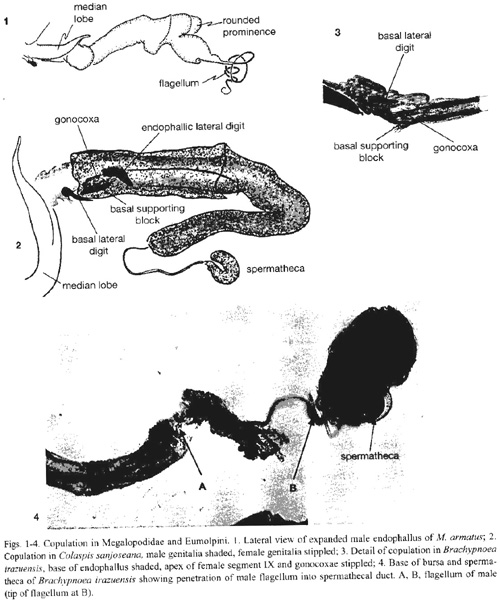
Megalopus armatus Lacordiare. Several pairs fell apart immediately when sprayed, with the endophallus withdrawn inside the median lobe, suggesting that during some stages of copulation the endophallus is probably not expanded. In one pair the entire endophallus was inflated and emerged from the female the moment they were sprayed. The endophallus was more or less cylindrical, and about three times the length of the median lobe (Fig. 1). It lacked sclerites, but nevertheless had a complex shape, including a pair of rounded distal prominences. The tip consisted of a long, thin sclerotized tube (the flagellum) that was nearly as long as the inflated endophallus, and which was of uniform diameter until it tapered at its distal end (Fig. 1). In the one pair that remained coupled when frozen, the endophallus was only partially inflated, and it had pulled partially free from the female, thus precluding determination of its alignment within her complex genital tract. Nevertheless, the length of the inflated endophallus was approximately that of the females vagina, and females spermatheca duct was a sclerotized, coiled tube whose internal diameter was approximately the external diameter of the males flagellum. The complementary sizes of male and female suggest that the flagellum passes through the long spermathecal duct of the female.
Chrysomelidae
Eumolpinae: Eumolpini
Colaspis sanjoseana Bechyné. Three pairs were examined. The male endophallus made an 180o turn near the base of the ovipositor, and filled the female vagina up to the entrance of the spermathecal duct (Fig. 2). The apex of the median lobe did not penetrate the female; the basal lateral digits lay folded just inside the apex of the females abdomen. The endophallic lateral digits were folded over the female gonocoxae, which in turn lay along lateral furrows of the males basal supporting block (Fig. 2). The male filament did not enter the spermathecal duct.
Brachypnoea irazuensis (Jacoby). Seven pairs were studied. The males basal lateral digits are sclerotized, but the endophallic lateral digits are membranous lobes above a thinly sclerotized basal supporting block. As in Colaspis, the basal lateral digits lay just inside the external opening of the female in copula. The female gonocoxae lay along either side of the endophallus in grooves formed by the upper lateral edges of the basal supporting block and the lower side of the endophallic lateral digit lobes (Fig. 3). The endophallus is inserted through the female ovipositor duct and vagina, and the apical sclerite lay at the mouth of the long, thin, and convoluted spermathecal duct. In one pair, the males flagellum was inserted a short distance into the very long, coiled spermathecal duct (Fig. 4).
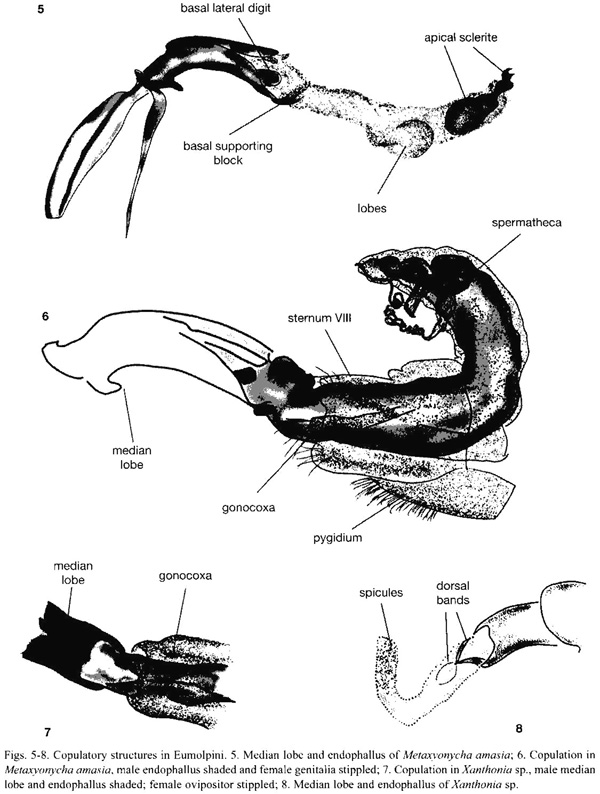
Metaxyonycha amasia Marshall. Nine pairs were collected feeding on leaves of Croton (Euphorbiaceae). The male basal lateral digits are sclerotized, but the endophallic lateral digits are membranous lobes. Both structures are close together and barely extend beyond the apex of the median lobe. The endophallus was inflated, and filled the females vagina, and the large apical sclerite was positioned against the opening of the spermathecal duct. At about two thirds of the length of the endophallus are a pair of lateral swellings and a smaller ventral swelling ("lobes" in Fig. 5). The female gonocoxae lay close to their normal positions and orientations at the tip of the abdomen. As in B. irazuensis, the gonocoxae lay on each side of the endophallus between the lobular endophallic lateral digits and the top of the basal supporting block (Fig. 6).
Chalcophana. One pair each of Chalcophana mutabilis Harold and Ch. semirufa Jacoby were frozen, but both separated during preservation. In both cases the males flagellum protruded beyond the apical sclerite and distal end of the inflated endophallus. Ch. mutablis separated completely, and the males flagellum protruded by almost the length of the endophallus. In the Ch. semirufa the females ovipositor tube was extended; the male apical sclerite was at the base of the ovipositor tube, and the flagellum protruded slightly.
Eumolpinae: Adoxini
Xanthonia sp. One pair preserved in copula showed a relatively simple copulatory system. The female had a relatively short, telescoping ovipositor and the male a membranous endophallus with two pairs of dorsal sclerotized flexible bands which probably function in supporting the everted endophallus (Fig. 7, 8). The female gonocoxae were pushed-or retracted-into the ovipositor (Fig. 7) and her dorsal hemisternites were twisted. Although lacking sclerites, the male endophallus had microspicules on the apical third (Fig. 8), and it was as difficult to pull from the female as the more elaborately armed endophalli of the preceding species.
Galerucinae: Alticini: Oedionychina
Alagoasa gemmata (Jacoby). The vaginal palpi are long, narrow, and curved, and were in transverse positions, curved around the apex of the males median lobe (Fig. 9). The walls of the genital orifice of the female are heavily muscled. In the copulating pair studied, the vagina had everted a short distance and entered the apex of the male median lobe. Sclerites at the tip of the median lobe were clamped around the vaginal membrane (Fig. 10). When separated, the females vaginal duct clearly showed longitudinal folds where it had been clamped in the males median lobe (arrow in Fig. 11).
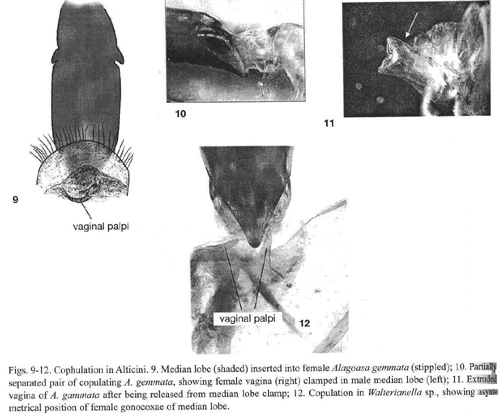
Walterianella sp. Two pairs belonging to different species were studied. In both the vaginal palpi were thin and are positioned asymmetrically on either side of the apex of the males median lobe (Fig. 12). In one pair, a fold of the female vagina was clamped in the males median lobe in the same manner as Alagoasa gemmata.
Galerucinae: Alticini: AlticinaAcanthonycha sp. The vaginal palpi were large and rectangular, bearing setae on their posterior median corners. The orifice of the male median lobe has two lateral sclerites and a small dorsal hook. The pair examined separated after being preserved, but the females vagina remained extruded and displayed longitudinal folds that matched the lateral sclerites of the males median lobe (as in Alagoasa gemmata, Fig. 11).
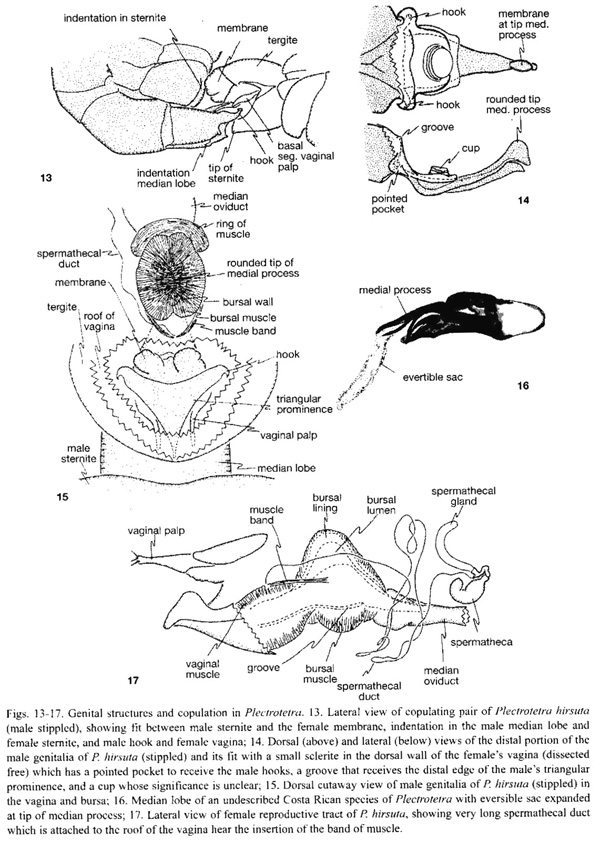
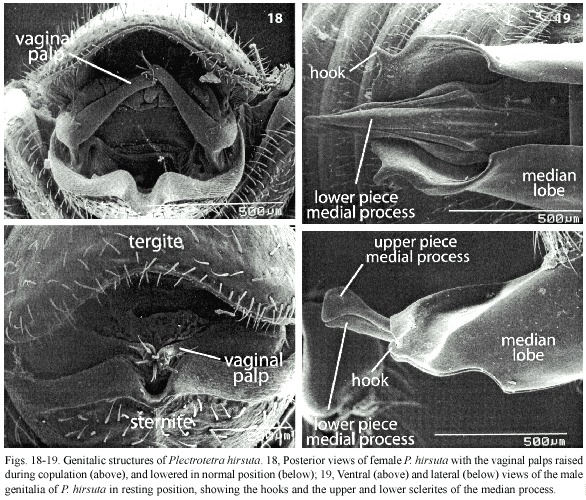
Plectrotetra hirsuta Jacoby. Five pairs were dissected. The central area of the males sternite five was concave. During copulation the soft tissue which projected posteriorly in some females was aligned with (but did not always reach) this concave surface (Fig. 13). The distal margin of his median lobe was deeply indented in the central portion, and during copulation the tip of the females sternite projected into this central indentation (Fig. 13). The tip of the median lobe bore a pair of dorsally projecting hooks (Fig. 13, 14, 19). Each of these hooks was inserted into a pocket in the wall of the vagina (Fig. 13, 14), near the articulation between the basal and distal segments of the vaginal palpi. These pockets were at the antero-lateral corners of a small sclerite on the dorsal wall of the vagina (Fig. 14). This sclerite also had a ventral projecting central ridge which fit against the distal margin of the median lobe (Fig. 14), and a shallow, rounded cup-like area. This cup did not fit against any male structure, nor was it connected directly with the mouth of the spermathecal duct, although it was very close to it.
At the base of the highly sclerotized, complexly sculptured medial process there was a triangular prominence with its tip directed basally (Fig. 15). The slightly curved distal segment of each vaginal palp lay along the somewhat indented lateral margin of the triangular prominence of the medial process in all dissected pairs (Fig. 15), but in one pair that separated after being frozen the distal segments of the palpi were folded dorsally and anteriorly (Fig. 18).
The distal portion of the male medial process was composed of two pieces, and there were two membranous areas associated with it. One membranous area was just dorsal to the base of the distal portion, and it was possible to move the medial process slightly with respect to the median lobe in this area. In one pair this membrane was slightly inflated ("membrane" in Fig. 15), but in all others was not inflated at all. The mouth of the long, thin and complexly folded spermathecal duct opened on the roof of the vagina near the area where this membranous area of the male was positioned during copulation (Fig. 15), and a small band of muscles ran from this area to the bursa ("muscle band" in Fig. 15, 17). There was a second, smaller membranous area on the dorsal surface of the tip of the medial process which was not inflated in any of the copulating pairs. It could be extended, however, into a long narrow sac in a different, undescribed Plectrotetra (Fig. 16).During copulation the two pieces of the medial process lay in different cavities in the female. The tip of the larger, upper rounded piece was lodged firmly in the blind end of the very heavily muscled bursal cavity, dorsal to the junction between the vagina and the oviduct (site labelled "bursal lumen" in Fig. 17). The smaller, thinner lower piece fit into the deep groove ("groove" in Fig. 17) on the ventral surface of the vaginal wall. The muscles surrounding the bursa were so thick that it appeared to be surrounded by a brown ring or cover.
Galerucinae: Galerucini
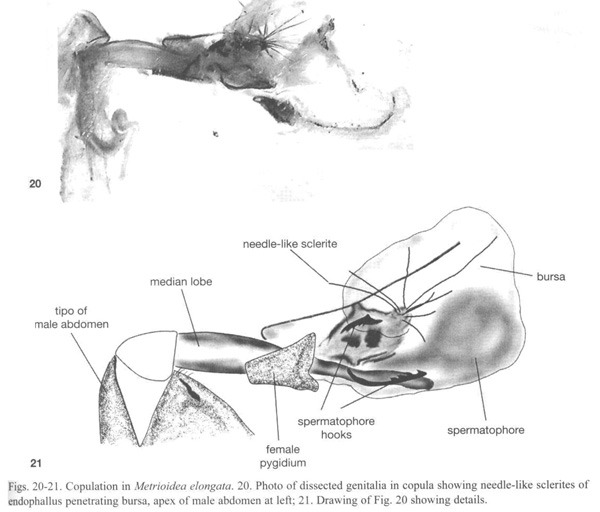
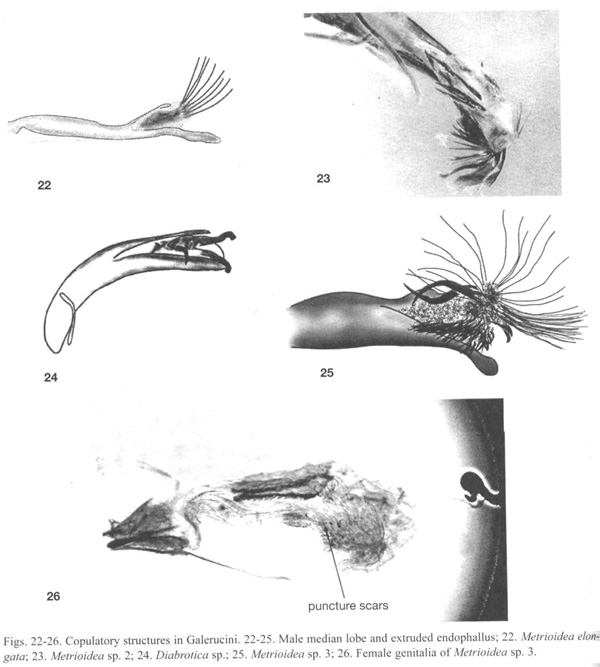
Metrioidea elongatus (Jacoby) (Luperina), One pair was frozen in copula. The male median lobe is long and narrow and was inserted deeply into the female bursa (Fig. 20). The group of long needle-like sclerites at the tip of the endophallus (Fig. 21, 22) opened into a fan-like arrangement inside the female bursa. Several sclerites pierced the wall of the bursa. The male was apparently in the act of placing a spermatophore inside the female bursa (Fig. 21). The female genitalia was also armed. Above and below the median lobe were two pairs of female sclerites with large saw-teeth directed internally (Fig. 21). We theorize that these are for opening the spermatophore as in the signum of some Lepidoptera (Drummond 1984). The male and female genitalia of non-copulating individuals of two other unidentified Metrioidea (sp. 2, Fig. 23 and sp. 3, Fig. 25, 26), and the male genitalia of an unidentified Diabrotica species (Fig. 24) also showed spines on the endophallus and scars on the female bursal wall.
Discussion
The species of this study are taxonomically dispersed, and the scope is very small with respect to the family Chrysomelidae. Our sample sizes were also small, and the evident mobility of male and female structures observed in other studies of copulation in chrysomelids (Eberhard and Karido 1996, Tallamy et al. 2002) suggests that we may have only documented some of possibly multiple genitalic positions. Nevertheless, there is a clear overall message: the morphological mesh between male and female genitalia during copulation in chrysomelids is very diverse.
Megalopodidae
Data on M. armatus are of particular interest because Megalopodidae (formerly included as a subfamily in Chrysomelidae) is a probable sister group of Chrysomelidae (Reid 2000). Our observations suggest that inserting and maintaining the tip of the median lobe in the vagina, inserting the flagellum into the spermathecal duct, and a lack of endophallic sclerites may be plesiomorphic in chrysomelids. We suggest that even in those groups (all eumolpines) in which we found the median outside the female in copulating pairs, it is probably inserted at some early stage of copulation; it seems likely that insertion is needed to provide purchase to drive the soft endophallus into the female.
Chrysomelidae
Eumolpinae. Common themes from Eumolpinae are an elongate endophallus (see also Mann and Crowson 1996), a long thin flagellum which may be inserted into the spermathecal duct (a trait shared with Cassidinae), and a long endophallus which is extended the length of the females elongate vagina. At least four different endophallus traits may serve to anchor the male within the female. In Colaspis spp., the endophallic lateral digits of the male engaged the female gonocoxae, and in C. pseudofavosa Riley the gonocoxae were apparently displaced inward during copulation Flowers (1999). In Metaxyonycha, where the endophallic lateral digits do not, or scarcely, enter the female, three inflated areas near the apical sclerite (Fig. 5) could serve the function of keeping the endophallus in position inside the female (similar inflated areas occur in M. armatus). Finally, in Xanthonia, the spiculate apex of the male endophalus (Fig. 8) could serve to anchor the endophallus within the female. Another possible, non-exclusive function for all of these traits is to provide the female with stimulation. The apical sclerite in Eumolpinae, located at the tip of the everted endophallus, is generally a small twisted sclerite whose exact shape varies between and within general (Flowers 1999). Possible functions for this sclerite are to guide and steady the flagellum as it is inserted into the spermathecal duct, and to deliver stimuli to the female.
Galerucinae: Alticini. Our miniscule sample of species in this diverse tribe (more than 200 genera are described from the Neotropics alone, Scherer 1988) shows striking variation.
The mesh between rigid male and female structures was more elaborate in Plectrotetra hirsuta than in any other species. Some of the male structures in P. hirsuta which fit against the female were relatively external (concave sternite VII, indentation at tip of median lobe). Farther inside the female, hooks on the distal lateral margins of the males median lobe engaged weakly sclerotized pockets in the wall of the vagina, the distal edge of a triangular prominence of the male fit into a groove in a small vaginal sclerite, the females vaginal palpi fit into grooves along the sides of this triangular prominence, the distal tip of the dorsal piece of the males medial process lodged tightly in the rounded semi-rigid blind end of the bursa, and the ventral piece of his medial process fit in a groove in the wall of the vagina.
The similar sizes of the oviduct and the membranous sac which emerged from the tip of the dorsal piece of the medial process (Fig. 16), and the close (though not perfect) juxtaposition of the males medial process with the junction of the oviduct with the bursa suggest that this male sac is probably inflated into the oviduct. The thick collar of muscle at this junction ("ring of muscle" in Fig. 15) hints at a female ability to exclude the male sac from her oviduct.
Several functional aspects of copulation in P. hirsuta are still not clear. For instance, why should the male introduce his genitalia substantially deeper into the female than the entrance to her sperm storage organ, and why should he have a sac which extends up her oviduct? Why should the female have such a thick layer of muscle surrounding the bursa, and a thickened bursal wall (Fig. 17) with a separate lumen that is walled off from the vagina? What is the function of the band of muscle ending near the mouth of the spermathecal duct ("muscle band" in Figs. 15, 17)?
Unlike the Galerucini or Eumolpinae (and most other members of the Animal Kingdom), in three alticine genera of our study, Acanthonycha, Alagoasa and Walterianella, it was the female which apparently "entered" the male, extruding her vaginal duct a short way up the opening at the apex of the median lobe (Fig. 10,12). Konstantinov (1998) found, as we did in other alticines, that in Aphthona formosana (Chen) the tip of the median lobe was in close contact with the vaginal palpi. He regarded this as an "internal courtship device". In the three species in this study, such "courtship" could be especially important, as the female must be induced to extend her vaginal duct into the male before the male can use his movable sclerites to clamp a fold of the vagina. Clamping was achieved both by the lateral sclerites hinging inward, and by the dorsal sclerites moving downward. In the pairs that came apart in
Acanthonycha sp. and Walterianella sp., the male sclerites were in the "open" position, suggesting that the pairs were in the process of separating at the moment of preservation. Details of what happens during copulation once clamping is achieved remain to be discovered. It is also possible that this "female penetration" of the male could be due to a reaction of the female to being frozen.
Galerucinae: Galerucini. On discovering the complicated and bizarre morphology of copulation in Metrioidea elongata, we dissected males of several other Metrioidea and other Galerucini. Similar endophallic armament was found in two other species of Metrioidea (Fig. 23, 25), but were not present in a third species. A species Luperina near Metrioidea had a series large hooks that folded out and over as the endophallus was everted, while the species of Diabrotica (Fig. 24) deployed three hooks from its endophallus, as seems to also occur during copulation in Diabrotica undecimpunctata howardi (D. Tallamy, pers. comm.). Sharp spines similar to those we observed in Metrioidea are illustrated in the figures of unextruded endophalli of Old World genera Aulacophora, Monolepta, Diorhabda, and Mimastra of Galerucini (Mann and Crowson 1996), and are pictured for Old World galerucine genera closely related to Metrioidea (Wagner 2003). Crudgington and Siva-Jothy (2000) described endophallic spines in a very distantly related chrysomelid, Callisobruchus maculatus (Bruchinae). These spines also penetrated the bursal wall, where they left characteristic puncture scars. We found similar scars in the bursae of the female Metrioidea sp. 3 (Fig. 26) as well as in the female of Metrioidea sp. 2. During copulation female Callisobruchus kicked at the male in apparent attempts to dislodge him. Similar kicking occurs in Colaspis floridana Blake and an undescribed Costa Rican species of Hylax (both Eumolpini) (R.W. Flowers, unpublished).
What could be the adaptive value of such spines which produce physical damage to the female? In the blowfly Lucilia sericata where the male has a an aedeagal structures capable of cutting into the female, the male accessory gland contains chemicals that reduce subsequent female mating when injected through these wounds into her haemolymph (Merritt 1989). In Callisobruchus, penetration of the female bursa shortens the life and reduces the fitness of the female, but it is possible that it confers advantages on the male by increasing the likelihood that his sperm will be used to fertilize the females eggs (Crudgington and Siva-Jothy 2000). Another, non-exclusive possibility is that it anchors the males genitalia in the female, as suggested by the behavior of two copulating Metrioidea elongata when they were collected. When they fell from oak foliage and fell onto a beating sheet, they flipped and tumbled energetically, but the male did not lose his grip on the female. Escape reactions in many beetles include rapid dropping and tumbling. In some galerucines with endophallic hooks, such as Diabrotica undecimpunctata howardi copulation lasts 3-4 hours (Krysan 1999). Some species may thus have a high probability of being interrupted by predators. Stimulation of the female is a further non-exclusive function.
General summary
When our observations are combined with previous studies, even further diversity is revealed. The cassidine Chelymorpha alternans Bohman (Rodriguez et al. 2004) is similar to Megalopus armatus in having a long, thin tubular male structure on the endophallus that penetrates a long spermathecal duct, but it may differ in also depositing a soft "spermatophore" in the bursa. The alticine Macrohaltica jamaicensis (Fabricius) (Eberhard and Kariko 1996) has a relatively simple endophallus, which serves as a mold for the spermatophore, the details of whose form differ between two species of this genus (W. Eberhard, unpublished); one endophallic sclerite serves to brace the endophallus against the tip of the median lobe inside the female when the endophallus is inflated, while a pair of sclerites may help give the spermatophore mold its form, and thus produce a large-headed spermatophore which may be more difficult for the female to discard. In another alticine,
Aphthona formosana Chen, in contrast, the endophallus penetrates only part way into the vagina, and both sclerotized structures on the endophallus and species-specific ridges and depressions on the median lobe rest against the vaginal palpi and may stimulate them (Konstantinov 1998). In Phenrica pallidicornis (Jacoby) the endophallus is large, membranous and inflated, although its function during copulation is not known (R.W. Flowers, unpublished). By contrast, in the Oedionychina and Acanthonycha described above, the endophallus is very short and when artificially inflated barely extends beyond the end of the median lobe; a very short endophallus is also known in some other Oedionychina (Duckett 1999, Duckett and Dazo 2004)
The inflated endophallus of the eumolpine Colaspis pseudofavosa is long and thin, and has a pair of digits (the endophallic lateral digits) that apparently serve still another function, bracing open the tip of the females ovipositor tube deep within her body (Flowers 1999). The endophallus in this species penetrates at least part way into the females bursa, but is much too thick to enter her thin spermathecal duct.
In summary, different male genitalic structures in different species of Chrysomeloidea are thought to hook or slide into slots in the walls of the female vagina, to brace the males inflated endophallus, against his median lobe, to form a mold for a spermatophore, to brace open the tip of the female ovipositor tube to allow other portions of the endophallus to penetrate deeper, to stimulate the females vaginal palpi, to form grooves in which her vaginal palpi can lie, to penetrate very long and complexly coiled spermathecal ducts, and to pierce the walls of the females bursa. It is clear, even from the present tiny sample of species, that much diversity in both form and function awaits discovery in chrysomelid genitalia, and "fitting together" in Chrysomelidae involves many unexpected ins and outs.
Acknowledgments
We thank the Escuela de Biología, Universidad de Costa Rica, the Área de Conservación Guanacaste, and the Instituto Nacional de Biodiversidad for providing facilities during this study. This study was funded in part by the Fulbright Program, U.S. Embassy in Costa Rica, the Costa Rica National Biodiversity Inventory, the Biodiversity Resources Development Project, GEF/World Bank to the first author; by a grant (FLAX02-03) from CSREES, USDA to Florida A&M University; and by the Smithsonian Tropical Research Institute.
Resumen
Se describen los coajustes mecánicos entre las estructuras genitales de machos y hembras de 11 especies neotropicales de Chrysomelidae y una especie de Megalopodidae. Las parejas fueron congeladas durante la cópula y después disectadas. En Megalopus armatus (Megalopodidae) el macho tiene un endofalo largo, con protuberancias membranosas complejas, y un flagelo terminal que probablemente penetra el largo del ducto de la espermateca hasta el lumen de la espermateca. En la subfamilia Eumolpinae, las hembras tienen ovipositores telescópicos, a través de los cuales los endofali de los machos pasan para llegar hasta o cerca a la boca del ducto de la espermateca. Es probable que el largo y delgado flagelo se inserta en el ducto de la espermateca. El endofalo del macho se apoya dentro de la hembra por varias estructuras, las cuales incluyen dos pares de apéndices laterales y apéndices apicales (ambos pares laterales son esclerotizados en Colaspis sanjoseana, únicamente el par basal en Brachypnoea irazuensis), un par de hinchamientos membranosos (en Metaxyonycha amasia), y espículas apicales sobre el endofalo (en Xanthonia). En la subfamilia Galerucinae, los machos de Metrioidea y Diabrotica (tribu Galerucini) tienen endofalos relativamente cortos, pero adornados con varios ganchos, espinas y agujas. En Metrioidea elongata las largas espinas endofálicas se abrieron dentro de la hembra, y penetraron las paredes de su bursa. En la tribu Alticini, el endofalo del macho de dos especies, Alagoasa gemmata y Walterianella sp., es muy corto y no entró al cuerpo de la hembra. En su lugar, la parte apical de la bursa de la hembra fue evertida, y fue agarrada por escleritos del lóbulo mediano del macho. Los genitales de Plectrotetra hirsuta penetraron profundamente en la hembra, llegando probablemente hasta el oviducto mediano, más profundo que la boca del ducto de la espermateca. Estructuras de ambos sexos, tanto membranosas como rígidas, se entrelazaron en forma compleja. Al combinar estas observaciones con otras descripciones publicadas previamente, se revela una gran diversidad en la morfología copulatoria de Chrysomeloidea.
References
Bechyné, J. 1997. Evaluación de los datos sobre los Phytophaga dañinos en Venezuela (Coleoptera). Parte I. Bol. Entomol. Venez. (Serie Monografías No. 1): 1-278+vi p. [ Links ]
Crudgington, H.S. & M.T. Siva-Jothy. 2000. Genital damage, kicking and early death. Nature 407: 855-856. [ Links ]
Drummond, B.A. 1984. Multiple mating and sperm competition in the Lepidoptera, p. 291-370. In R.L. Smith (ed.). Sperm competition and the evolution of animal mating systems. Academic, New York, USA. [ Links ]
Duckett, C.N. 1999. A preliminary cladistic analysis of the subtribe Disonychina with special emphasis on the series Paralactica (Chrysomelidae: Galerucinae: Alticini), p. 71-93. In M.L. Cox (ed.). Advances in Chrysomelidae Biology 1. Backhuys, Leiden, The Netherlands. [ Links ]
Duckett, C.N. & J.D. Dazo. 2004. A new species of flea beetle in the genus Alagoasa Bechyné (Coleoptera: Chrysomelidae: Galerucinae). Coleopterists Bull. 56: 84-96. [ Links ]
Eberhard, W.G. 1985. Sexual selection and animal genitalia. Harvard University, Cambridge, Massachusetts, USA. 244+x p. [ Links ]
Eberhard, W.G. 1996. Female control: sexual selection by cryptic female choice. Princeton University, Princeton, USA. 501+xiv p. [ Links ]
Eberhard, W.G. & S. Kariko.1996. Copulation behavior inside and outside the beetle Macrohaltica jamaicensis. (Coleoptera: Chrysomelidae). J. Ethol. 14: 59-72. [ Links ]
Flowers, R.W. 1999. Internal structure and phylogenetic importance of male genitalia in Eumolpinae, p. 71-93. In M.L. Cox (ed.). Advances in Chrysomelid Biology 1. Backhuys, Leiden, The Netherlands. [ Links ]
Konstantinov, A.S. 1998. On the structure and function of the female genitalia in flea beetles (Coleoptera: Chrysomelidae: Alticinae). Proc. Ent. Soc. Wash. 100: 353-360. [ Links ]
Krysan, J.L. 1999. Selected topics in the biology of Diabrotica, p. 71-93. In M.L. Cox (ed.). Advances in Chrysomelidae Biology 1. Backhuys, Leiden, The Netherlands. [ Links ]
Lew, A.C. & H.J. Ball. 1979. The mating behavior of the western corn rootworm Diabrotica virgifera (Coleoptera: Chrysomelidae). Ann. Entomol. Soc. Am. 72: 391-393. [ Links ]
Mann, J.S. & R.A. Crowson. 1996. Internal sac structure and phylogeny of Chrysomelidae, p. 291-316. In P.H.A. Jolivet & M.L. Cox (eds.). Chrysomelid biology, Vol. 1: The classification, phylogeny and genetics. SBP Academic, Amsterdam, The Netherlands. [ Links ]
Merrett, D.J. 1989. The morphology of the phallosome and accessory gland material transfer during copulation in the blowfly, Lucilia cuprina (Insecta, Diptera). Zoomorphology 108: 359-366. [ Links ]
Reid, C.A.M. 2000. Spilopyrinae Chapuis: a new subfamily in the Chrysomeelidae and its systematic placement (Coleoptera). Inver. Tax. 14: 837-862. [ Links ]
Rodriguez, V., D. Windson & W.G. Eberhard. 2004. Experimental demonstrations of a sexually selected advantage for longer male genitalia in the tortoise beetle, Chelymorpha alternans, p. 739-748. In P. Jolivet, J.A. Santiago-Blay & M. Schmitt (eds.). New developments in the biology of Chrysomelidae. SBP Academic, The Hague, The Netherlands. [ Links ]
Scherer, G. 1988. The origins of the Alticinae, p. 115-130. In P. Jolivet, E. Petitpierre & T.H. Hsiao (eds.) Biology of Chrysomelidae. Kluwer, The Netherlands. [ Links ]
Shapiro, A.M. & A.H. Porter. 1989. The lock-and-key hypothesis: evolutionary and biosystematic interpretation of insect genitalia. Ann. Rev. Ent. 34: 231-245. [ Links ]
Tallamy, D.W., B.E. Powell & J.A. McCafferty. 2002. Male traits under cryptic female choice in the spotted cucumber beetle (Coleoptera: Chrysomelidae). Behav. Ecol. 13: 511-518. [ Links ]
Wagner, T. 2003. Present status of a taxonomic revision of Aftrtropical Monolepta and related groups (Galerucinae), p. 133-146. In D.G. Furth (ed.) Special topics in leaf beetle biology. Proc. 5th Int. Sym. On the Chrysomelidae. Pensoft, Sofia, Moscow. [ Links ]