Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.53 suppl.1 San José May. 2005
Heavy metals in sediments,mussels and oysters from Trinidad and Venezuela
L.Rojas de Astudillo1 ,I.Chang Yen2 &I.Bekele3
1 Departament of Chemistry,Núcleo de Sucre,Universidad de Oriente,Cumaná,Estado Sucre,Venezuela; lrojas40@yahoo.com.
2 Department of Chemistry,University of the West Indies,St.Augustine,Trinidad.
3 Department of Food Production,University of the West Indies,St.Augustine,Trinidad.
Received 15-I-2004. Corrected 15-I-2005. Accepted 29-III-2005.
Abstract: The Gulf of Paria is bordered by both Trinidad and Venezuela,from which various metallic pollutants and other contaminants can originate.The Gulf is still a significant source of fish,crabs and shellfish for human consumption to both countries,where concerns over the quality of this marine environment have been long expressed but never properly addressed.In addition,the circulatory current patterns in the Gulf ensure that contaminants originating from either country are likely to affect both countries eventually.Heavy metals were determined in oysters (Crassostrea rhizophorae and C.virginica ),green mussels (Perna viridis )and sediments from the Gulf of Paria.Samples were obtained at four sites in Trinidad and three sites in Venezuela in the Gulf of Paria,in addition to comparative samples collected from three sites on the north coast of Venezuela.Edible tissues of twelve shellfish from each location were blended and aliquots digested with concentrated nitric acid, for extraction of cadmium,chromium,copper,lead,nickel and zinc.The solutions were analysed by flame atomic absorption spectroscopy.Mercury was extracted with a mixture of nitric,hydrochloric and sulphuric acids and determined by cold vapour atomic absorption.Sediments were oven-dried at 60°C, before being similarly extracted.Results showed that mercury in sediments at all sites in Trinidad and Venezuela exceeded NOAA and Canadian sediment quality guidelines,while cadmium, copper, nickel, lead and zinc also exceeded these guidelines at several sites.Heavy metal levels in oysters and green mussels varied widely with location. However,oysters from the Gulf of Paria contained significantly higher mean levels of cadmium,copper,nickel and zinc than those from the north coast of Venezuela, but this difference was not apparent in mussels.Cadmium, mercury and zinc in sediments were significantly correlated with those of mussels,but not of oysters,in which copper and zinc at several sites in the Gulf of Paria exceeded local maximum permissible levels (Cu =20 µg g-1 wet wt; Zn =50 µg g-1 wet wt)for human consumption. These findings indicate that while mussels may be better biological indicators of heavy metal pollution in sediments than oysters,the latter may provide copper and zinc contamination.Further research is needed to determine the most appropriate biological indicators of heavy metal and other pollutants in the local marine environment and to develop protocols for their use.
Keywords: Heavy metals,sediments,oysters,mussels,Gulf of Paria.
Sediments are an important source of metals to filter feeders,among them mussels and oysters (Schlekat et al.1992).The immobility of filter feeders makes these organisms ideal for monitoring changes in chemical concentrations at fixed locations.
Metal body loads of aquatic biota are often measured and used to evaluate ecological risks and potential sublethal effects (Phillips 1985, Rainbow 1995).Although body loads provide a simplistic indicator of toxic effects,they nonetheless yield information about potential exposure that can be used to assess both spatial and temporal trends in the health of aquatic ecosystems (Cohen et al.2001).
The green mussel Perna viridis and oysters (Crasostrea spp.),have been used as sentinel organisms in large-scale monitoring program (Philips 1985,Beliaeff et al.1998). P.viridis was discovered in Point Lisas,in the Gulf of Paria Trinidad,in the mid-1990 (Agard et al.1992),and since spread to Venezuela (Rylander et al.1996).This species and oysters (Crassostrea spp .)are widely used for human consumption,and are consequently of commercial importance.However,no trace metal data are available in terms of baseline concentrations,to enable future environmental impact assessment in Trinidad and Venezuela,thus necessitating the present study.
In this study,heavy metals in green mussel (P.viridis )and oyster (Crassostrea spp .) tissues and sediments from the Gulf of Paria were determined and correlated,to study possible relationships between metals in tissues and sediments.The sites on the north coast of Venezuela,which may be regarded as pristine, were selected as controls,for comparison with those in the Gulf of Paria,which receive discharges from a range of domestic and industrial sources.
Materials and methods
Sampling
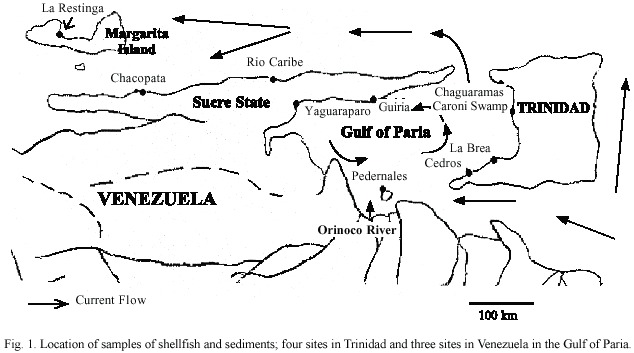
Samples of shellfish and sediments were obtained at four sites in Trinidad and three sites in Venezuela in the Gulf of Paria,in addition to comparative samples collected from three sites on the north coast of Venezuela (Fig.1).At all locations,the samples of Perna viridis were found in a size range of 9-12 cm shell length and oysters with 4-10 cm shell length.At most of the sites,divers were used to obtain the samples;while at others,collection was done by leaning out of a pirogue or climbing onto mangrove.Samples were randomly collected from about 20 m2 at each site.At least 20 individuals each of oysters and/or mussels were collected at each site.All sampling was carried out in May,2000.Since shellfish are normally consumed without depuration in both Trinidad and Venezuela,samples were immediately transported to the laboratory for processing.All shellfish were washed externally with a brush and distilled water to minimise contamination of tissue and then stored frozen until analysis.
Determinations of cadmium,chromium, copper,lead,nickel and zinc
The whole soft tissues of twelve mussels from each location were shucked into preweighed Pyrex beakers.All beakers were previously washed with detergent,rinsed with distilled water,soaked in 5%nitric acid,rinsed with distilled water and oven-dried.Prior to collection of their tissues,byssal threads were first removed from P.viridis .Each composite sample was blended to produce a homogenized mixture.Three replicates of approximately 5 g of each slurry,were weighed into boiling tubes and dried at 105 °C overnight.Concentrated analytical grade nitric acid (10 mL)was added to each sample and left to pre-digest overnight at room temperature in a fume hood.Reagent blanks were processed simultaneously.The boiling tubes were placed on a heating block to reflux at 130 °C for 6 hr.After cooling,the digests were diluted with 5 ml deionized water and filtered through Whatman #542 filters, into 25 ml volumetric flasks.Each solution was made up to volume with deionized water rinses of the residues.The prepared solutions were analysed for cadmium, chromium, copper, iron, lead,nickel and zinc,by flame atomic absorption spectroscopy with deuterium continuum background correction.In-date commercial metal calibration standard (BDH,Poole,U.K.) was used to prepare fresh calibration standards daily.A Varian Model SpectrAA-300 was used for all analyses.Oyster tissues were analysed by the same procedure.
Using three replicates (5 g)of wet tissue (
Determination of mercury
Samples were prepared for mercury analysis,using a modification of the method of Singh (1988).Triplicate 5 g aliquots of oyster and mussel macerates were weighed into 50 ml conical flasks.To each macerate,concentrated nitric acid (10 ml)was added,and samples were left to pre-digest overnight at room temperature in a fume hood.A small glass funnel was placed in the mouth of each conical flask,to prevent loss of sample,and the samples refluxed on a hot plate at 130 °C for 3 hr.Concentrated sulphuric acid (2.5 ml)and concentrated hydrochloric acid (1.0 ml)were then added to each sample,which was allowed to reflux at 130 °C for 3 hr more.Potassium manganate (VII)solution (5%m/V)was added drop-wise to each sample until a permanent pink coloration resulted. Hydroxylamine solution (50 µl,10%m/V)was added to destroy the excess potassium manganate (VII).The digests were filtered into 25 ml volumetric flasks, through Whatman #542 filters,and made up to volume with deionized water rinses of the residues.Mercury in mussels and oysters was determined by cold vapour atomic absorption spectrophotometry.
The procedure of mineralization of mercury in shellfish and sediment samples by Singh (1988)was also optimised,based on the use of a sequential (rather than simultaneous)mixture of nitric,sulphuric and hydrochloric acids.
Methods were validated with Standard Reference Materials,namely SRM 1566a oyster tissue (National Institute of Standards and Technology,MD,USA);lobster hepatopancreas (TORT-2)and dogfish muscle (DORM-2), from the National Research Council of Canada (Nova Scotia,Canada).
These SRM were subsequently analysed with each batch of samples,to monitor and control the quality of the analyses.
Determination of metals in sediments
Surface sediment samples were collected using an Ekman dredge at the same sites of collected mussels and oysters.Composites of several samples were taken over approximately 10 m2 at each site,placed in tightly sealed acid-washed plastic containers and stored at -4 °C until required for analysis.Sediments were dried at 60 °C to constant weight and subsequently sieved to  63 µm.Heavy metals, either of natural or anthropogenic origin,tend to be adsorbed to silt particles in sediments (
63 µm.Heavy metals, either of natural or anthropogenic origin,tend to be adsorbed to silt particles in sediments ( 63 µm),which have been used in recent studies on heavy metals in sediments (Gonzalez et al. 1999, Millward et al.1999,Soares et al.1999, Szefer et al.1999,Wenxin et al.1999).Also, dry rather than wet sieving was selected,since it is more convenient and adequate for assessing metal content variations in different sediment samples (Soares et al.1999).Triplicate subsamples (0.5 g)of each dried sediment were analyzed for metal analysis,using the same procedure used for mussels.
63 µm),which have been used in recent studies on heavy metals in sediments (Gonzalez et al. 1999, Millward et al.1999,Soares et al.1999, Szefer et al.1999,Wenxin et al.1999).Also, dry rather than wet sieving was selected,since it is more convenient and adequate for assessing metal content variations in different sediment samples (Soares et al.1999).Triplicate subsamples (0.5 g)of each dried sediment were analyzed for metal analysis,using the same procedure used for mussels.
An estuarine certified reference sediment (NIST Standard Reference Material 1646)was used to validate this method for the determination of the metals selected.This reference material was subsequently analyzed with each batch of sediment samples,to ensure consistently reliable results of analysis.
Results
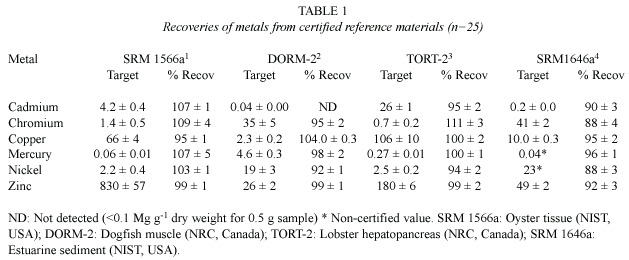
In Table 1,the results of analysis of the four Certified Reference Materials using the optimized procedures are shown.The results were compiled from the initial results of method validation and the subsequent quality control,using CRM samples analysed with each batch of samples.
In oysters,concentrations of zinc and copper showed significant variation (Student t,p<0.01)between different sites (Table 2). Oysters from La Brea and Cedros had the highest concentrations of copper,which exceeded the local maximum permissible levels (MPL) of 20 µg.g-1 wet weight (Anonymous 1998).
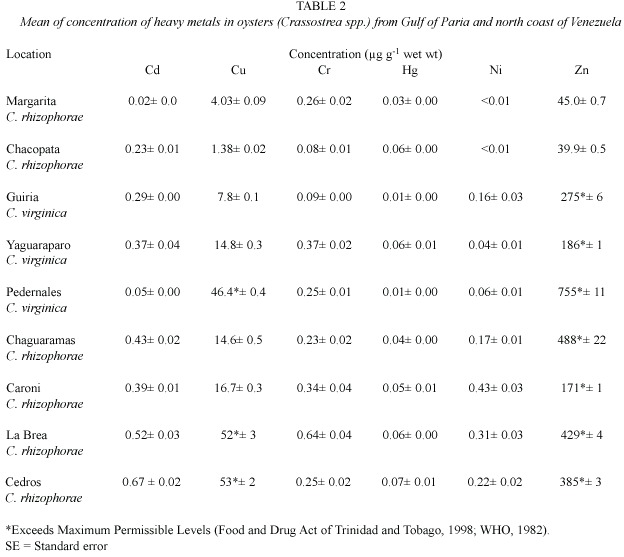
La Brea oysters contained the highest concentration of chromium,but did not exceed the World Health Organization recommended MPL of 13.0 µg.g-1 wet weight.However,zinc concentrations in oysters from all sites from the Gulf of Paria between Trinidad and Venezuela exceeded the MPL of 50 µg.g-1 wet weight.
P.viridis from Chaguaramas had the highest concentrations of mercury and zinc, but these concentrations were lower than the MPL (Table 3).
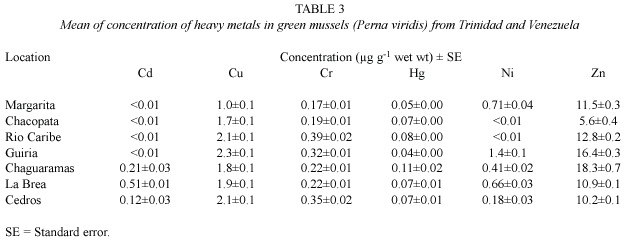
Data for metal concentrations in the sediment samples collected during the dry season,May 2000,are presented in Table 4.The ranges of concentrations (µg g-1 dry weight)of heavy metals found in sediments were:cadmium (<0.01-1.44), copper (5.1-21.9),chromium,(10-40),mercury (0.36-1.60)nickel (4.7-24.1),lead (0.7-37.3)and zinc (48-158).The highest concentrations of mercury,lead and zinc were in sediments of Chaguaramas in northern Trinidad.Cadmium was detected in La Brea and Cedros,at concentrations higher than all the other sites (Table 4).
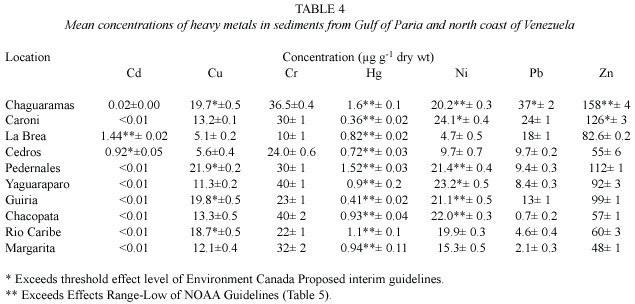
The highest concentration of chromium was found in sediment from Yaguaraparo and Chacopata in Venezuela,and the lowest at La Brea in Trinidad.Similarly,the highest concentration of copper was found in Pedernales in Venezuela,and the lowest at La Brea. Nickel was highest in Caroni Swamp sediment, but lowest at La Brea.Significantly,all sites with the highest individual concentrations of heavy metals were located in the Gulf of Paria (Table 4).In comparison,sediments from the north coast of Venezuela,namely Margarita, Chacopata and Rio Caribe had high concentrations of chromium and nickel (Table 4).
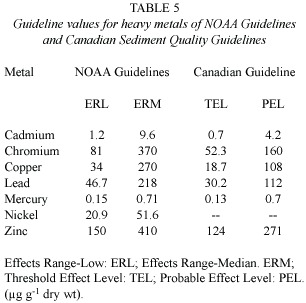
The threshold effects level (TEL)and Effects Range-Low (ERL)(Long et al.1995) for a given sediment parameter are the concentrations below which adverse biological effects are expected to occur only rarely.TEL is generally recommended as the proposed interim Canadian Sediment Quality Guidelines (Anonymous 2002),while ERL values are used in the NOAA Guidelines (Long et al.1995).
Because sediments in Trinidad and Venezuela were variable in their heavy metal concentrations,it was important to determine whether the concentrations found pose a threat to marine life.This was assessed firstly by comparison with sediment quality criteria and secondly by correlation of the contaminated sediments with oysters (Crassostrea spp .)and mussels (P.viridis ).
Since Trinidad and Venezuela have no established sediment quality guidelines at this time,the US National Oceanic and Atmospheric Administration (NOAA)and Canadian guidelines were used as interim measures to assess whether the concentrations of heavy metals in sediments could have adverse biological impacts (Table 5).
Sediment results show that mercury at all sites sampled in Trinidad and Venezuela exceeded the TEL of Canadian Sediment Quality Guideline and ERL of NOAA Guidelines. Similarly,as seen in Table 4,cadmium at two sites,copper at four sites,nickel at six sites, lead at one site and zinc at two sites exceeded their respective either TEL or ERL values.This indicates that the existing concentrations of metals in these sediments are sufficiently high to cause adverse biological effects.
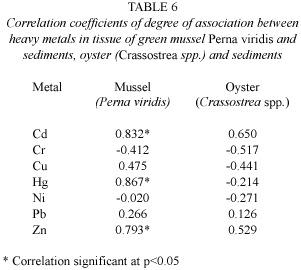
No significant correlation was found (Pearsons r,p>0.05)in metal concentrations between oysters and sediments (Table 6),in agreement with previous reports on oysters and sediments (Presley et al. 1990, Marin-Mezquita et al.1997,Hayes et al.1998,Gonzalez et al. 1999).However,for P.viridis tissue and sediment concentrations,correlations were statistically significant (Pearson s r,p<0.05)for Hg,Cd and Zn (Table 6).Regression analyses show that mercury, cadmium and zinc in sediments explained 75.2%, 69.29%and 62.9%,respectively,of the variance in mercury,cadmium and zinc in P.viridis .
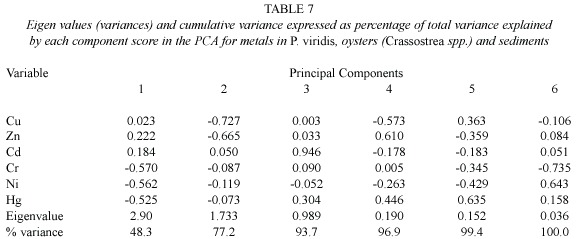
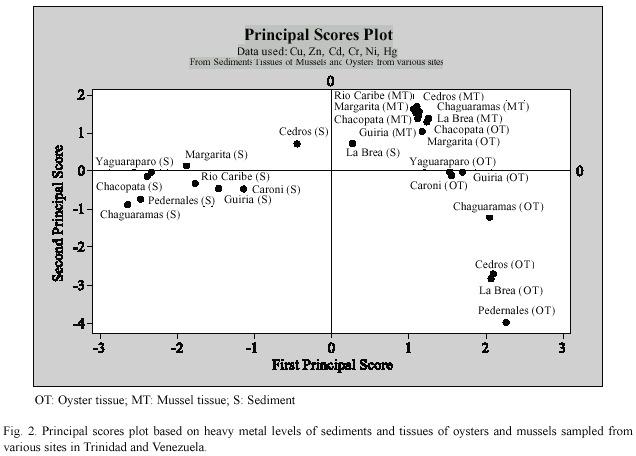
Principal component analysis (PCA)was carried out to examine the pattern of relationship among all metals in sediments,mussel and oyster tissues obtained from various sites from the Gulf of Paria.The first two PCA axes were selected because they explain the majority of variance of the heavy metals in sediment,in the green mussel (Perna viridis )and oysters (Crassostrea spp .).The similarities and differences among the samples (both sediment and tissue)can be seen in score plots (Fig. 2),which display the positions of the samples in the new coordinates.Similar samples are located together (Zitko 1996).
The eigen values (variances)and the cumulative variance expressed as percentage of total variance explained by each component score in the PCA for metals in P.viridis ,oysters (Crassostrea spp .)and sediments are presented in Table 7.The first two principal components explained 77.2%of the total variance,with the major percentage of variance accounted for by the first component (48.3%).The first component (PC-1)is positively related to the concentrations of Cd,Cu and Zn,but negatively related to the concentrations of Cr,Ni and Hg.The second component (PC-2),on the other hand,is only positively related to the concentration of Cd.
The principal score plots based on heavy metals in oyster and mussel tissue and sediments from sites in the Gulf of Paria are shown in Fig.2.Except for sediments from Cedros and La Brea,sediment samples are clustered in the left quadrant and are clearly separated from oyster and mussel tissue.This indicates that those sites are correlated by high concentrations of Ni,Cr and Hg,as they showed little within-group variation in these metals.In contrast,Cedros and La Brea sediments generally contain lower concentrations of Cu,Cr,Ni and Zn and elevated levels of cadmium than other sites. However,these two sites are also separated from each other by differences in their metal contents.
Oysters from Cedros and La Brea are clustered together in the lower right quadrant, separated from Pedernales,due to differences in Cu and Zn concentrations.Oysters from the other sites are found in smaller sub-clusters in the upper right quadrant.Chacopata and Margarita from the north coast of Venezuela; Yaguaraparo,Guiria and Caroni in the central Gulf of Paria;and Chaguaramas in the Northern Gulf of Paria are separated from the other three groups.The separation of sites in the positive quadrant of PC-1 is explained by the difference of concentrations of cadmium, copper and zinc in oyster and mussel tissue (Tables 2 and 3).The plots of scores of the first two components for heavy metals in tissue of Perna viridis show the similarity in metal concentrations and ratios,and are reflected in their tendency to cluster together.
Discussion
The results of analysis of the oyster,dogfish muscle,lobster hepatopancreas and estuarine sediment CRM agreed well with certified values,with recoveries ranging from 88%to 111% for the metals studied.Some values of chromium were found to be slightly higher than the reference values for SRM 1566a and TORT-2, and may have been caused by spectral interference (Sakao and Uchida 1999). Nevertheless, based on these results,no corrections of sample values were considered necessary.
The Gulf of Paria is a marine ecosystem, which receives runoff from lands used for petroleum and other industrial (iron and steel, fertilizers,methanol)and agricultural purposes. In the Gulf of Paria,bordered by Trinidad and Venezuela, C.virginica and C.rhizophorae are found,the former along the west coast,while the latter occurs along the east coast of the Gulf of Paria.C.rhizophorae also occurs on the north coast of Venezuela and Margarita Island. This distribution of oyster species may depend on environmental conditions,such as temperature,salinity and the degree of turbidity of the water (Bartol et al.1999,Laboy-Nieves et al. 2001,Chapman 2002).
In general,in this study,P.viridis contained lower heavy metal concentrations than oysters. These results are similar to results obtained from two "Mussel Watch "-type monitoring programs:the Réseau National d Observation de la qualité du milieu marin (RNO),the French monitoring network,and the Mussel Watch Project of the U.S.National Status and Trends (NS&T)Program.In oysters and mussels small differences for Hg and Cd,but more than tenfold differences for Cu and Zn were reported (Beliaeff et al.1998).
Oysters accumulate metals such as copper and zinc and can tolerate very high metal concentrations, without apparent detrimental effects (Lin and Hsie 1999,Soto-Jimenez et al.2001).However,in P.viridis,zinc concentrations exhibited a much narrower range than other metals,indicating that P.viridis can partially regulate Zn concentration in its soft tissue over a wide range of Zn bioavailabilities (Chan 1988,Chong and Wang 2001).The ability of bivalves to eliminate heavy metals,such as Zn,Cd,Pb and Cu,is well known.In any event,release of soluble and particulate materials is the prime mechanism by which marine organisms redistribute pollutants in their environment. However, the contributions of these mechanisms to the total heavy metal content in bivalves are still uncertain (Han et al.1993).
Total metal concentrations in sediments were generally similar to previous studies in the Gulf of Paria,with highest metal levels in sediments occurring close to areas containing industrial sites (Hall and Chang-Yen 1986, Mohammed et al.2001).The high concentrations of chromium and nickel found in sediments from the north coast of Venezuela, namely Margarita,Chacopata and Rio Caribe may differ in origin,since chromium levels are naturally high in local soils and sediments, while nickel is a lithophilic element,with large inputs from natural crust or soil-derived material,as well as from industrial activities such as fossil fuel combustion (Percy and Borland 1985).The elevated concentrations of mercury in sediments from the Gulf of Paria and Northern Venezuela may be due to the transport of this metal with run-off from gold mining operations in the Orinoco River watershed (Klekowski et al.1999).It is also possible that mercury pollution similarly originates from the Amazon-basin.Other site-specific investigations have found that mercury concentrations in water,sediments,and biota of the Brazilian Amazon and Suriname exceed global averages, and that these concentrations are a direct result of widespread mercury amalgamation mining operations conducted by small-scale prospectors (Bidone et al.1997,Mol et al.2001).
Similarly,as seen in Table 4,cadmium at two sites,copper at four sites,nickel at six sites,lead at one site and zinc at two sites exceeded their respective TEL or ERL values. This indicates that the existing concentrations of metals in these sediments are sufficiently high to cause adverse biological effects.
No significant correlation was found in metal concentrations between oysters and sediments (Table 6),in agreement with previous reports on oysters and sediments (Presley et al.1990,Marin-Mezquita et al.1997,Hayes et al.1998,Gonzalez et al.1999).This suggests that changes in the sediment metal loading do not influence metal concentration in oysters (Hayes et al.1998). Nevertheless,oyster tissues contain Cu and Zn at much greater concentrations than those found in sediments from several sites,indicating that these metals are probably absorbed for use in their metabolism.Vazquez et al.(1995)and Huanxin et al.(2000)reported similar findings on Cu and Zn in C.virginica and sediments.
Correlation coefficients of degree of association between heavy metals in tissue of green mussel Perna viridis and sediments were statistically significant for Hg,Cd and Zn (Table 6).These findings imply that sediments are a major source of cadmium,mercury and zinc to P.viridis .However,while sediments may be a major source of Zn to P.viridis,zinc may also come from other sources.It was demonstrated in a previous study (Chong and Wang 2001)that dissolved uptake from both water and food ingestion can contribute to zinc accumulation in P.viridis .These results suggest that suspended sediment particles may be a source of metals for filter-feeding animals. However,the relationship between metal partitioning to sediments and biological availability is not obvious,because the organic content of sedimentary particles and the acidic gut influence metal absorption in mussels (Gagnon and Fisher 1997b,Guppy 2001).
In comparison,chromium,mercury and nickel concentrations in both P.viridis and oyster tissue were lower than those in sediments. In general,marine shellfish appear to have a low tendency for chromium bioaccumulation (Saiz-Salinas et al.1996,Gibb et al.1996, Boening 1999,Chong and Wang 2001).
Despite these apparently low levels of mercury in P.viridis and oyster (Crassostrea spp .) tissue (60 to 100 ng.g-1 wet wt.),they are still a potential public health concern (Kawaguchi et al.1999),since mercury is a toxic metal that bioamplifies through food webs (Gagnon and Fisher 1997a).While low concentrations of mercury were found in sediments of Caroni Swamp on the south-western coastline of Trinidad,concentrations of mercury in Scarlet Ibis were about six times higher than in sediments from the Swamp where the birds normally feed (Klekowski et al.1999).
The results of this study,to measure the relationship among all metals in sediments, mussel and oyster tissues,indicate that the variability of metals in oysters is probably more site dependent than species –specific (Daskalakis 1996). However,all Perna viridis tissue samples from Trinidad and Venezuela are clustered in the upper right quadrant.This indicates that P.viridis mussels tend to regulate the levels of heavy metals in their tissue,and may not reflect the levels in sediments to which they are exposed. These results (Fig.2)show that the tendency towards heavy metal accumulation in oyster and mussel is different.This may be due to oysters and mussels having different capacities to regulate or accumulate heavy metals (Reinfelder et al.1997,Chong and Wang 2001, Soto-Jimenez et al.2001).
In particular,the high concentrations of lead,zinc and mercury detected in Chaguaramas sediments give cause for concern,since they will eventually affect the rest of the Gulf of Paria,due the circulatory patterns in the Gulf (Gopaul and Wolf 1996).
Comparable baseline studies are still needed for all coastal zones and estuaries throughout the Gulf of Paria.The geochemistry of sediments is complex,and in order to interpret contaminant concentrations measured in sediments it is necessary to understand how hydrology and other non-contaminant sediment properties may affect contaminant concentrations.
Because sediments are dynamic and particle ingestion is an important exposure route (Chapman 2002),monitoring of mussel and oyster tissue should occur monthly or at least bimonthly,to assess the extent of metal pollution in the Gulf of Paria.
Conclusions
The results of this study indicate that P.viridis mussels may be better indicators of environmental pollution than Crassostreid oysters.However,the ability of oysters to bio-accumulate copper and zinc may be used to reflect longer term exposure to environmental contamination by these metals.The application of either of these organisms as biological indicators of heavy metal contamination in local coastal waters will require further investigations to develop the protocols for their use. This is planned for the near future,to develop a monitoring system using local organisms,to monitor the quality of coastal environments in the Caribbean.
Acknowledgments
The financial support from Consejo de Investigación from Universidad de Oriente, Venezuela,The University of the West Indies,Trinidad,and Fondo Nacional de Investigaciones Científicas y Tecnológicas (FONACIT),Venezuela,is acknowledged.We thank the Institute of Marine Affairs,Trinidad and the Department of Life Sciences,University of The West Indies,for the assistance in collection of shellfish samples.
Resumen
El Golfo de Paria está rodeado por Trinidad y Venezuela y es una fuente significativa de peces y moluscos para el consumo humano.Se extrajo muestras de ostras (Crassostrea rhizophorae y C.virginica ),mejillones (Perna viridis )y sedimentos de cuatro sitios de Trinidad y tres de Venezuela en el golfo,así como muestras de tres localidades de la costa norte de Venezuela.El tejido comestible homogenizado de 12 moluscos de cada localidad fue usado para la extracción de metales pesados.El cadmio, cromo,cobre,plomo,níquel y zinc fueron analizados por espectroscopía de absorción atómica a la llama y el mercurio fue determinado por vapor al frío.Las ostras del Golfo de Paria presentaron concentraciones significativamente más altas de cadmio,cobre,níquel y zinc que las de la costa norte de Venezuela;pero esa diferencia no fue aparente en los mejillones.El mercurio en los sedimentos en todos los sitios y también el cadmio,cobre,níquel,plomo y zinc de varios sitios excedieron los niveles permisibles de las normas canadienses y estadounidenses.Los valores de cadmio, mercurio y zinc en los sedimentos se correlacionaron significativamente con los de los mejillones,pero no con los de las ostras.En las ostras los valores de cobre y zinc en varios sitios en el Golfo de Paria excedieron los límites máximos permitidos para el consumo humano.Los mejillones pueden ser mejores indicadores de contaminación de metales pesados en sedimentos,pero las ostras pueden indicar mejor la contaminación por cobre y zinc.Se necesita determinar el indicador biológico más apropiado en la determinación de metales pesados y otros contaminantes en el ambiente marino local,y desarrollar protocolos para su uso.
Keywords:Metales pesados,sedimentos,ostras,mejillones,Golfo de Paria.
References
Agard,J.B.,R.Kishore &B.Bayne.1992.Perna viridis (Linnaeus,1758):First record of the indo-pacific green mussel (Mollusc:Bivalvia )in the Caribbean. Carib.Mar.Stud.3:59-60. [ Links ]
Anonymous.1998.Republic of Trinidad and Tobago, Legal Supplement Part II,Food and Drugs Act 37(140):1221-1255. [ Links ]
Bartol,I.K.,R.Mann &M.Luckenbach.1999.Growth and mortality of oysters (Crassostrea virginica )on constructed intertidal reefs:effects of tidal height and substrate level J.Exp.Mar.Biol.Ecol.237:157-184. [ Links ]
Beliaeff,B.,T.P.O Connor &D.Claisse.1998.Comparison of chemical concentrations in mussels and oysters from the United States and France.Environ.Monit. Asses.49:87-95. [ Links ]
Bidone,E.O,Z.C.Castillo,T.J.S.Santos,T.M.C.Souza &L.D.Lacerda.1997.Fish contamination and human exposure to mercury in Tartarugalzinho River, Amapa State,Northern Amazon,Brazil:A screening approach.Water Air Pollut.97:9-15. [ Links ]
Boening,D.W.1999.An evaluation of bivalves as bio-monitors of heavy metals pollution in marine waters. Environ. Monit. Asses. 55:459-470. [ Links ]
Chan,H.M.1988.Accumulation and tolerance to cadmium,copper,lead and zinc by the green mussel Perna viridis. Mar. Ecol. Prog.Ser.48:295-303. [ Links ]
Chapman P.M.2002.Integrating toxicology and ecology: putting the "eco "into ecotoxicology.Mar.Pollut. Bull.44:7-15. [ Links ]
Chong,K.&W.X.Wang.2001.Comparative studies on the biokinetics of Cd,Cr and Zn in the green mussel Perna viridis and the Manila clam Ruditapes philippinarum.Environ.Pollut .115:107-121. [ Links ]
Cohen T.,S.Shane,H.Que &R.F.Ambrose.2001.Trace Metals in Fish and Invertebrates of Three California Coastal Wetlands. Mar.Pollut.Bull .42:224-232. [ Links ]
Daskalakis,K.D.1996.Variability of metal concentrations in oyster tissue and implications to biomonitoring. Mar. Pollut. Bull.32:794-801. [ Links ]
Gagnon,C.&N.S.Fisher.1997a.Bioavailability of sediment-bound methyl and inorganic mercury to a marine bivalve.Env.Sci.Technol.31:993-998. [ Links ]
Gagnon,C.&N.S.Fisher.1997b.The bioavailability of sediment-bound Cd,Co,and Ag to the mussel Mytilus edulis.Can.J.Fish.Aquat.Sci.54:147-156. [ Links ]
Gibb,J.,J.R.Alle &S.J.Hawkins.1996.The application of biomonitors for the assessment of mine-derived pollution on the West Coast of the Isle of man.Mar. Pollut.Bull.32:513-519. [ Links ]
Gonzalez,H.,M.Pomares,M.Ramirez &I.Torres.1999. Heavy metals in organism and sediments from the discharge zone of the submarine sewage outfall of Havana City Cuba.Mar.Pollut.Bull.38:1048-1105. [ Links ]
Gopaul,N.H.&J.Wolf.1996.A numerical model of tidal and wind-driven circulation in the Gulf of Paria. Carib.Mar.Stud.5:23-40. [ Links ]
Guppy,M.2001.Chemical Investigation in the green lipped mussel (Perna viridis )in the Gulf of Paria,Trinidad. PhD Thesis,Univ.West Indies,St.Augustine, Trinidad and Tobago.220 p. [ Links ]
Hall,L.&I.Chang-Yen.1986.Metals in sediments off Trinidad,West Indies.Mar.Pollut.Bull.17:274-276. [ Links ]
Han,B.C.,W.Jeng,Y.Tsai &M.Jeng.1993.Depuration of copper and zinc by green oysters and blue mussels of Taiwan.Environ.Pollut.82:93-97. [ Links ]
Hayes W.J.,I.J.Anderson,M.Z.Gaffor &J.Hurtado.1998. Trace metals in oyster and sediments of Botany Bay, Sidney.Sci.Total Environ.212:39-47. [ Links ]
Huanxin,W.,Z.Lejun &B.J.Presley.2000.Bioaccumulation of heavy metals in oyster (Crassostrea virginica )tissue and shell .Environ.Geol.39:1216-1226. [ Links ]
Kawaguchi,T.,D.Porter,D.Bushek &B.Jones.1999. Mercury in the American oyster Crassostrea virginica in South Carolina,USA,and public health concerns.Mar Pollut.Bull.38:324-327. [ Links ]
Klekowski,E.J.,S.A.Temple,A.M.Siung-Chang &K. Kumarsingh.1999.An association of mangrove mutation,scarlet ibis and mercury contamination in Trinidad,West Indies .Environ.Pollut.105: 185-189. [ Links ]
Laboy-Nieves,E.N.,E.Klein,J.E.Conde,F.Losada,J.J. Cruz &D.Bone.2001.Mass mortality of tropical marine communities in Morrocoy,Venezuela.Bull. Mar.Sci.68:163-179. [ Links ]
Lin,S.&I.-J.Y.Hsie.1999.Occurrences of green oyster and heavy metals contaminant levels in the Sien-San Area, Taiwan. Mar. Pollut.Bull.38:960-965. [ Links ]
Long,E.R.,D.D.MacDonald,S.L.Smith &F.D.Calder. 1995.Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments.Env.Mgmt.19:81-97. [ Links ]
Marin-Mezquita,L.,L.Baeza,O.Zapata-Perez &G.Gold- Bouchot.1997.Trace metals in the American oyster, Crassostrea virginica ,and sediments from the coastal lagoons Mecoacan,Carmen and Machona,Tabasco, Mexico.Chemosphere 34:2437-2450. [ Links ]
Millward,G.E.,C.Rowley,T.K.Sands,R.J.M.Howland & A.Pantiulin.1999.Metals in the sediments and mussels of the Chupa Estuary (White Sea)Russia .Estuar. Coastal Shelf Sci.48:13-25. [ Links ]
Mohammed,A.,I.Chang Yen &I.Bekele.2001.Heavy metal contamination in sediments of the Chaguaramas Peninsula. Inst.Mar.Affairs 8 th Ann.Res.Symp., Chaguaramas,Trinidad:6-7. [ Links ]
Mol J.H.,J.S.Ramlal,C.Lietar &M.Verloo.2001. Mercury Contamination in freshwater,estuarine,and marine fishes in relation to small-scale gold mining in Suriname,South America.Environ.Res.86: 183-197. [ Links ]
Percy,K.E.&S.A.Borland.1985.A multivariate analysis of element concentrations in Sphagnum magellanicum brid in the Maritime Provinces,Canada.Water Air Soil Pollut.25:331-338. [ Links ]
Philips,D.J.H.1985.Organochlorines and trace metals in green lipped mussels Perna viridis from Hong Kong waters:a test of indicator ability.Mar.Ecol.Prog. Ser.21:251-258 [ Links ]
Presley,B.J.,R.J.Taylor &P.N.Boothe.1990.Trace metals in Gulf of Mexico oysters.Sci.Total Environ.97: 551-593. [ Links ]
Rainbow,P.S.1995.Biomonitoring of heavy metal availability in the marine environment.Mar.Pollut.Bull. 31:183 -192. [ Links ]
Reinfelder,J.R.,W.X.Wang,S.N.Luoma &N.S.Fischer. 1997.Assimilation efficiencies and turnover rates of trace elements in marine bivalves:a comparison of oysters,clams and mussels .Mar.Biol.129:443-452. [ Links ]
Rylander,K.,J.Perez &J.A.Gomez.1996.Status of the green mussel,Perna viridis (Linnaeus,1758) (Mollusca:Mytilidae),in north-eastern Venezuela. Carib.Mar.Studies 5:86-87. [ Links ]
Saiz-Salinas,J.I.,J.M.Ruiz &G.France-Zubillaga.1996. Heavy metal levels in intertidal sediments and biota from the Bidasoa Estuary.Mar.Pollut.Bull.32:69-71. [ Links ]
Sakao,S.&H.Uchida.1999.Determination of trace elements in shellfish tissue samples by inductively coupled plasma mass spectrometry .Anal.Chim.Acta 382:215-223. [ Links ]
Schlekat,C.,B.L.McGee &E.Reinharz.1992.Testing sediment toxicity in Chesapeake Bay with the amphipod Leptocheirus plumulosus :an evaluation.Environ. Toxicol.Chem.11:225-236. [ Links ]
Singh,J.G.1988.A Study of heavy metals and hydrocarbons in fish,crab and mussels found in Trinidad.PhD Thesis, Univ.West Indies,St.Augustine,Trinidad and Tobago.333 p. [ Links ]
Soares,H.M.,R.A.Boaventura,A.A.Machado &J.C. Esteves Da Silva.1999.Sediments as monitors of heavy metal contamination in the Ave River basin (Portugal):multivariate analysis of data.Environ. Pollut .105:311-323. [ Links ]
Soto-Jimenez,M.,F.Paez-Osuna &F.Morales-Hernandez. 2001.Selected trace metals in oysters (Crassostrea iridescens )and sediments from the discharge zone of the submarine sewage outfall in Mazatlán Bay (southeast Gulf of California):chemical fractions and bioaccumulation factors. Environ.Pollut .114: 357-370. [ Links ]
Szefer P.,A.A.Ali,A.A.Ba-Haroon,A.A.Rajeh,J.Geldon &M.Nabrzyski.1999.Distribution and relationships of selected trace metals in molluscs and associated sediments from the Gulf of Aden,Yemen.Environ. Pollut.106:299-314. [ Links ]
Vazquez,F.G.,V.K.Sharma,V.H.Alexander &C.A. Frausto.1995.Metals in some lagoons of Mexico. Environ.Health Perspect .103 (Suppl.1):33-34. [ Links ]
Wenxin,L.,W.Zijian,W.Xianghua &T.Hongxia.1999. The application of preliminary sediment quality criteria to metal contamination in the Le An River. Environ.Pollut .105:355-366. [ Links ]
Internet references
Anonymous.2002.Canadian sediment quality guidelines for the protection of aquatic life.CCME (Canadian Council of Ministers of the Environment).Canada. (Downloaded:December 03,2002,http://www.ccme.ca/assets/pdf/e1_06.pdf). [ Links ]
Zitko,V.1996.Polynuclear aromatic hydrocarbons in the Gulf of Maine sediments visualized by Principal Component Analysis.(Downloaded:June 14,2002,http://www.nml.dartmouth.edu/rergom/NEWsWinter96/zitko.html) [ Links ]