Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.53 n.3-4 San José Sep. 2005
Birds of a high-altitude cloud forest in Alta Verapaz, Guatemala
Knut Eisermann1 & Ulrich Schulz
University of Applied Sciences Eberswalde, Fr. Ebertstr. 28, 16225 Eberswalde, Germany.
1 Current address and address of correspondence: Knut Eisermann. P.O. Box 098 Periférico. Guatemala, Ciudad Guatemala, Centroamérica. Tel.: 502-5906 6479; knut.eisermann@proeval-raxmu.org
Received 03-III-2005. Corrected 18-VII-2005. Accepted 28-VII-2005.
Abstract: The Northern Central American Highlands have been recognized as endemic bird area, but little is known about bird communities in Guatemalan cloud forests. From 1997 to 2001 a total of 142 bird species were recorded between 2 000 and 2 400 masl in cloud forest and agricultural clearings on Montaña Caquipec (Alta Verapaz, Guatemala). The bird community is described based on line transect counts within the forest. Pooling census data from undisturbed and disturbed forest, the Gray-breasted Wood-Wren (Henicorhina leucophrys) was found to be the most abundant species, followed in descending order by the Common Bush-Tanager (Chlorospingus ophthalmicus), the Paltry Tyrannulet (Zimmerius vilissimus), the Yellowish Flycatcher (Empidonax flavescens), the Ruddy-capped Nightingale-Thrush (Catharus frantzii), and the Amethyst-throated Hummingbird (Lampornis amethystinus). Bird communities in undisturbed and disturbed forest were found to be similar (Sørensen similarity index 0.85), indicating low human impact. Of all recorded species, ~27% were Nearctic-Neotropical migratory birds. The most abundant one was the Wilsons Warbler (Wilsonia pusilla). The Montaña Caquipec is an important area for bird conservation, which is indicated by the presence of four species listed in the IUCN Red List (Highland Guan Penelopina nigra, Resplendent Quetzal Pharomachrus mocinno, Pink-headed Warbler Ergaticus versicolor, Golden-cheeked Warbler Dendroica chrysoparia), and 42 Mesoamerican endemics, of which 14 species are endemic to the Central American Highlands. The results presented here will be useful as baseline data for a long-term monitoring. Rev. Biol. Trop. 53(3-4): 577-594. Epub 2005 Oct 3.
Key words: Bird community, cloud forest, conservation, Guatemala, Nearctic-Neotropical migrants, species richness.
Tropical rainforests have been labeled as some of the most endangered biomes on a global scale (Dobson 1996). While public interest is mainly focused on lowland rainforests (Wilson 1992, Primack 1993, Dobson 1996, Primack and Corlett 2005), the overall area of cloud forests in the world has been more greatly reduced than lowland forests in the last few decades (Doumenge et al. 1995, Hamilton et al. 1995). Between 1990 and 2000, Guatemala had an annual deforestation rate of 1.7% of remaining forests, equaling 485 km2 (FAO 2003).
Humid broadleaf forests within the condensation zone, which is usually above 1 000 m, are known as cloud forests (Richards 1996, Brown and Kappelle 2001). Characteristic northern Mesoamerican cloud forest birds, like the Resplendent Quetzal (Pharomachrus mocinno) and the Slate-colored Solitaire (Myadestes unicolor), range in altitude from ~1 000- 3 000 m. At the lower range limit, these species are associated with many lowland rainforest species with an upper range limit at 1 500-1 800 m (Howell and Webb 1995). High species turnover rates have been observed along an altitudinal transect in the Sierra Madre (Navarro 1992), and there is almost no similarity in bird species composition between lowland broadleaf forests and cloud forests above 2 000 m (pers. obs.), which can therefore be considered as the habitat of cloud forest specialists.
Although the Guatemalan highlands have previously been included in an endemic bird area (Bibby et al. 1992b, Long 1995, Wege and Long 1995, Stattersfield et al. 1998), there is no comprehensive description of bird communities widely available. This study was carried out as a thesis research (Eisermann 1999) and aims to increase the knowledge on the structure of bird communities in high-altitude cloud forests in Guatemala.
In order to decrease human impact on the cloud forest, local conservation institutions provide agricultural support and alternative income opportunities to the local people (Schulz and Unger 2000). Birds have been used as indicators for the effectiveness of conservation efforts in biomonitoring programs (Kremen et al. 1994). In order to measure the impact of the efforts of several conservation institutions at Caquipec, a long-term monitoring program of the avifauna will be implemented based on the results of this study.
Materials and methods
Study area
The Montaña Caquipec (15°23N, 90°11W) is a karst mountain (Weyl 1980) which is characterized by a rugged topography, located ~45 km southeast of the city of Cobán, department Alta Verapaz. The study area includes cloud forest and open agricultural habitats, at elevation of 2 000 - 2 400 m.
The area is often enveloped in clouds with an annual precipitation of ~4 000 mm and a mean annual temperature of 15°C (MAGA 2002). Primary vegetation is an evergreen humid broadleaf forest, with canopy heights between 25 and 40 m. The tree community is species rich, including different species of the laurel family (Lauraceae), oaks (Quercus spp.), and yellowwood (Podocarpus sp.). The forest canopy on ridges is dominated by oaks (Quercus spp.). A low-canopy (5 m) elfin forest (Cavendishia sp. and other Ericaceae) is found in small patches on ridges most exposed to wind. Treeferns (Dicksoniaceae and Cyathaceae), various shrubs (e.g. Gesneriaceae, Melastomataceae) and small palms (Chamaedorea spp.) dominate the forest understory in most areas. Due to the high humidity in the forest, most of the trunks and branches are heavily covered (<15 cm deep) with mosses and liverworts (Bryophyta), ferns (Filicatae), orchids (Orchidaceae), bromeliads (Bromeliaceae) and other epiphytes of the families Ericaceae, Campanulaceae, Piperaceae, and Araceae.
The Montaña Caquipec has been mostly deforested, leaving a remnant forest fragment of ~12 km² on the least accessible slopes of the mountain peaks. Within this fragment there are clearings of up to 75 ha occupied by small indigenous settlements. The residents of Caquipec belong to the Maya Qeqchi ethnic group. They are subsistence farmers who mainly depend on the cultivation of corn (Zea mayz) and beans (Phaseolus sp.) by means of slash-and-burn agriculture. After a few sub-sequent crop cycles, fields are left fallow for about 5 years, resulting in a large land requirement per farmer. Due to this factor and population increase, the slash-and-burn agricultural methods are unsustainable and have resulted in deforestation of all but the most inaccessible slopes. People live in traditional settlements with simple huts dispersed widely in agricultural clearings. Houses are constructed using the stems of treeferns and timber from the forest. People also use the forest for a variety of subsistence purposes including firewood, hunting, medicinal and edible plants, and tourism. The secondary growth adjacent to cultivated areas is used for cattle raising, the harvest of a sedge (Carex sp.) for roof material, and the fruit of the arrayan-shrub (Myrica cerifera), which is used to produce a natural wax for candles (Schulz and Unger 2000).
The forest appears generally undisturbed, except in some parts where previous timber extraction created an interrupted canopy. Considering birds as highly mobile animals, there is no truly pristine habitat available due to the small size of the remaining forest area.
Methods
The bird community of the cloud forest and open habitat was studied for 208 days from September, 1997 to October, 1998.
A definition of terms used in this report follows:
Undisturbed forest: Cloud forest with a closed canopy, without signs of timber extraction.
Disturbed forest: Cloud forest with canopy gaps caused by selective timber extraction for subsistence use.
Open habitat: Agricultural clearings and secondary scrub associated with fallow fields.
Nearctic birds: Nearctic-Neotropical migrants, transient or wintering species which breed in the Nearctic region.
Transient: Nearctic species in the process of migration, present at Caquipec just temporary during the northern winter.
Vagrant: Occasionally observed species, which are resident in the region.
Species nomenclature refers to AOU (1998) and its supplements, names of species treated in this report are affected by Banks et al. (2003, 2004). We treat the Central American form of the Sharp-shinned Hawk (Accipiter striatus chionogaster) separated from the North American form (Accipiter striatus striatus).
Two field methods were applied: casual observations and line-transect counts.
Casual observations
From September, 1997 to February, 1998 qualitative species composition data were collected using the ad libitum method (Lehner 1978). The principal investigator (KE) walked along existing trails in undisturbed forest, disturbed forest, and open habitat types, between one hour before sunrise until three hours after sunset. Birds were identified by voice or visual signs. The principal investigator was able to familiarize himself with the calls and songs of all resident and migratory bird species during the course of the ad libitum surveys, which was later essential in order to conduct the audiovisual transect counts. During 208 days of field work, a total effort of 1 250 hr of survey were conducted throughout the study site. Eight of the 143 species were added to the species list through casual observations made on additional visits from 1999-2001 (Eisermann, pers. observ., P. Kaestner, M. Schweighöfer, R. Haupt, pers. comm.).
Transect counts
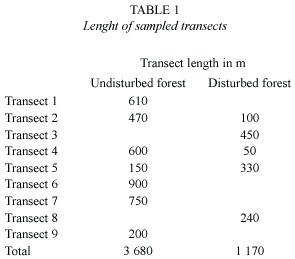
All line-transect sampling was conducted by the principal investigator, between March and August 1998 when favorable weather conditions permitted (no rain, calm). Transect sampling methods followed Bibby et al. (1992a). Nine transects between 200 m and 900 m long were flagged on an existing trail network (Fig. 1, Table 1). The total length of all transect lines was 4 850 m, of which 3 680 m sampled undisturbed forest and 1 170 m sampled disturbed forest. All individuals seen or heard within a perpendicular distance of 30 m from the transect line were recorded. Sampling was repeated two to nine times per transect, between 5:30 and 9:00 a.m. The census taker walked at an average speed of 90 m per 15 min. Total length of sampled transects was 31.79 km. Because the effective strip width along the transects was 60 m, the survey design effectively sampled an area of 29.1 ha, representing ~2.4% of the total forest area. Three of the transects crossed both forest types, but no sampling was conducted within the distance of 50 m to the habitat edge.
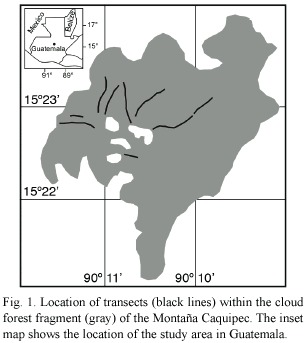
Data Analysis
Species Richness
To predict species richness we applied a first-order jackknife estimator to the line transect data (Krebs 1999), using the software "Biodiversity Professional" (McAleece el al. 1997). Each transect was treated as sample unit. Calculation is based on presence/absence. We excluded the records of Nearctic species from this analysis; so the estimate indicates species richness of breeding species only.
Dominance
To describe the community we calculated dominance ranking using the following equation (modified after Mühlenberg 1993):
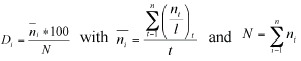
where Di is the dominance of species i, ni is the mean number of individuals of species i per 100 m transects (across all transects), N is the sum of all ni, t is the number of transects, l is the total length (in 100 m units) of all counts along one transect.
The relative abundance values used for this analysis were based on the frequency of observation per 100 m of sampling effort (n/100 m) on the line transects during all months of the survey (March-August). We assumed no significant changes in abundance due to the brief sampling period, which coincided with the peak of reproductive activity.
Abundance
We compared species abundance between undisturbed and disturbed forest using a permutation test for independent samples based on mean differences. Following Rice (1989) we adapted significance at the alpha level for the multiple comparisons at 0.025. For these calculations we used the statistical package SSS (Engel 1998). Each transect section representing a particular forest type was used as a replicate unit (7 sections in undisturbed and 5 in disturbed forest, Table 1). The same abundance estimate used for the dominance ranking analysis was used for this comparison. In order to describe 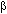
Results
Inventory and species richness
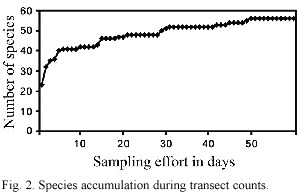
In the current study, a total of 142 bird species were recorded on Montaña Caquipec at elevations between 2 000 and 2 400 m.
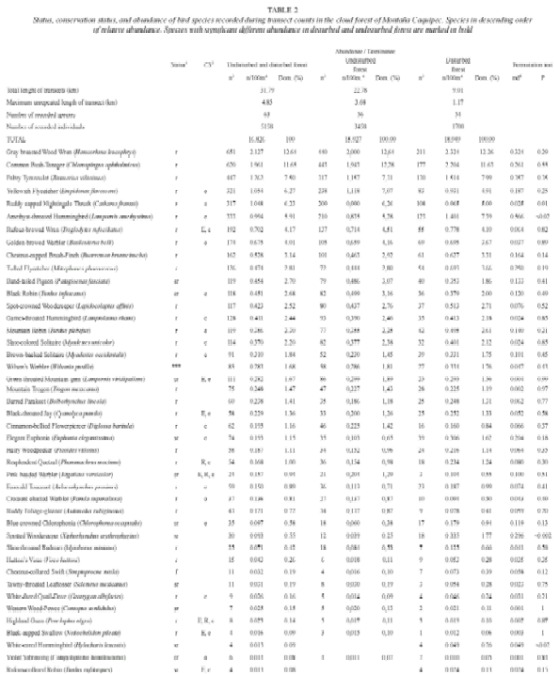
Table 2 shows the relative abundance of species obtained from transect counts, and table 3 shows relative abundance values for species which were only recorded during ad libitum sampling. The following bird families were found to be most species rich: Parulidae with 22 species, followed by Tyrannidae (13), Turdidae (12), Trochilidae (9), Emberizidae (8), and Vireonidae with 7 species.
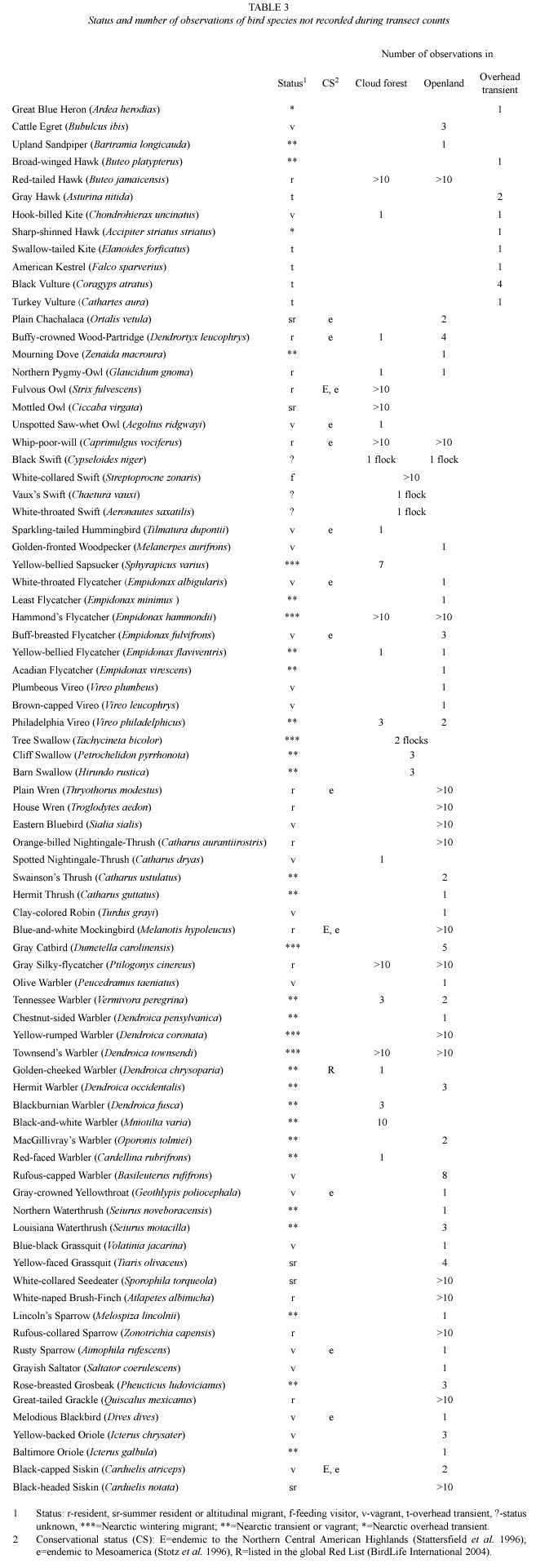
Ninety-three species (~65%) were recorded within the cloud forest (undisturbed and disturbed). Subtracting from this total the number of species classified as feeding visitors, vagrants, overhead transients, Nearctic-Neotropical migrants, and unclassified species, the remaining 60 species are assumed to be local breeders. These are classified as permanent residents, summer residents, or probable altitudinal migrants (Table 2). The predictions of species richness for resident forest-dwelling birds based on transect counts, provided a first-order jackknife value of 74 species.
Composition and habitat use
Of 93 species recorded in the cloud forest, ~47% were insectivores, ~18% omnivores, ~11% nectarivores, and ~9% carnivores. Approximately 40% (37 of 93) of all species observed in forest (disturbed and undisturbed) were recorded exclusively in this habitat and ~45% (44 of 98) of the species recorded in open habitat were observed exclusively there. Fifty six species were recorded in the forest and in the open habitat as well. The Sørensen similarity index value comparing the bird community between both forest types combined and open habitat was 0.53.
The Sørensen-index value comparing undisturbed and disturbed forest was 0.85, indicating a minor difference in species composition. When comparisons between the two forest types were made using the permutation test, only three species had a significant difference in abundance (Table 2). The total bird abundance of all species was not significantly different between disturbed and undisturbed forest (permutation test, mean differences -3.127, p = 0.10, based on 792 permutations).
The most abundant species with a dominance >5% of the total number of individuals were (in descending order): Gray-breasted Wood-Wren (Henicorhina leucophrys), Common Bush-Tanager (Chlorospingus ophthalmicus), Paltry Tyrannulet (Zimmerius vilissimus), Yellowish Flycatcher (Empidonax flavescens), Ruddy-capped Nightingale-Thrush (Catharus frantzii), and Amethyst-throated Hummingbird (Lampornis amethystinus).
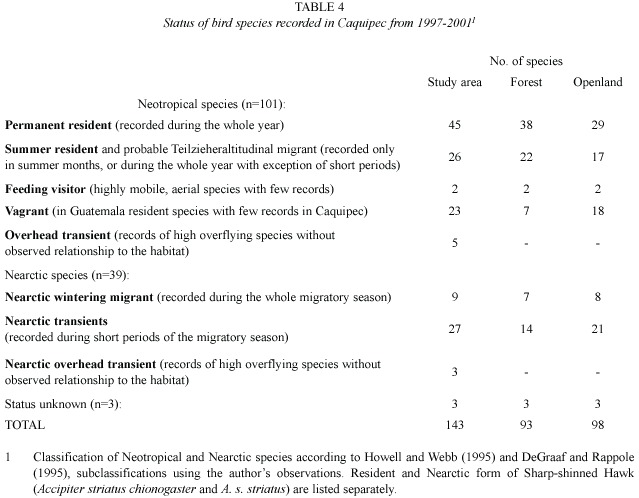
Migratory birds
Approximately a fourth of all recorded species (39 of 143 species, see Table 2 and Table 3) are classified as Nearctic-Neotropical migrants. Nine of these species were observed during the whole migratory season and are considered Nearctic wintering migrants. The remaining 30 Nearctic species are classified as transients. The number of Nearctic-Neotropical migratory species was found to be higher in open habitat than in the forested habitats (Table 4). Tables 2, 3 and 4 classify migratory species based on the pattern of presence over one year of sampling. The most abundant wintering Neartic species was the Wilsons Warbler (Wilsonia pusilla), which was recorded from beginning of September until mid-May. The Western Wood-Pewee (Contopus sordidulus) was the only summer resident, recorded from February to July.
Mixed-species flocks
We observed 30 mixed-species flocks during casual observations and recorded the associated species. Of 93 species recorded for combined forest types, 23 species (~25%) were observed foraging in mixed-species flocks (Table 5). Twelve species were wintering or transient Nearctic birds. The number of individuals detected in a single flock ranged from 3 to 30 containing a range of 2 to 6 species. The nucleus species was always the Common Bush-Tanager (Chlorospingus ophthalmicus), no other species was present in all flocks. Adjoining species were observed in all forest strata.

Discussion
Inventory and species richness
Species richness depends on various ecological factors. In general, it decreases with increasing latitude and altitude (Begon et al. 1990). Terborgh (1977) found a peak in species richness at the elevation of 1 500 m along an altitudinal transect on the eastern slope of the Peruvian Andes, where species richness decreased above this elevation. Navarro (1992) recorded a declining species richness from 680 m to 3 100 m in the Sierra Madre, Mexico. Therefore we expected species richness at Caquipec (>2 000 m) would be lower compared to species richness values of ~100 resident species recorded locally in undisturbed cloud forest at 1 500 m (Eisermann, unpub. data). In the current study we predicted a species richness of 74 species with jack-knife estimator, which is 14 species more than we obtained from transect counts and casual observations (60 species). Although the species accumulation curve from transect counts indicates that almost all species were detected (Fig. 2), several rare or vagrant species may be expected to contribute to species richness.
Comparison with other cloud forest avifaunas
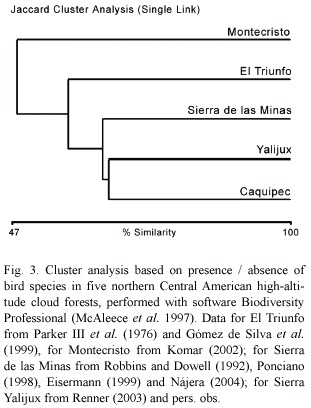
The distribution of cloud forest species in northern Central America is known on a large scale (Howell and Webb 1995), but information on relative abundance in bird communities is lacking. Currently no published description of a Guatemalan cloud forest bird community, treating all species, is widely available. Komar (2002) provided the first relative abundance description for a northern Central American cloud forest bird community at Cerro Montecristo (14°26 N 89°21 W), in northern El Salvador. An inventory is available for the El Triunfo cloud forest (15°39 N 92°48 W) in Chiapas, Mexico, without detailed data on relative abundance (Parker III et al. 1976, Gómez de Silva et al. 1999). Unpublished data are available from the Sierra de las Minas (15°05 N 89°57 W), department El Progreso, Guatemala (Robbins and Dowell 1992, Ponciano 1998, Eisermann 1999, Nájera 2004) and the Montaña Yalijux (15°23 N 90°04 W), Alta Verapaz in Guatemala (Renner 2003, pers. obs.). The cloud forests at all sites cover a similar altitudinal range as the Caquipec forest at elevations of 2 000-2 400 m (Montecristo: 2 000-2 350 m, El Triunfo: 1 870-2 450 m, Sierra de las Minas and Yalijux: 2 000-2 500 m). To compare the most common species between the five sites, we use the species detected by Komar (2002) at least once in a four-hour period of intensive searches at Montecristo; the species which were labeled numerous in Gomez de Silva et al. (1999) or common in Parker III et al. (1976) in El Triunfo; the species with a dominance >1% in Yalijux (Renner 2003); and the species classified as common and fairly common in Sierra de las Minas (Nájera 2004). From our data from the Montaña Caquipec we classify species with a relative abundance value of >0.28 per 100 m of transect as common, which equals 1 bird per 360 m of transect sampled (4 hours) and is therefore comparable with the effort of Komar (2002). Five of the most common species have been recorded at all sites: Rufous-browed Wren (Troglodytes rufociliatus), Gray-breasted Wood-Wren (Henicorhina leucophrys), Ruddy-capped Nightingale-Thrush (Catharus frantzii), Golden-browed Warbler (Basileuterus belli), and Common Bush-Tanager (Chlorospingus ophthalmicus). The Spotted Nightingale-Thrush (Catharus dryas), classified as common in Montecristo and El Triunfo, was not recorded or was classified as vagrant in all of the Guatemalan high-altitude cloud forest sites (one observation at Caquipec, P. Kaestner pers. comm., Robbins and Dowell 1992, Renner 2003). In Alta Verapaz, this species is common at lower elevations (<1 500 m, Eisermann, unpub. data). Although the cloud forest avifauna of northern Central America (eastern Chiapas, Guatemala, El Salvador and Honduras) has been described as similar based on regional species lists (Hernández-Baños et al. 1995), there are differences between the five cloud forest sites. Figure 3 shows the similarity of the bird communities of Caquipec, Yalijux, Sierra de las Minas, El Triunfo and Montecristo, taking into account all species recorded within the forest except Nearctic-Neotropical migrants and overhead transients. Yalijux is located ~11 km to the east of Caquipec. Both sites are connected by a narrow corridor of cloud forest, and bird communities are very similar. Higher dissimilarity seems to result from longer distances between sites. The Sierra de las Minas is located at a distance of ~35 km from Caquipec, separated by the Polochic valley. An interchange between bird populations of both sites has been observed on the Resplendent Quetzal (Pharomachrus mocinno), which moved apparently from the Sierra de las Minas eastwards to the Sierra de Chuacús and from there to Caquipec and Yalijux (Paiz 1996). The Sierra de los Cuchumatanes is an altitudinal corridor between Caquipec and El Triunfo, which is ~270 km away. The high dissimilarity of the bird community at Montecristo (Fig. 3, ~135 km from Caquipec), is probably caused by isolation from the Chiapas-Guatemalan highlands. Current descriptions of northern Central American cloud forest sites are insufficient to explain differences in the composition of the bird communities. All papers present data from short-term studies, thus annual or long-term population fluctuations are unknown. Long-term monitoring of bird populations and their habitats is necessary in order to identify factors that affect populations.
We observed a remarkably high relative abundance of the Amethyst-throated Hummingbird (Lampornis amethystinus) at Caquipec (0.99 birds per 100 m transect, dominance 5.9%, Tab. 2). In Montecristo, no hummingbird was recorded with a dominance >5% of all individuals (data from Komar 2002). In South American cloud forests, hummingbirds have been recorded within the most abundant species. Poulsen and Krabbe (1998) recorded three hummingbird species at some study sites above 3 000 m with more than 5% dominance. The highest dominance was recorded for the Tyrian Metaltail (Metallura tyrianthina) at the Chaucha site with 11.6%. Terborgh (1977) has shown that the dominance of the nectarivorous guild increases toward higher elevations and attributed this phenomenon to a more abundant and more constant supply of food resources in higher altitudes and a lack of food resources for other species.
Human Impact
Because there are no historical data available on the bird community of the Montaña Caquipec, the human impact on it is difficult to assess. Thirty nine species were recorded exclusively in open habitat and indicate increased species richness throughout the whole area, because this kind of habitat did not exist in the research area before human settlement. We assume that human activity has had some negative effects on species richness through the reduction of forest size and hunting. The Horned Guan (Oreophasis derbianus), for example, was not found in the current study. This endemic cloud forest cracid is very sensitive to disturbance, especially hunting (Howell and Webb 1995, González-García et al. 2001), and was still reported for the region of Cobán until the first half of the 20th century (Hellmayr and Conover 1942). Unfortunately no exact geographic data are available, but these reports are probably from Caquipec, since this mountain range is accessible by road since long and it is the highest mountain in Alta Verapaz. Thiollay (1984) and Peres (2000) have demonstrated that even low intensity hunting can heavily affect game populations and their natural predators, which could explain the absence of the Horned Guan, and the low abundance of the Highland Guan (Penelopina nigra) in the study area (Table 2).
In other tropical regions, little similarity was found between commercially exploited forest and pristine forest (Newmark 1991, Thiollay 1992, Mason 1996). In Caquipec, the high degree of similarity between the bird communities of undisturbed and disturbed forest indicates that the subsistence use of forest resources by Qeqchi communities may have low impact on the overall species composition.
Conservation
The Northern Central Amerian Highland, which includes the mountains of Chiapas, Guatemala, El Salvador and Honduras, is a recognized endemic bird areas (Bibby et al. 1992b, Stattersfield et al. 1998, Brown and Kappelle 2001). In the current study, 42 (~30%) of the recorded species are endemic to Mesoamerica and 14 species (~10%) are endemic to the Northern Central American Highlands. Twelve species of the latter group were recorded in cloud forest (Tables 2 and 3). Four species recorded at Caquipec are listed in the Red List of globally threatened species (BirdLife International 2004): Highland Guan (Penelopina nigra: Near Threatened), Resplendent Quetzal (Pharomachrus mocinno: Near Threatened), Pink-headed Warbler (Ergaticus versicolor: Vulnerable), and the migratory Golden-cheeked Warbler (Dendroica chrysoparia: Endangered). The high percentage species with a restricted range and the presence of four globally threatened species identify the Montaña Caquipec as an important area for bird conservation and emphasize the high importance of Guatemalan high-altitude cloud forests for conservation. Conservation efforts in these forests are insufficient in Guatemala. Although the Guatemalan system of protected areas covers almost 30% (31 930 km2) of the country (CONAP 2003), only ~2 235 km2 are cloud forests (~7% of protected areas). These include the Sierra de las Minas, some smaller areas in the southern volcanic belt, the Biotopo del Quetzal in Baja Verapaz and areas in the western highlands. Humid montane forests of the northernmost mountain chain of Guatemala are underrepresented in the system of protected areas. In the northern part of the department of Alta Verapaz there are only 5 protected areas with cloud forest. Their sizes are rather small, ranging from 48 to 1 366 ha, for a total of 26 km2 (CONAP 2003). We assume that such small areas will not support viable populations of many bird species. Altitudinal migration has been reported for many cloud forest bird species (Powell and Bjork 1994, Howell and Webb 1995, Paiz 1996, Winker et al. 1997). This has led to the recognition that an extension of conservation activities along the altitudinal gradient is essential for the effective conservation of many species. Conservation activities need to be extended into rural communities (Islebe and Véliz 2001). Various local NGOs have been active at Caquipec by supporting indigenous communities with agricultural and ecotourism projects, in order to generate alternative income and reduce the rate of deforestation (Schulz and Unger 2000). At present, we are unable to evaluate the impact of alternative forest use, such as tourism, on the habitat quality of the forest. A long-term monitoring is needed to be able to evaluate population trends and human impact. Data provided here will be useful baseline data for further research.
Acknowledgments
The period of familiarization with the bird voices would have been much longer without the invaluable support of Amy Seglund and Shawn Conner during an introductory field trip. We are grateful to the staff of BIDAS / Proyecto Eco-Quetzal (Cobán, Alta Verapaz) for its technical support and to David Unger for constructive discussion during the field work. Thanks to the Qeqchi indigenous people of the community of Chicacnab for their kind cooperation. We thank Jürgen Engel for statistical advice. We appreciate critical comments on the manuscript by an unknown reviewer and Claudia Avendaño, and the improvements in English usage made by Erick Baur through the Association of Field Ornithologists program of editorial assistance. Special thanks to Peter, Gisela and Käthe Eisermann for their financial support.
Resumen
Las alturas del norte de Centroamérica han sido reconocidas como región de aves endémicas, pero se conoce poco sobre las comunidades de aves en bosques nubosos de Guatemala. De 1997 a 2001 se han detectado 142 especies de aves entre 2 000 y 2 400 msnm en el bosque nuboso y áreas agrícolas en la Montaña Caquipec (Alta Verapaz, Guatemala). El patrón de la comunidad de aves se describe por medio de censos en transectos de línea. Combinando los datos de censos en bosque prístino y bosque perturbado se concluyó que Henicorhina leucophrys es la especie más abundante, seguida en orden descendente por Chlorospingus ophthalmicus, Zimmerius vilissimus, Empidonax flavescens, Catharus frantzii y Lampornis amethystinus. El índice de Sørensen de 0.85 entre bosque prístino y bosque perturbado indica un impacto relativamente bajo de la población humana local. El ~27% de todas las especies encontradas fueron aves migratorias neárticas, siendo la más abundante Wilsonia pusilla. La Montaña Caquipec es un área importante para la conservación de aves, lo cual es indicado por la presencia de cuatro especies incluidas en la Lista Roja de IUCN (Penelopina nigra, Pharomachrus mocinno, Ergaticus versicolor, Dendroica chrysoparia) y de 42 especies endémicas de Mesoamérica, de las cuales 14 son endémicas de las alturas norteñas de Mesoamérica. Estos resultados servirán como base para un monitoreo a largo plazo.
Palabras clave: Aves, bosque nuboso, conservación, Guatemala, migración, Neartico-Neotrópico migrants, riqueza de especies.
References
AOU. 1998. The American Ornithologists Union Checklist of North American Birds. American Ornithologists Union, Washington D.C. 829 p. [ Links ]
Banks, R.C., C. Cicero, J.L. Dunn, A.W. Kratter, P.C. Rasmussen, J.V. Remsen, Jr., J.A. Rising & D.F. Stotz. 2003. Fortyfourth supplement to the American Ornithologists Union checklist of North American birds. Auk 120: 923-931. [ Links ]
Banks, R.C., C. Cicero, J.L. Dunn, A.W. Kratter, P.C. Rasmussen, J.V. Remsen, Jr., J.A. Rising & D.F. Stotz. 2004. Fortyfifth supplement to the American Ornithologists Union checklist of North American birds. Auk 121: 985-995. [ Links ]
Begon, M., J.L. Harper & C. Townsend. 1990. Ecology - individuals, populations, communities. Blackwell, Oxford. 960 p. [ Links ]
Bibby, C.J., N.D. Burgess & D.A. Hill. 1992a. Bird census techniques. Academic, London. 257 p. [ Links ]
Bibby, C.J., N.J. Collar, M.J. Crosby, M.F. Heath, C. Imboden, T.H. Johnson, A.J. Long, A.J. Stattersfield, & S.J. Thirgood. 1992b. Putting biodiversity on the map: priority areas for global conservation. International Council for Bird Preservation, Cambridge, United Kingdom. 90 p. [ Links ]
BirdLife International. 2004. Threatened birds of the world 2004. CD-Rom. BirdLife International, Cambridge, United Kingdom. [ Links ]
Brown, A.D. & M. Kappelle. 2001. Introducción a los bosques nublados del neotrópico: una síntesis regional. p. 25-40. In M. Kappelle & A.D. Brown (eds.). Bosques nublados del neotrópico. Instituto Nacional de Biodiversidad, Santo Domingo de Heredia, Costa Rica. [ Links ]
CONAP. 2003. Informe nacional de areas protegidas de Guatemala. Concejo Nacional de Areas Protegidas, Presidencia de la República de Guatemala, Guatemala. 37 p. [ Links ]
DeGraaf, R.M. & J.H. Rappole. 1995. Neotropical miratory birds: natural history, distribution, and population change. Cornell University, Ithaca, New York. 676 p. [ Links ]
Dobson, A.P. 1996. Conservation and Biodiversity. Scientific American Library, Freeman, New York. 264 p. [ Links ]
Doumenge, C., D. Gilmour, M.R. Pérez & J. Blockhus. 1995. Tropical montane cloud forests: conservation status and management issues. pp. 24-37 In L.S Hamilton, J.O. Juvik & F.N. Scatena (eds.) Tropical montane cloud forest. Springer, New York. [ Links ]
Engel, J. 1998. SSS. Rubisoft Software, Eichenau, Germany. [ Links ]
Eisermann, K. 1999. Avifaunistisch-ökologische Untersuchungen in einer Nebelwaldregion Guatemalas als Grundlage für die Entwicklung eines Biomonitoringprogramms. Thesis, Univ. of Applied Sciences, Eberswalde, Germany. 164 p. [ Links ]
FAO. 2003. State of the worlds forests 2003. Food and Agriculture Organization of the United Nations, Rome. 168 p. [ Links ]
Gómez de Silva, H.G., F. González-García & M.P. Casilla-Trejo. 1999. Birds of the upper cloud forest of El Triunfo, Chiapas, Mexico. Ornitol. Neotrop. 10: 1-26. [ Links ]
González-García, F., D.M. Brooks & S.D. Strahl. 2001. Estado de conservación de los Cracidos en Mexico y Centro América. pp. 1-50 in D.M. Brooks & F. González-García (eds.). Cracid ecology and conservation in the new millenium. Misc. Pub. Houston Mus. Nat. Science, Publ. 2. [ Links ]
Hamilton, L.S., J.O. Juvik & F.N. Scatena. 1995. The Puerto Rico tropical cloud forest symposium: introduction and workshop synthesis. pp. 1-23 In L.S Hamilton, J.O. Juvik & F.N. Scatena (eds.) Tropical montane cloud forest. Springer, New York. [ Links ]
Hellmayr, C.E. & B. Conover. 1942. Catalogue of birds of the Americas and the adjacent islands. Part 1. Field Mus. Nat. His. 13, pt. 1, no. 1. [ Links ]
Hernández-Baños, B.E., A.T. Peterson, A.G. Navarro-Sigüenza & P. Escalante-Pliego. 1995. Bird faunas of the humid montane forests of Mesoamerica: biogeographic patterns and priorities for conservation. Bird Conserv. Int. 5: 251-277. [ Links ]
Howell, S.N.G. & S. Webb. 1995. A guide to the birds of Mexico and northern Central America. Oxford Univ., New York. 851 p. [ Links ]
Islebe, G.A. & M.E. Véliz Pérez. 2001. Guatemala. p. 231-241 In M. Kappelle & A.D. Brown (eds.). Bosques nublados del neotrópico. Instituto Nacional de Biodiversidad. Santo Domingo de Heredia, Costa Rica. [ Links ]
Krebs, C.J. 1999. Ecological methodology. Benjamin/Cummings, Menlo Park, California. 624 p. [ Links ]
Kremen, C., A.M. Merenlender, & D.D. Murphy. 1994. Ecological monitoring: a vital need for integrated conservation and development programs in the tropics. Conserv. Biol. 8: 388-397. [ Links ]
Komar, O. 2002. Birds of Montecristo National Park, El Salvador. Ornitol. Neotrop. 13: 167-193. [ Links ]
Lehner, P.N. 1978. Handbook of ethological methods. Garland STPM, New York. 403 p. [ Links ]
Long, A.J. 1995. The importance of tropical mountain cloud forests for endemic and threatened birds. pp. 79-105 In L. S. Hamilton, J.O. Juvik, & F. N. Scatena (eds.) Tropical montane cloud forest. Springer, New York. [ Links ]
MAGA. 2002. Atlas de Guatemala. CD-Rom. Ministerio de Agricultura, Ganadería y Alimentación, Guatemala. [ Links ]
Mason, D. 1996. Responses of Venezuelan understory birds to selective logging, enrichment strips, and vine cutting. Biotropica 28: 296-309. [ Links ]
Magurran, A.E. 1991. Ecological diversity and its measurement. Chapman and Hall, London. 179 p. [ Links ]
Mühlenberg, M. 1993. Freilandökologie. Quelle & Meyer, Heidelberg, Germany. 512 p. [ Links ]
Nájera, A. 2004. Diversidad y estructura de la avifauna en el bosque nuboso primario de Albores, Sierra de las Minas, Guatemala. Thesis, Univ. del Valle de Guatemala, Guatemala. 77 p. [ Links ]
Navarro S., A.G. 1992. Altitudinal distribution of birds in the Sierra Madre del Sur, Guerrero, Mexico. Condor 94: 29-39. [ Links ]
Newmark, W.D. 1991. Tropical forest fragmentation and the local extinction of understory birds in the eastern Usambara Mountains, Tanzania. Conserv. Biol. 5: 67-78. [ Links ]
Paiz, M.C. 1996. Migraciones estacionales del Quetzal (Pharomachrus mocinno mocinno de la Llave) en la Sierra de las Minas y sus alrededores: implicaciones para su conservación. Thesis, Univ. del Valle de Guatemala, Guatemala. 109 p. [ Links ]
Parker III, T.A., S. Hilty & M. Robbins. 1976. Birds of the El Triunfo cloud forest, Mexico, with notes on the Horned Guan and other species. American Birds 30: 779-782. [ Links ]
Peres, C.A. 2000. Effects of subsistence hunting on vertebrate community structure in Amazonian forests. Conserv. Biol. 14: 240-253. [ Links ]
Primack, R.B. 1993. Essentials of conservation biology. Sinauer, Sunderland, Massachusetts. 375 p. [ Links ]
Primack, R.B. & R.T.Corlett. 2005. Tropical Rain Forests - An Ecological and Biogeographical Comparison. Blackwell, Oxford, United Kingdom. 336 p. [ Links ]
Poulsen, B.O. & N. Krabbe. 1998. Avifaunal diversity of five high-altitude cloud forests on the Andean western slope of Ecuador: testing a rapid assessment method. J. Biogeography 25: 83-93. [ Links ]
Ponciano, J.M. 1998. Comunidades de aves en función de la distancia con la frontera agrícola en la Sierra de las Minas, Guatemala. Thesis, Univ. del Valle de Guatemala, Guatemala. 118 p. [ Links ]
Powell, G.V.N. & R.D. Bjork. 1994. Implication of altitudinal migration for conservation strategies to protect tropical biodiversity: A case study of the Quetzal (Pharomachrus mocinno) at Monte Verde, Costa Rica. Bird Conserv. Int. 4: 161-174. [ Links ]
Renner, S. 2003. Structure and diversity of cloud forest bird communities in Alta Verapaz, Guatemala, and implications for conservation. Diss. Thesis, Georg-August-Univ. Göttingen, Germany. 101 p. [ Links ]
Rice, W.R. 1989. Analyzing tables of statistical tests. Evolution 43: 223-225. [ Links ]
Richards, P.W. 1996. The tropical rain forest. Cambridge University, Cambridge, United Kingdom. 575 p. [ Links ]
Robbins, C.S. & B.A. Dowell. 1992. Report on bird survey at Sierra de las Minas, March 1992. Patuxent Wildlife Research Center. U.S. Fish & Wildlife Service, Laurel, Maryland. 22 p. [ Links ]
Schulz, U. & D. Unger. 2000. Integration von Landnutzung und Regenwaldschutz: eine Fallstudie aus Guatemala. p. 94-105. In R. Detsch (ed.) Aktuelle Strömungen in Landschaftsnutzung und Naturschutz. Wissenschaft & Technik, Berlin. 306 p. [ Links ]
Stattersfield, A.J., M.J. Crosby, A.J. Long & D.C. Wege. 1998. Endemic bird areas of the world: priorities for biodiversity conservation. BirdLife Conservation Series No. 7. BirdLife International, Cambridge, United Kingdom. 864 p. [ Links ]
Stiles, F.G. & A.F. Skutch. 1989. A guide to the birds of Costa Rica. Comstock/Cornell Univ., Ithaca, New York. 511 p. [ Links ]
Stotz, D.F., J.W. Fitzpatrick, T.A. Parker III, & D.K. Moskovits. 1996. Neotropical birds: ecology and conservation. Univ. of Chicago, Chicago. 481 p. [ Links ]
Terborgh, J. 1977. Bird species diversity on an Andean elevational gradient. Ecology 58: 1007-1019. [ Links ]
Thiollay, J.-M. 1984. Raptor community structure of a primary rain forest in French Guiana and effects of human hunting pressure. J. Raptor Res. 18: 117-122. [ Links ]
Thiollay, J.-M. 1992. Influence of selective logging on bird species diversity in a Guianan rain forest. Conserv. Biol. 6: 47-63. [ Links ]
Wege, D.C. & A.Long. 1995. Key areas for threatened birds in the Neotropics. Birdlife Conservation Series No. 5. Birdlife International, Cambridge, United Kingdom. 311 p. [ Links ]
Weyl, R. 1980. Geology of Central America. Borntraeger, Berlin und Stuttgart. 371 p. [ Links ]
Wilson, E.O. 1992. The Diversity of Life. Belknap, Harvard Univ., Cambridge, Massachusetts. [ Links ]
Winker, K., P. Escalante, J.H. Rappole, M.A. Ramos, R.J. Oehlenschlager & D.W. Warner. 1997. The evolution and conservation of Wetmores Bush-Tanager: periodic migration and lowland forest refugia in a "sedentary" Neotropical bird. Conserv. Biol. 11: 692-697. [ Links ]
Internet reference
McAleece, N., J. Lambshead, G. Patterson & J. Gage. 1997. Biodiversity Professional. The Natural History Museum and The Scottish Association For Marine Science. (available online at http://www.sams.ac.uk, site accessed 7 June 2005). [ Links ]