Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.53 n.3-4 San José Sep. 2005
Biomass production and nutritional value of Artemia sp. (Anostraca: Artemiidae) in Campeche, México
Teresita D.N.J. Maldonado-Montiel & Leticia G. Rodríguez-Canché*
Facultad de Ciencias Químico Biológicas, Universidad Autónoma de Campeche, Av. Agustín Melgar s/n Campeche 24030, Campeche, México. Tel +52 (981) 81 1 98 00 x 73002 Fax +52 (981) 81 198 00 x 73099
* Centro de Estudios de Desarrollo Sustentable y Aprovechamiento de la Vida Silvestre. Av. Agustín Melgar s/n Campeche 24030, Campeche, México. Tel +52 (981) 81 1 98 00 x 62500 Fax +52 (981) 81 198 00 x 62599; lgrodrig@mail.uacam.mx
Received 23-IV-2002. Corrected 13-X-2004 Accepted 10-XII-2004
Abstract: Biomass of the crustacean Artemia sp. has multiple uses. The biochemical composition and biomass production of Artemia grown from cysts produced by a native population from Real de Salinas were evaluated under laboratory conditions. Nauplii (instar I) were stocked at density of 10 nauplii/ml in 1.5 l tanks, fed with rice bran from day 2 to day 6, and with the microalgae Tetraselmis suecica from day 7 to day 15. At the end of the trial (day 15) the average length was 5.34 mm, biomass production was 15.72 g/l (wet weight), and survival was 79%. The proximal analysis and biochemical composition of Artemia biomass indicated that its nutrient percentages are closely similar to Artemia from other regions, making this species a suitable food for cultured fish and crustacean. Rev. Biol. Trop. 53(3-4): 447-454. Epub 2005 Oct 3.
Key words: Artemia, biomass production, biochemical composition, brine shrimp, culture, Mexico.
In Mexico, aquaculture activities are currently expanding. Campeche is a coastal state in southern Gulf of Mexico where aquaculture is gaining importance. Some endemic finfish and crustacean species seem to have aquaculture potential, but live food availability is one of the major constraints for the culture development of these species. Studies on native Artemia populations represent an alternative for the exploitation of natural resources favoring also the development of the local aquaculture industry. Potentially, Artemia is an excellent food source, which could provide quality feed for fish and crustaceans (Sorgeloos 1980) in sufficient amounts and at the proper times for the growing aquaculture industry of this country.
Both Artemia nauplii and adults have the great advantage of satisfying the nutritional requirements of a wide variety of organisms. (Espinosa-Fuentes et al. 1997). However, compared with freshly hatched nauplii, the nutritional value of on-grown and adult Artemia is superior (Léger et al. 1986).
As feed for cultured crustaceans, Artemia adults provide additional benefits as they have been used for induction, reinforcement of sexual maturation and for increasing of fertilization rates (Naessens et al. 1997, Wouters et al. 1998). Artemia biomass can also be applied as a dietary ingredient or gustatory attractant in artificial diets for fish and crustacean larvae.
The bioencapsulation technique provides interesting opportunities for using Artemia biomass not only as food attractant, but also as carrier for administration of various products to the predator, such as essential nutrients, pigments, hormones, and prophylactic or therapeutic agents (Léger et al. 1986, Majack et al. 2000, Malpica Sanchez et al. 2004.).
Controlled cultivation of Artemia biomass have distinct advantages over open cultivation; outdoors systems are subjected to environmental variables, resulting in changing culture conditions and fluctuating growth rates. In comparison, controlled cultivation has no environmental or space restrictions, facilitating production of specific growth stages (i.e. juveniles, pre-adults and adults) and, allowing greater quality control and harvesting that can be controlled to meet the needs and preferences of the predator species (Dhont et al. 1993).
Since selecting an appropriate Artemia population for cultivation depends on factors such as feed conversion efficiency, growth rate and protein content (Sorgeloos 1980). The aim of the present study was to evaluate, under laboratory conditions, the growth, biomass quality, and production of a local Artemia population from Real de Salinas, Campeche, México.
Materials and methods
Decapsulation and system setup: Artemia sp. (Anostraca (Sears 1817) Artemiidae (Grochowski 1896) (Leach 1819)) cysts were collected in the natural saltmarshes from Real de Salinas, Campeche, México using plastic spoons. Samples were filtered through a mesh (500 µm) in order to eliminate coarse debris. The cysts obtained were placed in buckets containing water from the saltmarshes previously filtered (100 µm). Once in the laboratory, the cysts were soaked into a 300g/l NaCl solution in order to dehydrate the cysts and eliminate impurities by gravity. This process was repeated to ensure that all coarse debris was removed.
Cysts were rinsed in freshwater and placed in conical containers also with freshwater. The purpose was to remove lighter particles by floatation. Cysts were collected at the bottom of the recipients using a fine mesh (150 µm) and then the mesh was gently squeezed in order to remove the excess of water. Cysts were then dried in a feed dryer at <40°C (Castro and De Lara 1991). Decapsulation was carried out in a solution of sea water:sodium hypochloride (1:1) (Castro, personal comm.) The cysts were incubated in seawater for 24 h. The resulting nauplii were stocked at 10 nauplii/ml into fresh conical recipients containing 1 L seawater (experimental units). The seawater in the units was previously filtered through 1 µm cartridge filter. The experimental units (three) were kept at room temperature and under constant aeration ensuring sufficient oxygenation and maintaining the feed in suspension. Dissolved oxygen, water temperature (YSI model 51 oxymeter), salinity (SR-1 compensated refractometer), and pH (pocket meter BOE-570 Boeckel) were recorded daily during the trial.
Feeding routine: Artemia were starved during the first 24 hr in order to allow yolk resorption. The nauplii were fed with a rice bran suspension (DAgostino 1980, Intriago and Jones 1993) from day 2 to 6 (5 days) of the trial. This suspension was prepared with 3 g of rice bran micronized with a screen (100 µm) and suspended in 1 L of seawater. Consequently it was homogenized using a kitchen blender and filtered (30 µm) before being cold stored (Dobbeleir et al. 1980). From day 7, the organisms were fed with the micro-algae Tetraselmis suecica at 200 000 cells/ml (Ahmadi et al. 1990, De Roeck-Holtzhauer et al. 1993, Odile et al. 1994) until the end of the trial at day 15 (9 days). The microalgae were cultivated in Guillard f2 medium (Guillard 1975). Rice bran particles and algal cells were counted using a hematocytometer. The feed volumes were calculated with the following formula modified from Alfonso (1993):
Required feed concentration-Unconsumed feed
FV= x Experimental unit volume
Feed concentration-Unconsumed feed
FV= Feed volume
To determine the weight of microalgae consumed during the trial, 50 ml aliquots (n=6) of T. suecica were filtered through precom-busted, pre-weighed fiberglass filters. These filters were then washed with distilled water and dried at 100°C for 4 hr. They were then weighed again in order to determine the dry weight of the microalgae cells. The quantity of rice bran administered was calculated from the proportion of rice bran included in the suspension (i.e. 3 g rice bran/1 l sea water). Feed conversion rate (FCR) (New 1987) and specific growth rate (SGR) were calculated (Lavens and Sorgeloos 1991).
Data collection: Average length was measured at the beginning of the trial, and daily from day 8 to day 15. For each measurement, 30 organisms were removed from the experimental units and the length was measured from the top of the head to the base of the caudal furca (Amat 1979), using an optical microscope (first measurement) or a dissection microscope equipped with a micrometric ruler.
Artemia total biomass was measured on days 1, 8, 11, 13 and 15. In order to weight the Artemia, the whole water volume of each experimental unit was filtered. On day 1, 100 µm filters were used and 200 µm filters for the remaining days. Excess water was removed with a cloth and the wet weight recorded with a 0.01 g precision analytical balance.
Proximal and biochemical analysis: In order to determine the nutritional value of the Artemia obtained in laboratory conditions, a proximal (AOAC 1984), amino acid and fatty acid analysis were carried out on the previously dried (<40°C) biomass. Amino acid analysis was carried out using high pressure liquid chromatography in a cation exchange resin, diluted with a pH gradient. (Beckman 1985). Fatty acid analysis was performed by gas chromatography. A Varian Aerograph 1400 series chromatographer was used with a 1/8" diameter by 1.5 m long column, an OV-101 packing at 1.5% with 0.5 ml of sample dissolved in chloroform and programmed temperature range 140°C to 270°C with increments of 6°/minute (McNair and Bonalli 1969). A biochemical analysis was also carried out for the wild Artemia sp. following the methodology described above.
Results
Average length on day 1 was 0.45 (± 0.03) mm and average maximum length was 5.24 (± 0.51) mm on day 15 (Fig. 1). Biomass increased from 0.280 g (initial total average) to 15.72 (± 1.05) g (final average) in 15 days (Fig. 2). A survival of 79%, a feed conversion rate of 0.25:1 and a specific growth rate of 1.35 were obtained. The rice bran particles/ml administered to the Artemia cultures were 250 000 particles/ml, out of which the consumption was 756 ml, equivalent to 1.89 x 108 particles. The amount of microalgae administered to the Artemia cultures were 200 000 cells/ml, out of which the consumption was 44.16 l, equivalent to 8.83 x 109 algal cells.
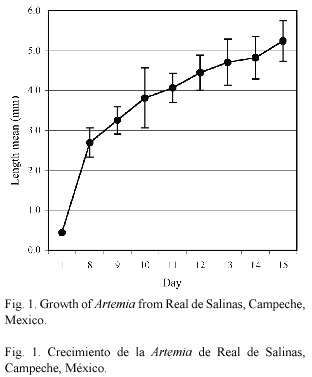
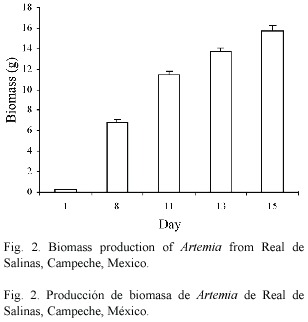
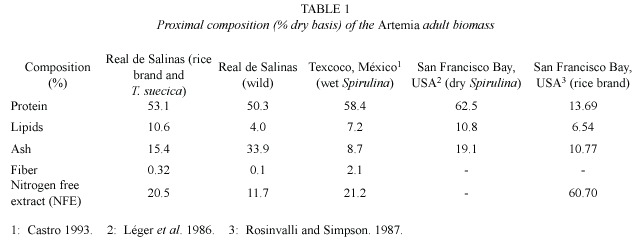
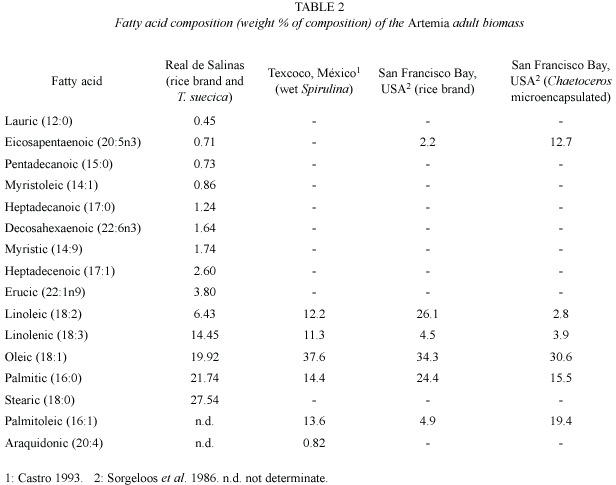
Table 1 shows the results of the proximal biomass analysis for both cultivated and wild Artemia from Real de Salinas, México. Table 2 shows the fatty acid composition, and Table 3 the amino acid composition of the cultivated Artemia. For comparison, data from other studies obtained with Artemia populations from Sosa Texcoco, México, and San Francisco Bay, U.S.A., have been included in the Tables.
Water quality in this study was considered optimal since no wide variations were observed for any of the variables. 5 mg/l of oxygen was recorded, a pH range of 7.7-8.5, temperature between 25-30°C, and salinity between 29-36 g/l.
Discussion
Dhont and Lavens (1996) have suggested that for an adequate production of Artemia in controlled conditions, water quality parameters should be maintained within an optimal range (salinity between 32-65 g/l, oxygen above 2 mg/l, temperature between 19-25°C, and pH between 6.5-8). The values recorded in this study indicated that the temperature was relatively higher and salinity lower than the reported as optimal by Dhont and Lavens (1996). However, these conditions did not seem to have limited Artemia biomass production in this study.
Dhont and Lavens (1996) reported than the final survival and biomass production of Geat Salt Lake Artemia cultures at the same density of this trial and using micronized feeds and live algae, was lower (72 % and 11.6 g/L, respectively) than this trial (79% and 15.72 g/L, respectively).
The survival (79%) after 15 days of growth in this trial feeding only 9 days with T. suecica is comparable with the best survival (80% and 90%) of Artemia after 23 days of culture feeding with the marine micoalga Phaeodactylum ticornutum with different nutrient concentrations (Fabregas et al. 1998) and the survival (65%) after 7 days of growth of Artemia fed with Tetraselmis sp (Luong-Van et al. 1999).
Espinoza-Fuentes et al. (1997) made experimental assays where Artemia is feeding with dry Spirulina. The density was of 6 nauplius/ ml. The biomass obtained in 15 days was 32.87-36.16 g /20 L (1.8 g / 1 L). Even if the density was lower than this trial, the biomass obtained in this trial was much higher and the food used cheaper than the Spirulina.
It seems that use of rice bran during the first five days of cultivation was adequate in the present trial. The carbohydrates in the rice bran seem to have contributed to the growth of Artemia since this species requires abundant carbohydrates during the first days of development (Johnson 1980).
The proximal analysis revealed that the protein content (%) was relatively similar to the wild Artemia and those reported for Artemia fed only on rich protein sources such as fresh Spirulina (Castro 1993) and dry Spirulina (Léger et al. 1986). Although wild Artemia lacks an external protein source, their natural protein content might be originated from feed sources that thrive in their natural environment.
However, the protein obtained in this study was 3.8 times higher than that obtained for A. franciscana when fed upon rice bran only (Rosinvalli and Simpson 1987). It is likely that the high protein content of Artemia in this study was positively influenced by the inclusion of T. suecica during the last 9 days of the experiment. These results suggests that the diet (carbohydrate and protein sources) used here were in good balance.
The similarity in lipids percentage between the cultivated Real de Salinas (10.6%) and San Francisco bay populations fed only on dry Spirulina (10.8%) (Léger et al. 1986) is noteworthy. However, it is important to emphasize that the feeds administered in the present study (rice bran and T. suecica) are more economic than the dry Spirulina used for the San Francisco population.
The ash content of the wild Artemia was higher (50%) than the content obtained for their experimentally raised homologues at the end of the experiment. This is probably consequence of the wild populations feeding regime, which is mainly based on organic particulate matter (OPM) that can cause ash accumulation in the telopodites and the digestive tube, thus increasing the ash proportion and lowering that of other nutritional elements (Gozalbo and Amat 1988). Nevertheless, the ash content of the Artemia grown in the laboratory in this study was not substantially different from that reported for other Artemia grown also under controlled conditions with different feed supplements (Castro 1993, Léger et al. 1986, Rosinvalli and Simpson 1987).
The effect of nutrient concentration, the factor explaining most of the variance in length and survival of the Artemia, cannot be solely explained on the basis of an increase of the protein/lipid or protein/carbohydrate ratios. Other biochemical parameters are important and responsible for the differences found in the cultures of Artemia (Fabregas et al. 1998)
Sakamoto et al. (1982) have argued that the biochemical composition of the Artemia biomass reflects the diet administered. Eicosapentaenoic (EPA) and decosaexaenoic (DHA) fatty acids are considered essential components of the diet of marine organisms (Kanazawa et al. 1979, Watanabe 1993). Eicosanoid production from arachidonic acid (n-6 fatty acid) is modulated by EPA, and failure to supply these two essential fatty acid in the appropriate balance may result in adverse biochemical responses when fed to the predator organisms (Sargent 1995 in Smith et al. 2002). In the present study EPA reached a value of only 0.71%, and DHA of 1.64%. Lavens and Sorgeloos (1991) found that when Artemia was cultivated with agricultural subproducts, the harvest contained small amounts of EPA and DHA acid. Therefore, it is reasonably to assume that the low amounts of EPA and DHA found here are likely to be due to the use of rice bran in the diet. This is also true for the cases of the linoleic and linolenic acid, which were found in relatively high concentrations.
Léger et al. (1986) has suggested that other factors such as the developmental stages of the animals, population-based differences, and the type, quality and quantity of food available can also affect the fatty and amino acid composition in Artemia. To discern the effect of such factors on the fatty acid composition found here is beyond the aims of this study.
The biochemical composition of Artemia fulfills the nutritional requirements of aquaculture freshwater species (Watanabe et al. 1978, 1980). However, the low content of some fatty acids may limit its use as live food for marine species. Bioencapsulation could be used to enrich the Artemia (Sakamoto et al. 1982) in order to make it suitable for marine species or to be used in specific formulated diets.
When the essential amino acid (EAA) profile of the Artemia biomass from Real de Salinas are compared with those obtained for Artemia from Sosa Texcoco, México as San Francisco, USA, it becomes clear that seven out of eight EAA are higher in the former.
This comparison may be more meaningful with the cultivated Sosa Texcoco population, since these were fed with the highly nutritive and expensive Spirulina. If the comparison is made between the EAA Artemia values of the present study and the EAA requirements for crustacean and fishes (Tacon 1987), it can be seen that the nutritional requirements of most aquaculture domesticated animals would be totally or almost totally fulfilled using this Artemia sp. as feed or as part of a balanced diet.
Tacon (1987) has stated that the protein quality of feed ingredients depends chiefly on their amino acid composition and its biological availability. However, the quality of the feed cannot be determined by just a chemical analysis.
A high protein level alone has no relation to the quality of the feed. The real quality of the feed must be evaluated from the growth, feed conversion and survival of the target species (C.P. Shrimp News 1995).
The SGR, the FCR, the survival and the high nutritional value of Artemia observed in this study, coupled with the simple technology used, strongly suggest that the mixed feeding regime of rice bran and T. suecica under given culture conditions are adequate for biomass production of this population of Artemia with great potential for aquaculture applications
Acknowledgments
The authors thank Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO) for financial support and Universidad Nacional Autónoma de México (UNAM) for providing the facilities. We also thank Miguel Olvera Novoa and Heréndira Cortés Torres for comments and corrections on the manuscript, and for language advise.
Resumen
El crustáceo Artemia spp. tiene múltiples usos en acuicultura. El potencial de producción de biomasa de Artemia sp. de Real de Salinas, Campeche, México en condiciones controladas es desconocida. En el presente trabajo, se evaluó la producción de biomasa de la población de Real de Salinas en condiciones de laboratorio y su composición bioquímica. Los nauplios (instar I) fueron sembrados a una densidad de 10/ml en unidades experimentales (tres) de 1.5 l. Del día 2 al 6 del experimento, los animales fueron alimentados con salvado de arroz y del día 7 al final del ensayo (día 15) con la microalga T. suecica. La longitud total promedio de Artemia al final del ensayo fue de 5.34 mm, la producción de biomasa fue de 15.72 g/l (peso húmedo) y una sobrevivencia de 79%. El análisis proximal y la composición bioquímica de la biomasa de Artemia, indicó que el porcentaje de nutrientes son adecuados para ser utilizada como alimento de peces y crustáceos.
Palabras clave: Artemia, producción de biomasa, composición bioquímica, camarón de la salmuera, cultivo, México.
References
Ahmadi, M.R., H. Leibovitz & K.L., Simpson.1990. Nutrient composition of the Iranian brine shrimp (Artemia uromiana). Comp. Biochem. Physiol. 95B: 225-228. [ Links ]
Alfonso, E. 1993. Larvicultura. Manual del II curso internacional de producción de postlarvas de camarones peneidos del Atlántico de América. Facultad de Ciencias, UNAM, México, D.F. p. 39-79. [ Links ]
Amat, F.D. 1979. Diferenciación y distribución de las poblaciones de Artemia (Crustáceo branquiópodo) de España. Tesis Doctoral. Universidad Barcelona, Barcelona, España. [ Links ]
AOAC. Association of Official Analytical Chemists, 1984. Arlington, VA., 1141 p. [ Links ]
Beckman. 1985. The system 6300 series high performance aminoacids analyzer. Instruction manual. Spinco Division of Beckman, Division Instrument, Palo Alto, California. [ Links ]
Castro, T.B. 1993. Biología y cultivo de Artemia francisacana en el ex Lago de Texcoco, de Ecatepec, Estado de México. Tesis Doctoral. Universidad Nacional Autónoma de México. [ Links ]
Castro, J. M. & R.A. De Lara. 1991. Manual de Técnicas para el manejo de quistes de Artemia sp. Universidad Autónoma Metropolitana Unidad Xochimilco, México, D.F., 47 p. [ Links ]
C.P. Shrimp News. 1995. How well do you know your shrimp feed. C.P. Group. C. P. Shrimp News 3. [ Links ]
DAgostino, A. 1980. The vital requirements of Artemia: physiology and nutrition, p. 55-82. In G. Persoone, P. Sorgeloos, O. Roels & E. Jaspers (eds.). The Brine Shrimp Artemia, Universa Press, Wetteren, Belgium. [ Links ]
De Roeck-Holtzhauer, Y., C. Claire, F. Bresdin, L. Amicel & A. Derrien. 1993. Vitamin, free amino acid and fatty acid compositions of some marine planktonic microal-gae used in Aquaculture. Bot. Mar. 36: 321-325. [ Links ]
Dhont, J. & P. Lavens. 1996. Tank production and use of ongrown Artemia, p. 164-195. In P. Lavens & P. Sorgeloos (eds.). Manual on the Production and Use of Live Food for Aquaculture. FAO Fisheries Technical Paper 361, Rome. [ Links ]
Dhont, J., P. Lavens & P. Sorgeloos. 1993. Preparation and use of Artemia as food for shrimp and prawn larvae, p. 61-93. In J.P. McVey (ed.). CRC Handbook of Mariculture. Crustacean Aquaculture (2nd edition) CRC Press, Inc., Boca Raton, Florida, USA. [ Links ]
Dobbeleir, J., N. Adam, E. Bossuyt, E. Bruggeman & P. Sorgeloos. 1980. New aspects of the use of inert diets for high density culturing of brine shrimp, p. 165-174. In G. Persoone, P. Sorgeloos, O. Roels & E. Jaspers (eds.). The Brine Shrimp Artemia. Ecology, Culturing, Use in Aquaculture. Vol. 3. Universa, Wetteren, Belgium. [ Links ]
Espinosa-Fuentes, A., A. Ortega-Salas & A. Laguarda-Figueras. 1997. Two experimental assays to produce biomass of Artemia franciscana (Anostraca). Rev. Biol. Trop. 44(3): 565-572. [ Links ]
Fabregas, J., Otero, A., Morales, E.D., Arredondo-Vega, B.O., Patiño, M. 1998. Modification of the nutritive value of Phaeodactylum tricornutum for Artemia in semicontinuous cultures. Aquaculture 169: 167-176. [ Links ]
Gozalbo, A. & F. Amat. 1988. Composición bioquímica de biomasas silvestres de Artemia (Crustácea, Branchiopoda, Anostraca). Invest. Pesq. 52 (3): 375-385. [ Links ]
Guillard, R.R.L. 1975. Culture of phytoplancton for feeding marine invertebrates, p. 29-60. In W. L. Smith & M. H. Chanley (eds.). Culture of marine invertebrate animals. Plenum, New York, NY. [ Links ]
Intriago, P. & D. Jones. 1993. Bacteria as food for Artemia. Aquaculture 113: 115-127. [ Links ]
Johnson, D. 1980. Evaluation of various diets for optimal growth and survival of selected life stages of Artemia, p. 185-192. In G. Persoone, P. Sorgeloos, O. Roels, & E. Jaspers (eds.). The Brine Shrimp Artemia, Vol. 3. Universa, Wetteren, Belgium. [ Links ]
Kanazawa, A., S. Teshima & M. Endo. 1979. Relationship between essential fatty acid requirements of aquatic animals and the capacity for bioconversion of linolenic acid to highly unsaturated fatty acids. Comp. Biochem. Physiol. 63 B: 295-298. [ Links ]
Lavens, P. & P. Sorgeloos. 1991. Production of Artemia in culture tanks, p. 317-350. In R.A. Brown, P. Sorgeloos & C.N.A. Trotman (eds.). Artemia Biology. CRC, Boca Raton, Florida, USA. [ Links ]
Léger, P.H., D.A. Bengtson, K.L. Simpson & P. Sorgeloos. 1986. The use and nutritional value of Artemia as a food source. Oceanogr. Mar. Biol. Ann. Rev. 24: 521-623. [ Links ]
Luong-Vang, T., S.M. Renaud, D.L. Parry. 1999. Evaluation of recently isolated Australian tropical microalgae for the enrichement of the dietary value of brine shrimp, Artemia nauplii. Aquaculture 170: 161-173. [ Links ]
Malpica Sanchez, A., T. Castro Barrera, H. Sandoval Trujillo, J. Castro Mejía, R. DeLara Andrade & G Castro Mejía. 2004. Composición del contenido de ácidos grasos en tres poblaciones mexicanas de Artemia franciscana de aguas epicontinentales. Rev. Biol. Trop. 52: 297-300. [ Links ]
Majack, T.J., M.B. Rust, K.C. Massee, G.W. Kissil, R.W. Hardy & M.E. Peterson. 2000. Bioencapsulation of erythromicin using adult brine shrimp, Artemia franciscana (Latreille). J. Fish. Dis. 23: 71-76. [ Links ]
Mc Nair, H.M. & E.J. Bonalli. 1969. Basic gas chromatography. Varian Instrument, Division California. [ Links ]
Naessens, E., P. Lavens, L. Gómez, C.L., Browdy, K. McGovern-Hopkins, A.W. Spencer, K. Kawahigashi, & P. Sorgeloos.1997. Maturation performance of Penaeus vannamei cofed Artemia biomass preparations. Aquaculture 155: 87-101. [ Links ]
New, M.B. 1987. Feed and feeding of fish and shrimp. Aquaculture Development and Coordination Programme. ADCP/REP/87/26. United Nations Development Programme. Food and Agriculture Organization of the United Nations, Rome, 275 p. [ Links ]
Odile, S.M., C. Claire, A. Derrien, L. Coiffard & D. Roeck-Holtzhauer. 1994. Fatty acid composition of some marine microalgae. Phytochemistry 36 (3): 691-693. [ Links ]
Rosinvalli, P.C. & K.L. Simpson. 1987. The brine shrimp Artemia as a protein for humans, p. 503-514. In P. Sorgeloos, D. Bengston, W. Decleir and E. Jaspers (eds.). Artemia Research and its Applications. Vol. 3 Ecology Culturing. Use in Aquaculture. Wetteren, Belgium. [ Links ]
Sakamoto, M., L. Holland & D.A. Jones. 1982. Modification of the nutritional composition of Artemia by incorporation PUFAs using micro-encapsulated diets. Aquaculture 28: 311-320. [ Links ]
Smith, G.G., A.J.Ritar, Ch. F. Phleger, M.N. Nelson, B. Mooney, P.D. Nichols P.R. Hart. 2002. Changes in gut content and composition of juvenile Artemia after oil enrichment and during starvation. Aquaculture 208: 137-158. [ Links ]
Sorgeloos, P. 1980. The use of brine shrimp Artemia in Aquaculture, p. 25-45. In G. Persoone, P. Sorgeloos, O. Roels & E. Jaspers (eds.). The Brine Shrimp Artemia. Vol. 3. Ecology, Culturing, Use in Aquaculture, Universa Press, Wetteren, Belgium. [ Links ]
Tacon, A.G.J. 1987. Nutrición y Alimentación de Peces y Camarones Cultivados. Manual de Capacitación. 1. Nutrientes Esenciales. GCP/RLA/075/ITA. FAO, Brasilia, Brasil, 116 p. [ Links ]
Watanabe, T., F. Oowa, C. Kitajima & S. Fujita. 1978. Nutritional quality of brine shrimp, Artemia salina, as a living feed from the viewpoint of essential fatty acids for fish. Bull. Jap. Soc. Sci. Fish. 44: 1115-1121. [ Links ]
Watanabe, T., F. Oowa, C. Kitajima & S. Fujita. 1980. Relationship between dietary value of brine shrimp Artemia salina and their content of w3 highly unsaturated fatty acids. Bull. Jap. Soc. Sci. Fish. 46: 35-41. [ Links ]
Watanabe, T. 1993. Importance of decosahexaenoic acid in marine larval fish. J. World Aquacult. Soc. 24: 152-161. [ Links ]
Wouters R., Molina, C., Lavens, P. & Calderón, J. 2001. Lipid composition and vitamin content of wild female Litopenaeus vannamei in different stages of sexual maturation. Aquaculture 198 (3-4): 307-323. [ Links ]














