Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.52 suppl.1 San José Sep. 2004
in Bahía de La Paz, Gulf of California
I. Gárate-Lizárraga1 , J.J. Bustillos-Guzmán2 , K. Erler3 , M.S. Muñetón-Gómez1 , B. Luckas3 & A. Tripp-Quezada1
1 Centro Interdisciplinario de Ciencias Marinas, IPN, Apdo. Postal 592, C.P. 23000, La Paz, Baja California Sur, México; igarate@ipn.mx
2 Centro de Investigaciones Biológicas del Noroeste. Apdo. Postal 128, La Paz, Baja California Sur, México.
3 Friedrich-Schiller University, Faculty of Biology and Pharmacy, Department of Food Chemistry, Dornburgerstraße 25, 07743 Jena, Germany.
Recibido 13-VIII-2004. Corregido 30-VIII-2004. Aceptado 01-IX-2004.
Abstract
Occurrence and toxic profiles of paralytic shellfish toxins (PST) in the chocolata clam Megapitaria squalida were investigated. From December 2001 to December 2002, 25 clams were obtained monthly from Bahía de La Paz, Gulf of California. Additionally, net (20 µm) and bottle phytoplankton samples were also collected to identify toxic species. Toxins were analyzed by HPLC with post-column oxidation and fluorescence detection. Toxicity in the clam was low and varied from 0.14 to 5.46 µg/STXeq/100 g. Toxicity was detected in December, March, April, June, and August. Toxin profile was composed mainly by STX, GTX2, GTX3, dcGTX2, dcGTX3, C2, dcSTX and B1. Gymnodinium catenatum was the only PST-producing dinoflagellate identified in the phytoplankton samples throughout the study period. G. catenatum was observed mainly in net samples from December 2001 to December 2002; however, in bottle samples, G. catenatum was only observed in five months. Highest abundance (2 600 cells l-1 ) was observed in March and the lowest (160 cells l-1 ) in June. G. catenatum mainly formed two-cell chains and rarely four or eight. The presence of PST in net phytoplankton samples support the fact that G. catenatum is the main source of PST in the clams. This study represents the first report of PST toxins in the chocolata clam from Bahía de La Paz.
Key words: Megapitaria squalida, Gymnodinium catenatum, PST, La Paz Bay, Gulf of California.
Palabras clave: Megapitaria squalida, Gymnodinium catenatum, PST, Bahía de La Paz, Golfo de California.
Paralytic shellfish poisoning (PSP) is probably better known than other shellfish poisonings (diarrheic, neurotoxic, and amnesic) and is a significant public health concern worldwide. PSP is usually a consequence of eating toxic bivalve shellfish that have ingested, by filter feeding, large quantities of toxic dinoflagellates in plankton. In México, few cases of PSP have been recorded. The first detected case of PSP food poisoning from shellfish was in the late 1970s in Bahía de Mazatlán, a coastal lagoon in the south-eastern Gulf of California (Mee et al. 1986). The PSP vector was attributed to the dinoflagellate Gymnodinium catenatum Graham. Thereafter, isolated reports of PSP in oysters, clams, and scallops have been reported for several coastal lagoons, Bahía de Mazatlán, Manzanillo, Bahía Concepción, and Acapulco (Cortés-Altamirano et al. 1999, Figueroa-Torres and Zepeda-Esquivel 2001, Gárate-Lizárraga et al. 2004). Besides G. catenatum as a causative species, other dinoflagellates, such as Alexandrium catenella (Whedon and Kofoid) Balech, and Pyrodinium bahamense var. compressum (Böhm) Steidinger, Tester & Taylor, have been linked to PSP episodes (Cortés-Altamirano et al. 1996, Sierra-Beltrán et al. 1996, Cortés-Altamirano and Alonso-Rodríguez 1997, Mancilla-Cabrera et al. 2000, Gárate-Lizárraga et al. 2004). Also, a recent massive mortality of nauplii and adult shrimp occurred in February-May 2001, coinciding with G. catenatum red tides (Alonso-Rodriguez and Páez-Osuna 2003).
Records of phytoplankton blooming species in Bahía de La Paz are scarce and the species responsible for such blooms are the protozoan Mesodinium rubrum Lohman, dinoflagellates Gonyaulax polygramma Stein, Prorocentrum rhathymum Loeblich III, Sherley & Schmidt, Scrippsiella trochoidea (Stein) Loeblich, and Cochlodinium polykrikoides Margalef, and the diatoms Rhizosolenia debyana Peragallo, and Chaetoceros debilis Cleve (Gárate-Lizárraga and Martínez-López 1997, Gárate-Lizárraga et al. 2001, 2003). No paralytic shellfish toxin producer species have been previously recorded in this bay, however, we believe that the lack of a regular monitoring program is the cause for the absence of PSP records. Herein we present the first evidence of paralytic shellfish toxins (PST) in the chocolata clam (Megapitaria squalida Sowerby) and the presence of G. catenatum in this bay.
Material and methods
Bahía de La Paz is the largest bay on the western side of the Gulf of California and clams were collected in natural schools near El Mogote sand bar (24º1031 N, 110º21 W) (Fig. 1). Twenty five specimens of the chocolata clam (Megapitaria squalida) were monthly collected by scuba diving, always in the same site sampling, from December 2001 to December 2002. Clams were labeled and stored in ice and shipped to the laboratory, where they were stored at -20°C until analysis. Simultaneously, surface water temperature was recorded using a Kalhlsico thermometer. Surface net (20 µm mesh) and bottle samples were also monthly collected to identify toxic species and count cells. Samples were fixed with a lugol solution. Some net phytoplankton samples were filtered through GF/F Whatman filters and frozen, in order to analyze paralytic shellfish toxins.
About 200-250 mg of shellfish tissue (fresh material) was homogenized and stored at 4º C before analysis. Extraction of PSP toxins were done by adding 4 ml acetic acid (0.03 N) to 200 mg shellfish tissue and sonicated (35 kHz) for 5 minutes in a ice bath and then clarified by centrifugation (3 000 rpm for 5 min) and filtration of the supernatant with a single-use syringe filter (0.45 µm). An aliquot (150 µl) of the clarified extract was mixed with hydrochloric acid (1 N; 35 µl) and heated for 15 min (90°C) to convert N-sulfocarbomoyl toxins into their related carbomoyl toxins (Yu et al. 1998). Finally, 10 µl of the extracts (with and without hydrolysis) were injected into the HPLC system. The chromatographic system consisted of an AS-4000 intelligent autosampler and L-6200A intelligent pump (both Merck-Hitachi), two LC-9A pumps (Shimadzu) used for delivery of post column reaction solutions, an RF551 fluorescence detector (Shimadzu), an 1 ml CRX390 post-column reaction unit (Pickering Laboratories) a D-6000 HPLC-manager (Merck-Hitachi), and a 250 x 4.6 mm column packed with 5 µm Supelcosil-C18 DB (Supelco No. 58355). PSP toxins were detected using the excitation and emission wave-length 333 nm and 390 nm, respectively.
Chromatography was performed as previously described (Yu et al. 1998). Briefly, an ion-pair buffer gradient composed of a solution of octanesulfonic acid and ammonium phosphate at pH 6.9 and acetonitrile to separate PST (paralytic shellfish toxins). After post-column oxidation with alkaline periodic acid, the resulting products were detected with a fluorescence detector. Identification of PSP toxins was carried out by comparing chromatograms obtained from sample extracts with those resulting after injection of standard solutions obtained from the National Research Council Canada. Quantification of PSP toxins content was carried out by comparing peak areas in chromatograms of sample extracts with corresponding calibration graphs. PSP content of clams examined were expressed as µg STXeq 100 g soft tissue as well as molar percent (mol %).
Phytoplankton bottle samples were sedimented in 25 ml settling chambers and examined with a phase contrast inverted microscope (Hasle 1978). Toxic species abundance and it identification was made simultaneously. Phytoplankton collected by net hauls was examined exhaustively with a phase contrast microscope. Special emphasis was paid to identification of PSP toxin producers or blooming species, using standard reference works (Dodge 1982, Fukuyo et al. 1990, Balech 1988, Steidinger and Tangen 1996).
Results
Diatoms and dinoflagellates were by far the most important phytoplankton groups. From dinoflagellates, the only red tide blooming species were: Alexandrium affine (Inoe and Fukuyo) Balech, Ceratium furca (Ehrenberg) Claparède et Lachmann, Gonyaulax digitalis (Pouchet) Kofoid, G. catenatum, Prorocentrum micans Ehrenberg, Scrippsiella trochoidea and C. polykrikoides. G. catenatum was the only PSP toxin producing dinoflagellate identified. This species had not been previously reported in this bay and was observed mainly in net samples from December 2001 to December 2002; however, in bottle samples G. catenatum was only during five months. Highest abundance (2 600 cells l-1 ) was observed in March and the lowest (160 cells l-1 ) was observed in June. G. catenatum mainly forming two-cell chains and rarely four-cell chains.
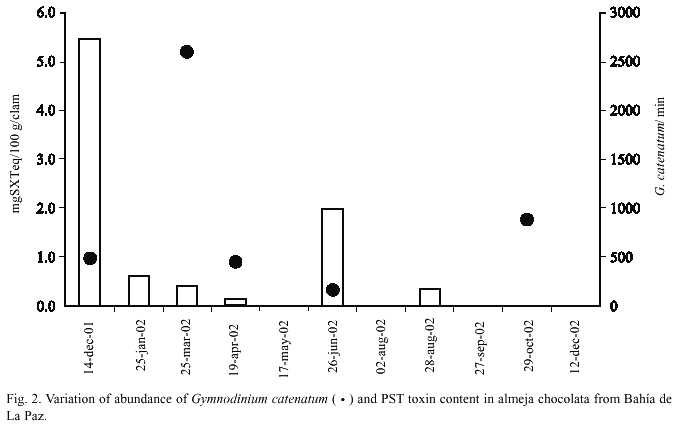
PST were found only during six months and the toxicity level in the chocolata clams varied from 0.14 to 5.46 µg STX equivalent/100 g soft tissues (Fig. 2). These values are very low compared to the regulatory limit (80 µg STX equivalent/100 g soft tissue) proposed by the US Food and Drug Administration. Because G. catenatum was the only PSP toxin producing dinoflagellate identified in the phytoplankton samples, toxicity in clams are most probably linked to it. The low toxicity values found in clams in coincidence with low abundances of G. catenatum also supports this suggestion.
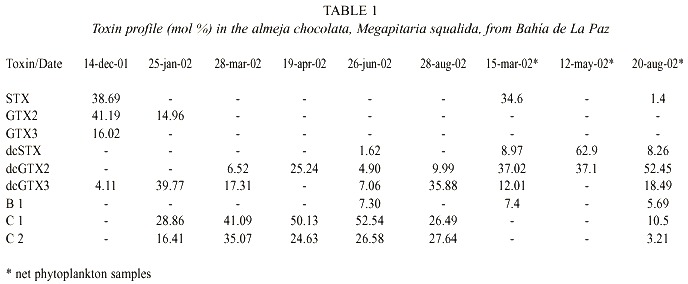
Toxin profiles in clams include nine toxins (Table 1). From these, the more potent toxins STX (Saxitoxin), GTX2 (Gonyautoxin-2), and GTX3 (Gonyautoxin-3) were dominant (more than 90% on a molar basis) in the first sample date. Thereafter, the C1 (N-sulfo-gonyautoxin-1) and C2 (N-sulfo-gonyautoxin-2) from the group of the N-sulfocarbamoyl toxins were the more important toxins, contributing in some cases more than 79% of the total toxin content. The B1 (N-sulfo neo-saxitoxin) toxin was also present and represented less than 8% on a molar basis of the total toxin contents. The decarbamoyl gonyautoxin 2 and 3 (dcGTX2 and dcGTX3) were the second toxin group in order of importance contributing from 4.11 to 39.77% and from 4.9 to 25.2% of the total toxin content, respectively. Molar contribution of the decarbamoyl-saxitoxin (dcSTX) was observed only in June. Toxin profile in net phytoplankton samples was composed by seven toxins, being dcGTX2, dcGTX3 and B1 common in both kind of samples (Table 1).
Discussion
G. catenatum is a naked dinoflagellate broadly distributed along the Pacific coast of México and has been linked to paralytic shellfish poisoning and human intoxications (Mee et al. 1986), however, this is the first record of this species in Bahía de La Paz. The abundances of G. catenatum in this bay were very low when comparing to those abundance values reported in the previous studies in the Gulf of California (Mee et al. 1986, Cortés-Altamirano et al. 1999). This suggests that environmental conditions were not appropriate for its proliferation (Fraga et al. 1998) although G. catenatum occurred at temperatures ranging from 18 to 26°C, similar to the temperature range reported for Bahía de Mazatlán and Bahía Concepción (Cortés-Altamirano et al. 1999, Gárate-Lizárraga et al. 2001).
Paralytic toxin profile in clams was composed by nine toxins. C1 and C2 toxins were the most important toxins, contributing in some cases more than 79% of the total toxin content. In a molar basis, DcGTX2 and DcGTX2 toxins were the most important analogues in net phytoplankton samples. The presence of C1, C2, and DcGTX2, has been reported for several G. catenatum strains from the region of Bahía Concepción (a coastal bay situated in the middle of the Baja California Peninsula), and in a molar basis, represented more than 80% of the toxins (Band-Schmidt et al. 2004). These results and the singular presence of G. catenatum in seawater samples also suggest that this dinoflagellate is the cause of the toxicity in the clam samples. This fact was corroborated by comparing the toxin profile observed in net phytoplankton samples with these found in clams. However, other PSP producers may be acting as vector as it is suggested by the presence of GTX2 and GTX3 at the beginning of the sampling period. Recent personal observations in net phytoplankton samples collected in Bahía de La Paz revealed the presence of Alexandrium monilatum (Howell) Taylor, A. tamiyavanichii Balech and A. catenella (Whedon and Kofoid) Balech, three toxic species not previously recorded in this area. Unfortunately, long-term records of phytoplankton from the bay are unavailable to corroborate this hypothesis.
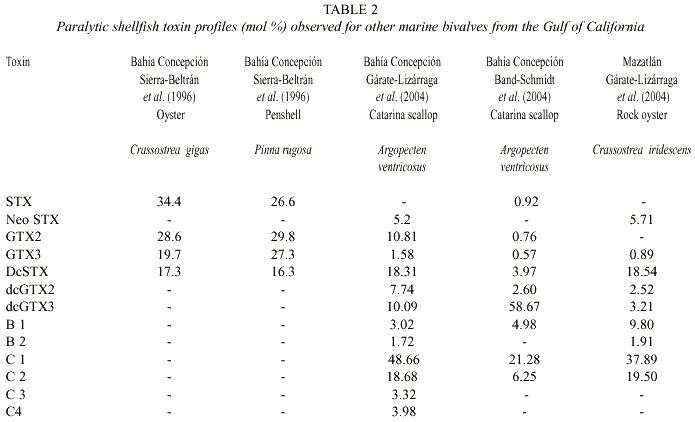
When comparing the toxin profiles in our shellfish samples with records from other coastal lagoons around the Gulf of California where G. catenatum is also present, it is clear that they are quite similar (Table 2). Seven toxins were recorded in common in these samples: GTX3, dcSTX, dcGTX2, dcGTX3, B1, C1, and C2. STX (38.69 mol %) was present in the chocolata clam, oysters and clams and NeoSTX (Neo-saxitoxin) was only found in samples of catarina scallop, and rock oyster from Bahía Concepción and Bahía de Mazatlán (Gárate-Lizárraga et al. 2004). The toxin profiles are quite different from shellfish that fed naturally with A. catenella (Sierra-Beltrán et al. 1996). This variability may reflect interspecific differences in the metabolism of ingested toxins or the different sources of toxins. The only report of the presence of these latter two toxins in marine scallops being linked to G. catenatum is from Takatani et al. (1998), who found NeoSTX (0.1% mol) and STX in very low molar percentage (0.1) in marine bivalves from Japan.
When bivalves recent ingest toxin-containing dinoflagellates, they typically contain high proportions of C1-C2 toxins (Oshima et al. 1989, 1990, Mackenzie and Beauchamp 2000, Gárate-Lizárraga et al. 2004). The clam M. squalida showed a high proportion of N-sulfocarbamoyl toxins (between 16.4 to >50%), suggesting recent ingestion of PSP-containing organisms or a low capacity to transform carbamate toxins (Hummert et al. 1997). The high content of decarbomoyl (dc) derivatives, toxins known to be obtained through enzymatic conversion from other PSP toxin types in shellfish (Bricelj and Shumway 1998, Jaime et al. 2002), in the scallop, and in its presumable prey, G. catenatum, support the hypothesis of a low toxin bioconversion capacity of this scallop.
This is the first record of PST present in this bay and in the chocolata clams. These results are important since seashell management regulation for the Gulf of California does not exist and therefore the presence of toxins, up to now in low concentrations, may be considered. The low values of PST recorded explain the null intoxication in shell consumption organisms from this area. However, in places were incidence of G. catenatum is higher, as in the Bahía de Mazatlán, PSP is also more frequent (Mee et al. 1976, Gárate-Lizárraga et al. 2004). This clam species is distributed from the Laguna Ojo de Liebre, México to Mancora, Perú (Keen 1971) where human population consume this mollusk. This wide distribution offers an excellent opportunity to study and characterize toxins in different regions linked to G. catenatum or other paralytic shellfish toxin producers. Our group continues monitoring studies of toxic phytoplankton and paralytic toxins in other bivalves of commercial importance.
Acknowledgments
This work was supported by the Instituto Politécnico Nacional (CEGEPI grants 20010320; 200203333). Partial financial support was also provided by DLR (German Center for Air and Airspace) and Consejo Nacional de Ciencia y Tecnología (México) (Project "Biología y Filogenia Molecular de Dinoflagelados del Pacífico Mexicano". I.G.L. is COFFA, EDI and CONACyT (Fellowship grant 138138). The text was improved by the CIBNOR editing staff. We also thank Clara Ramírez Jaúregui for her collaboration in facilitating the bibliography and Rosalba Alonso for her criticisms of the manuscripts.
Resumen
Se investigó la ocurrencia así como los perfiles de toxinas paralíticas (PST) en la almeja chocolata Megapitaria squalida (Sowerby, 1835), de la cual se recolectaron mensualmente 25 ejemplares de diciembre del 2001 a diciembre del 2000 en La Bahía de La Paz, Golfo de California. Simultáneamente, se obtuvieron muestras de fitoplancton de botella y de red (20 µM) para identificar especies tóxicas, así como para detectar la presencia de toxinas paralíticas. Las toxinas se analizaron por HPLC con una oxidación post-columna y detección fluorescente. La toxicidad en las almejas fue baja y varió de 0.14 a 5.46 µg/STXeq/100 g y se detectó en diciembre, marzo, abril, junio y agosto. El perfil de toxinas estuvo compuesto principalmente por STX, GTX2, GTX3, dcGTX2, dcGTX3, C2, dcSTX y B1, siendo similar al perfil observado en las muestras de red. Gymnodinium catenatum fue la única especie productora de toxinas paralíticas identificada en las muestras de fitoplancton a través de todo el período del estudio. G. catenatum se observó principalmente en muestras de red correspondientes a diciembre 2001-diciembre 2002; sin embargo, en muestras de botella, G. Catenatum solo fue observado en cinco meses. La abundancia más alta (2 600 céls l-1 ) se encontró en marzo y la más baja (160 céls l-1 ) en junio. G. catenatum se presentó formando cadenas de dos células y raramente de cuatro u ocho. La presencia de toxinas en las muestras de red donde esta especie es frecuente, apoya la idea de que G. catenatum sea la fuente principal de PST en las almejas. Este estudio representa el primer reporte de toxinas paralíticas en la almeja chocolata de Bahía de La Paz.
References
Alonso-Rodríguez, R. & F. Paéz-Osuna. 2003. Nutrients, phytoplankton and harmful algal blooms in shrimp ponds: a review with special reference to the situation in the Gulf of California. Aquaculture 219: 317-336. [ Links ]
Band-Schmidt, C.J., J.J. Bustillos-Guzmán, I. Gárate-Lizárraga, C. Lechuga-Deveze, K. Reinhardt & B. Luckas. 2004. Profiles of paralytic shellfish toxin in strains of the dinoflagellate Gymnodinium catenatum and the scallop Argopecten ventricosus from Bahía Concepción, Gulf of California, Mexico. Harmful Algae (In press). [ Links ]
Balech, E. 1988. Los Dinoflagelados del Atlántico Sudoccidental. Publ. Espec. Inst. Esp. Oceanogr. Nº 1. Madrid, pp. 310. [ Links ]
Bricelj, V.M. & S.E. Shumway. 1998. Paralytic shellfish toxins in bivalve molluscs: occurrence, transfer kinetics, and biotransformations. Rev. Fish. Sci. 6: 315-383. [ Links ]
Cortés-Altamirano, R. & R. Alonso-Rodríguez. 1997. Mareas rojas durante 1997 en la Bahía de Mazatlán, Sinaloa, México. Cien. Mar. 15: 31-37. [ Links ]
Cortés-Altamirano, R., D.U. Hernández-Becerril & R. Luna-Soria. 1996. Red tides in México: Areview, pp. 101-105. In T. Yasumoto, Y. Oshima & Y. Fukuyo (eds.). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission, UNESCO, Sendai, Japan. [ Links ]
Cortés-Altamirano, R., A. Núñez-Pastén & N. Pastén-Miranda. 1999. Abundancia anual de Gymnodinium catenatum Graham dinoflagelado tóxico de la costa este del Golfo de California. Cien. Mar 35: 51-56. [ Links ]
Dodge, J.D. 1982. Marine Dinoflagellates of the British Isles. Her Majestys Stationery Office, London. pp. 303. [ Links ]
Figueroa-Torres, M.G. & M.A. Zepeda-Esquivel. 2001. Mareas rojas del Puerto Interior, Colima, México. Sci. Nat. 3: 39-52. [ Links ]
Fraga, S., S.M. Gallagher & D.M. Anderson.1998. Chain-forming dinoflagellates: an adaptation to red tides, pp. 281-284. In T. Okaichi, D.M. Anderson & T. Nemoto (eds.). Red Tides: Biology, Environmental Science and Toxicology. Elsevier, New York. [ Links ]
Fukuyo, Y., H. Takano, M. Chihara & K. Matsuoka. 1990. Red Tide Organisms in Japan. An Illustrated Taxonomic Guide. Uchida Rokakuho, Tokyo. pp. 407. [ Links ]
Gárate-Lizárraga, I. & A. Martínez-López. 1997. Primer registro de una marea roja de Prorocentrum mexicanum (Prorocentaceae) en aguas del Golfo de California. Rev. Biol. Trop. 45: 1263. [ Links ]
Gárate-Lizárraga, I., M.L. Hernández-Orozco, C. Band-Schmidt & G. Serrano-Casillas. 2001. Red tides along the coasts of Baja California Sur, México (1984 to 2001). Oceánides 16: 127-134. [ Links ]
Gárate-Lizárraga, I., D.A. Siqueiros-Beltrones & V. Maldonado- López. 2003. First record of a Rhizosolenia debyana bloom in the Gulf of California, México. Pac. Sci. 57: 141-145. [ Links ]
Gárate-Lizárraga, I., J.J. Bustillos-Guzmán, R. Alonso-Rodríguez & B. Luckas. 2004. Comparative paralytic shellfish toxin profiles in two marine bivalves during outbreaks of Gymnodinium catenatum (Dinophyceae) in the Gulf of California. Mar. Pollut. Bull. 48: 397-402. [ Links ]
Hasle, G.R. 1978. Using the inverted microscope, pp. 196. In A. Sournia (ed.). Phytoplankton manual. UNESCO, Paris. [ Links ]
Hummert, C., M. Ritscher, K. Reinhardt & B. Luckas. 1997. Analysis of the characteristic PSP profiles of Pyrodinium bahamense and several strains of Alexandrium by HPLC based on ion-pair chromatographic separation, post-column oxidation, and fluorescence detection. Chromatographia 45: 312-316. [ Links ]
Jaime, E., G. Gerts & B. Luckas. 2002. Accumulation and detoxification of paralytic shellfish poisoning in different shellfish exposed to a toxic Alexandrium fundyense strain. Abstract. X International Conference on Harmful Algae. St. Petersburg, Florida. 139 p. [ Links ]
Keen, A.M. 1971. Sea Shells of Tropical West America. Marine Mollusks from Baja California, México to Perú. Standford University, USA. pp. 1064. [ Links ]
Mancilla-Cabrera, E., C. Ramírez-Camarena & L. Muñoz-Cabrera. 2000. Primer registro de Gymnodinium catenatum Graham (Gymnodiniaceae) como causante de marea roja en la Bahía de Acapulco, Gro., México, pp. 85-86. In R. Ríos-Jara, E. Juárez-Carrillo, M. Pérez-Peña, E. López-Iriarte, E.G. Robles-Jarero, D.U. Hernández-Becerril & M. Silva-Briano (eds.). Estudios sobre el plancton marino en México y el Caribe, Sociedad Mexicana de Planctología y Universidad de Guadalajara, México. [ Links ]
Mee, L.D., M. Espinosa & G. Díaz. 1986. Paralytic shellfish poisoning with a Gymnodinium catenatum red tide on the Pacific coast of Mexico. Mar. Environ. Res. 19: 17-92. [ Links ]
Oshima, Y., M. Hirota, T. Yasumoto, G.M. Hallegraeff, S.I. Blackburn & D.A. Steffensen. 1989. Production of paralytic shellfish toxins by the dinoflagellate Alexandrium minutum. Halim from Australia. Bull. Jap. Soc. Sci. Fish. 55: 920-925. [ Links ]
Oshima, Y., K. Sugino, M. Hirota & T. Yasumoto.1990. Comparative studies on paralytic shellfish toxin profiles of dinoflagellates and bivalves, pp. 391-396. In E. Graneli, B. Sundstrom, L. Elder & D.M. Anderson (eds.). Toxic Marine Phytoplankton. Elsevier, New York. [ Links ]
Sierra-Beltrán, A.P., M.L. Morquecho-Escamilla, C. Lechuga-Devéze & J.L. Ochoa. 1996. PSP monitoring program at Baja California Sur, Mexico, pp. 105- 108. In T. Yasumoto, Y. Oshima & Y. Fukuyo (eds.). Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris. [ Links ]
Steidinger, K.A. & K. Tangen. 1996. Dinoflagellates, pp. 387-584. In C. Thomas (ed.). Identifying Marine Diatoms and Dinoflagellates. Academic, San Diego. [ Links ]
Takatani, T., H. Akaeda, T. Kaku, M. Miyamoto, H. Mukai & T. Noguchi. 1998. Paralytic shellfish poison infestation to oyster Crassostrea gigas due to dinoflagellate Gymnodinium catenatum in Amakusa Islands, Kumamoto Prefecture. J. Food Hyg. Soc. Jap. 39: 292-295. [ Links ]
Yu, R., C. Hummert, B. Luckas, P.Y. Qian & M.J. Zhou. 1998. A modified HPLC method for analysis of PSP toxins in algae and shellfish from China. Chromatographia 48: 671-676. [ Links ]
Internet references
Mackenzie, L. & T. Beauchamp. 2001. Gymnodinium catenatum in New Zealand: a new problem for public health and the shellfish industry (Downloaded: August 17th , 2004, http://www.cawthron.org.nz/Assets/Gcat.pdf). [ Links ]