Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.52 suppl.1 San José Sep. 2004
T.R. Tosteson
Department of Marine Sciences, University of Puerto Rico-Mayaguez, Isla Magueyes Marine Station, P.O. Box 908 Lajas P.R. 00667. Tel./Fax: (787) 899-2564; cigua@aol.com
Recibido 31-X-2002. Corregido 27-VIII-2003. Aceptado 11-XII-2003.
Abstract
Analyses of ciguatoxicity in the great barracuda Sphyraena barracuda and quantity of toxic benthic dinoflagellates on coastal reefs (correlated with the number of cases of human ciguatera intoxications in Puerto Rico) were used to construct a model formulated on data obtained during the period of 1985-1988. The validity of the proposed model has been questioned by recent data obtained during the period of 1990-2000. Barracuda ciguatoxicity no longer showed a prominent seasonality while the fraction of randomly caught barracuda that were ciguatoxic significantly increased during this period. These two changes, accompanied by the discovery that ciguatoxic fish contained a variety of multiple toxins, appear to be correlated with the steadily increasing periods of elevated sea surface temperatures in this region.
Key words: Ciguatera, seasonality, sea surface temperatures, multiple toxins.
Palabras clave: Ciguatera, estacionalidad, temperatura de superificie marina, toxinas múltiples.
Ciguatera fish poisoning has been reported in the Caribbean Sea since the late 18th century (Halstead 1967). Estimates of the number of human ciguatera intoxications based on data obtained from public health services on the Caribbean islands are regarded as underestimates, for generally 0.1% or less of intoxicated persons consult a physician (Tosteson 1995). Surveys conducted in the area of Puerto Rico and the American Virgin Islands from the late 1970s through mid 1980s and more recently (1990-1996) suggest that an estimated 16 to 18 thousand people were poisoned per year during these periods (Tosteson 1995).
Seasonal fluctuations in ciguatoxicity of the great barracuda, Sphyraena barracuda, randomly caught off the southwest coast of Puerto Rico and the blooms and toxicities of benthic dinoflagellates found on the coastal reefs in this region generated a model that correlated well with fluctuations in the reported human ciguatera intoxications in Puerto Rico as a whole during the period of 1985-1988 (Tosteson et al. 1988, Tosteson 1995, Tosteson et al. 1998).
Materials and methods
Large S. barracuda (5 to 10 kg) were randomly caught in the offshore coastal waters of southwest Puerto Rico during the course of this study. The tissues of the individual barracuda were extracted and their ciguatoxicity determined in mice using reported procedures (Tosteson et al. 1988, Tosteson 1995, Ballantine et al. 1988). While data concerning the density and toxicity of benthic dinoflagellates found on the coastal reefs in the area of the Marine Station is not cited directly here, during the course of this study dinoflagellates were harvested, microscopically examined, extracted and assayed for their toxicity in white Swiss mice using reported procedures (Ballantine et al. 1988, Tosteson et al. 1989).
Sea surface temperatures (SSTs) were taken daily during the morning at the Marine Station during the course of this study. Recorded temperatures correlate well with those taken at mid-day on coastal reef sites along the southwest coast of Puerto Rico (Ballantine et al. 1988). The total number of days during which the SST was >29.5°C was noted during each of the months of this study.
Results
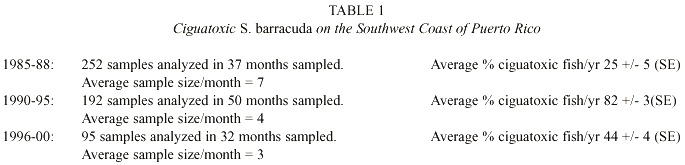
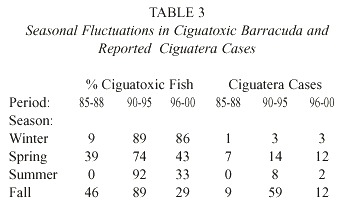
Table 1 summarizes the yearly distribution of ciguatoxicity among the 539 barracuda randomly caught off the southwest coast of Puerto Rico from 1985 through 2000. The yearly rate of barracuda ciguatoxicity increased significantly during these three sampling periods. Table 2 illustrates the correlation of barracuda ciguatoxicity with the total cases of human ciguatera intoxications reported to the Department of Health of Puerto Rico during each of the periods described in Table 1. The average percentage of ciguatoxic barracuda/year in each of the three periods sampled is linearly correlated with the average number of human ciguatera cases/year reported to the Department of Health (correlation coefficient r = 0.995). These data indicate that the yearly frequency of barracuda ciguatoxicity is a useful bioindicator of the frequency of human intoxications with ciguatera. During the initial period of this study (1985- 1988) 252 barracuda specimens were analyzed for their ciguatoxicity. There was a marked seasonal fluctuation in the frequency of barracuda ciguatoxicity. Thus, in the winter (December and January) and summer (June and July) the percentage of captured barracuda that were ciguatoxic was negligible. The average percentage of ciguatoxic barracuda caught in the spring (February, March, April and May) and fall (August, September, October and November) were 35 and 46% respectively. The original seasonal model of ciguatera was based on these data. Two major developments have altered the continued validity of this model. These changes were: (1) the gradual loss of seasonal fluctuations in percentage of ciguatoxic barracuda and (2) significant increases in the fraction of ciguatoxic barracuda caught along the southwest coast of Puerto Rico in all seasons in 1990-2000. Table 3 illustrates the change in the seasonal pattern of barracuda ciguatoxicity during the three periods of this study. The seasonality of barracuda ciguatoxicity was clearly gone in barracuda samples studied in 1990-1995 and 1996-2000. However, it is of interest to note that while not as pronounced, the seasonal fluctuations of reported cases of human ciguatera intoxications in Puerto Rico as a whole remained during both of these periods. The persistence of some seasonal fluctuations in reported cases of ciguatera poisonings may in large part be due to the eating habits of the people in Puerto Rico with respect to fish and the major holidays (i.e. Easter and Thanksgiving) in the spring and fall seasons of the year.
Discussion
Increases in the fraction of captured barracuda that were ciguatoxic and changes in the seasonal frequencies of ciguatoxic barracuda may reflect an increasing complexity of multiple toxins brought about by changes in environmental temperatures that have taken place during the period of this study. The predominant ciguatoxic fishes in the Pacific Ocean and the Caribbean Sea have multiple toxins (Legrand et al. 1992, Lewis et al. 1991, Tosteson et al. 1992, Tosteson et al. 1995, Pottier et al. 2002). The multiple toxins found in ciguatoxic fish are no doubt responsible for the broad and variable spectra of medical symptoms found in human beings intoxicated with ciguatera. Both the variety and nature of these toxins differ in fishes and marine invertebrates found in the various tropical oceans of the earth. The multiple toxins found in ciguatoxic fish in the Caribbean and the toxic benthic dinoflagellate vectors of this poisoning may be the result of variable environmental conditions and/or variable microbial symbionts in these microalgal vectors (Tosteson et al. 1992, Tosteson et al. 1995).
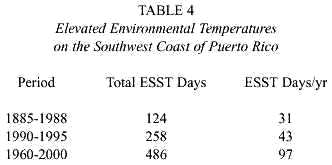
Analyses of seawater temperatures recorded at the Marine Station on the southwest coast of Puerto Rico since 1966 indicated that the six-year period of 1990-1995 was the warmest period in the last 30 years of recorded sea surface temperatures in this area (Tosteson et al. 1998). The indicator used to evaluate changes in ambient SSTs in each of the three periods of the study reported here was the number of days in which the SSTs were elevated to temperatures >29.5°C. (ESST). Table 4 summarizes the number of ESST days during each of the three study periods. The ESST days/year steadily and significantly increased during the course of this study. It is of interest to note that both the total number of reported cases of human ciguatera intoxications and the percentage of ciguatoxicity in captured barracuda also increased (Table 2).
Several factors may account for the correlation between increasing sea surface water temperatures and ciguatoxicity in fish. In general, tropical marine organisms live at the upper limits of their thermal tolerance. Thus, changes of two or three degrees in ambient temperature would be expected to produce marked responses in tropical marine biota. These responses are reflected in changes in respiration, metabolic rates, circulating hormones and predatory activity in a variety of fishes (Senthilkumaran and Joy 1995, Beddow et al. 1995). Elevated sea surface temperatures would also be expected to alter the growth patterns of the dinoflagellates and their associated microbial flora, vectors of the "ciguatoxins" in the ciguatera food chain (Ballantine et al. 1988, Pitcher et al. 1993, Oshima et al. 1993, Lewis et al. 1993). Increased temperatures (30°C) have been shown to reduce the toxicity and alter the symbiotic bacterial flora associated with laboratory cultured Ostreopsis lenticulars, the predominant toxic benthic dinoflagellate vector of ciguatera in the barracuda found along the southwest coast of Puerto Rico (Ashton et al. 2001). Changing environmental temperatures would be expected to have several important effects on the ciguatera food chain, namely, (1) alterations in the production and variety of toxins introduced by microbial vectors of ciguatoxicity into the barracuda and (2) modifications in the subsequent metabolism of these products in ciguatoxic fish, resulting in a variety of multiple toxins that subsequently produce complex ciguatera intoxications when transmitted to human consumers. The thermal envelope of the marine environment appears to have a broad and comprehensive effect on the changing paradigm of ciguatera in the Caribbean.
Acknowledgments
The author would like to thank Carmen de Seda and the staff of the Epidemiology Divison of the Departement of Health of the Commonwealth of Puerto Rico for providing the data on the reported number of cases of human ciguatera intoxications in Puerto Rico and Mayra Ashton for her help in the preparation of this manuscript.
Resumen
Pruebas de ciguatoxicidad en la gran barracuda, Sphyraena barracuda y la cantidad de dinoflagelados bentónicos tóxicos en los arrecifes de la costa están correlacionados con el número de casos de intoxicaciones por ciguatera en humanos, en Puerto Rico. Estos hechos fueron utilizados para construir un modelo que fue formulado con la información recopilada durante el período de 1985- 1988. La validez de este modelo propuesto ha sido cuestionada debido a la información recientemente obtenida durante el período 1990-2000. La ciguatoxicidad en la barracuda ya no demostraba una marcada asociación con las temporadas mientras que la cantidad de barracuda ciguatóxica capturada al azar tuvo un aumento significativo durante este período. Estos dos cambios estuvieron acompañados por el descubrimiento de que peces ciguatóxicos contienen una gran variedad de toxinas que parecen
estar relacionadas con los períodos de elevadas temperaturas en la superficie del mar. Estas temperaturas continúan aumentando de manera que a su vez continúan en un aumento estable en esta región.
References
Ashton, M., T. Tosteson & C. Tosteson. 2003. The effect of elevated temperature on the toxicity of the laboratory cultured dinoflagellate Ostreopsis lenticularis (Dinophyceae). Rev. Biol. Trop. 51 (Supl. 6): 1-6. [ Links ]
Ballantine, D.L., T.R. Tosteson, H.D. Durst & A.T. Bardales. 1988. Population dynamics and toxicity of natural populations of benthic dinoflagellates in southwest Puerto Rico. J. Exp. Mar. Biol. Ecol. 119: 201-212. [ Links ]
Beddow, T.A., J.L. Van Leeuwen & I.A. Johnson. 1995. Swimming kinematics of fast starts are altered by temperature acclimation in the marine fish Myoxocephalus scorpius. J. Exp. Biol. 198: 203-208. [ Links ]
Halstead, B.W. 1967. Poisonous and Venomous Marine Animals of the World. U.S. Government Printing Office. Washington D.C. [ Links ]
Legrand, A.M., M. Fukui, P. Cruchet, Y. Ishibashi & Y. Yasumoto. 1992. Characterization of ciguatoxins from different fish species and wild Gambierdiscus toxicus, pp. 25-32. In T.R. Tosteson (ed.). Proc. Third Inter. Conf. Cigua. Fish Poisoning, Polyscience Publications Inc., Quebec, Canada. [ Links ]
Lewis, N.I., S.S. Bates, J.L. McLachlan & J.C. Smith. 1993. Temperature effects on growth, domoic acid production, and morphology of the diatom Nitzschia pungens f. multiseries, pp. 601-606. In T.J. Smayda & Y. Shimizu (eds.). Toxic Phytoplankton Blooms in the Sea, Elsevier, New York. [ Links ]
Lewis R.J., M. Sellin, M.A. Poli, R.S. Norton, J.K. MacLeod & M.M. Sheil. 1991. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 29: 1115-1127. [ Links ]
Oshima, Y., H. Itakura, K.-C. Lee, T. Yasumoto, S. Blackburn & G. Hallegraff. 1993. Toxin production by the dinoflagellate Gymnodinium catenatum, pp. 907- 912. In T.J. Smayda & Y. Shimizu (eds.). Toxic Phytoplankton Blooms in the Sea, Elsevier, New York. [ Links ]
Pitcher, G.C., D.A. Horstman & D. Calder. 1993. Formation and decay of red tide blooms in the southern Benguela upwelling system during the summer of 1990/91, pp. 317-322. In T. J. Smayda and & Y. Shimizu (eds.). Toxic Phytoplankton Blooms in the Sea. Elsevier, New York. [ Links ]
Pottier, I., J.P. Vernoux, A. Jones & R.J. Lewis. 2002. Characterization of multiple Caribbean ciguatoxins and congeners in individual specimens of horse-eye jack (Caranx latus) by high performance liquid chromatography/ mass spectrometry. Toxicon 40: 929-940. [ Links ]
Senthilkumaran, B. & K.P. Joy. 1995. Changes in hypothalamic catecholamines, dopamine-beta- hydroxylase, and phenylethanolamine-N-methyl transferase in the catfish Heteropneustes fossilis in relation to season, raised photoperiod and temperature, ovariectomy and estradiol-17-beta replacement. Gen. Comp. Endocrin. 97: 121-134. [ Links ]
Tosteson, T.R., D.L. Ballantine & H.D. Durst. 1988. Ciguatoxic barracuda frequency in southwest Puerto Rico. Toxicon 26: 795-801. [ Links ]
Tosteson, T.R., D.L. Ballantine, C.G. Tosteson, V. Hensley & J. Bardales. 1989. Associated bacterial flora, culture growth and toxicity of the benthic dinoflagellates, Ostreopsis lenticularis and Gambierdiscus toxicus. J. Appl. Environ. Microbiol. 55: 137-141. [ Links ]
Tosteson, T.R., R.A. Edwards & D.G. Baden. 1992. Caribbean ciguatera and polyether dinoflagellate toxins: Correlation of ciguatoxin with standard toxins. pp. 89-102. In T.R. Tosteson (ed.). Proc. Third Inter. Conf. Cigua. Fish Poisoning. Polyscience Publications Inc., Quebec, Canada. [ Links ]
Tosteson, T.R. 1995. The diversity and origins of toxins in ciguatera fish poisoning. P.R. Health Sci. J. 14: 117-129. [ Links ]
Tosteson, T.R., R.F. Bard, I.M. Gonzalez, D.L. Ballantine & G.S. Bignami. 1995. Toxin Diversity in the Ciguatera Food Chain, pp. 73-87. In Y. Hokama, P.J. Scheuer & T.Y. Takeshi (eds.). Proceedings of the International Symposium on Ciguatera and Marine Natural Products Asian Pacific Research Foundation, Honolulu, Hawaii. [ Links ]
Tosteson, T.R., D.L. Ballantine & A. Winter. 1998. Sea surface temperature, Benthic dinoflagellate toxicity and toxin transmission in the ciguatera food chain, pp. 48-49. In B. Reguera, J. Blanco, M.L. Fernández & E. Wyatt (eds.). Harmful Algae. Xunta de Galicia, Spain, & IOC, UNESCO. [ Links ]