Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.2 San José Jun. 2003
Distribution and abundance of Syacium ovale larvae (Pleuronectiformes: Paralichthyidae) in the Gulf of California
Gerardo Aceves-Medina 1, Ricardo J. Saldierna-Martínez 2 & Enrique A. González 2
Received 25-IV-2001. Corrected 05-XI-2001. Accepted 10-III-2002.
Abstract
The spawning season of the tonguefish Syacium ovale (Günter 1864) was determined by an analysis of the distribution of preflexion stage larvae in the Gulf of California. The larvae were collected during eight oceanographic surveys between 1984 and 1987. The spawning of this species starts in early summer and ends at the beginning of fall, with the highest reproductive activity in mid summer. The central and southern regions of the Gulf are the most important reproductive area. Spawning is associated with high sea surface temperatures and low plankton biomass, both of which are characteristics of the tropical current that invades the study area during summer.
Keywords: Syacium ovale, ichthyoplankton, spawning areas and periods, Gulf of California.
Paralichthyidae is one of the families of pleuronectiform fishes captured with high frequency in both commercial fisheries and exploratory trawls of demersal fish (Van der Heiden 1985, Coronado and Amezcua 1988), and in plankton trawls in the Eastern Tropical Pacific (Ahlstrom 1972).
Syacium is the most abundant paralichthyidae genus (Coronado and Amezcua 1988); however, although it is considered one of the most significant potential demersal fishery resources on the continental shelf of the Gulf of California (Amezcua 1985, Van der Heiden 1985), because of its small size (25 cm maximum) its commercial use is currently limited to local consumption by artisanal or subsistence fisheries (Hensley 1995).
Among the four Syacium species in the Eastern Tropical Pacific, only S. latifrons and S. ovale are found in the Gulf of California, the last inhabiting soft bottom between 9 m and 40 m depth (Hensley 1995). The planktonic eggs and larvae are found primary in the upper few meters of the water column (Moser and Sumida 1996), and are among the most abundant ichthyoplankton taxa collected during pelagic fish larvae trawls in the Gulf of California (Ahlstrom 1972). The highest adult abundance of S. ovale in the Pacific coast of southwest of México is in spring (Amezcua 1985), but larvae are most abundant in summer (Ahlstrom 1972). The remaining biology of this species is almost unknown. In this work we analyzed the distribution and abundance of the larvae in the Gulf of California to obtain indirect data about reproductive periods and areas, and the role of temperature in this part of the S. ovale life cycle.
Materials and methods
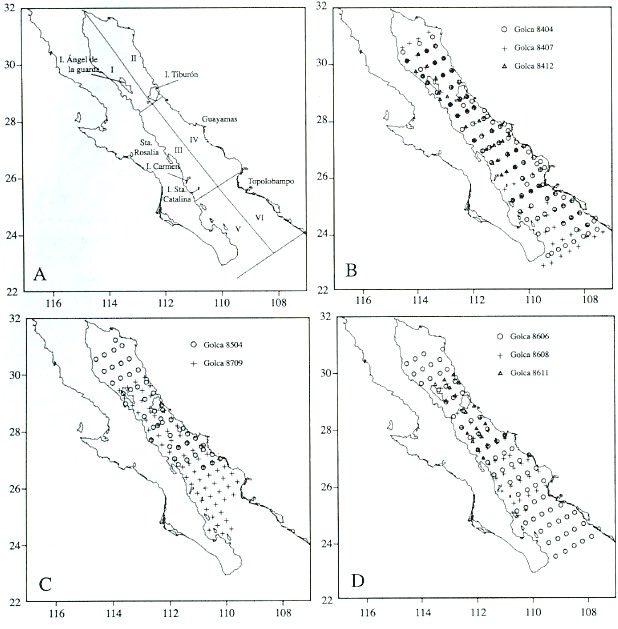
The Gulf of California is located between 23° and 32° N latitude, and has been split into several regions defined by hydrographic and topographic differences that can have an influence on distribution patterns of marine organisms. Gilbert and Allen (1943) and Round (1967) identified four latitudinal zones in the Gulf of California on the basis of oceanographic elements and phytoplankton communities. Smith et al. (1976) split the Gulf into eight regions according to sardine larval concentrations. In this work, we combined both schemes and defined six regions for the analysis of our data (Fig. 1A).
We divided the Gulf into three latitudinal regions: 1) the northern region, the head of the Gulf south to Islas Tiburón and Angel de la Guarda, characterized by shallow depths (average 200 m) compared to the central and southern regions, and a fauna primarily of temperate affinity (Brinton et al. 1986); 2) the central region, extending south to an imaginary line between north of Topolobampo and north of Isla San José; and 3) the southern region, extending south to an imaginary line between Punta San Miguel and Cabo San Lucas. Both central and southern regions have predominantly tropical fauna. A convergence zone and an eddy-like circulation pattern (Emilson and Alatorre 1980) at the common border between central and southern regions produce significant differences in phytoplankton communities (Round 1967, Brinton et al. 1986).
Each of these regions is divided into two zones, one on the eastern side characterized by having a broad continental shelf and principally sandy beaches, and the other on the western side characterized by a narrow continental shelf and rocky beaches. There are upwellings on the eastern side, principally in the central region of the Gulf during the winter months, whereas summer upwelling is present on the western coast, but of minor intensity.
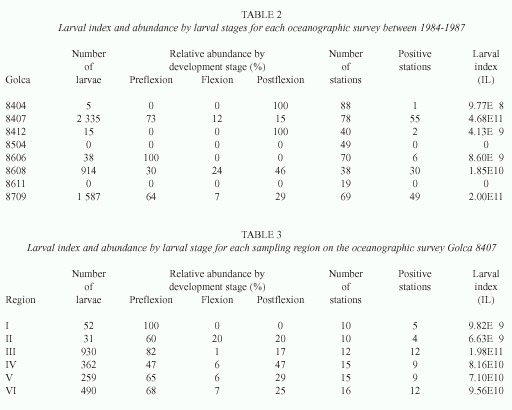
Biological material was obtained from eight oceanographic surveys with variable coverage in the Gulf of California between 1984 and 1987 (Fig. 1B-D). The March-April, July-August 1984, and June 1986 surveys (Golca-8404, -8407, and -8606) extended over the whole area from the head to the southern limit with 88, 78, and 70 sampling stations respectively. The September 1987 survey (Golca-8709) extended partially into the northern and southern region, and covered the entire central region with 69 sampling stations. The November- December 1984 and August 1986 surveys (Golca-8412 and -8608) extended from the north throughout the central region and partially into the southern region with 40 and 38 sampling stations respectively. The March-April 1985 and November 1986 surveys (Golca-8504 and -8611) covered only the northern and central regions with 49 and 19 sampling stations, respectively.
The zooplankton samples were taken using Bongo nets, with 333 µm and 505 µm mesh and flowmeters at the mouth to estimate the filtered water volume. Kramer et al. (1972) and Smith and Richardson (1979) described the sampling method in detail. Sea surface temperature (sst) was measured with a bucket frame thermometer, and plankton biomass was estimated in the laboratory using the displacement volume method (Beers 1976).
Fish larvae were separated from the zooplankton samples collected with the 505 µm mesh net. Syacium ovale larvae were identified according to Ahlstrom et al. (1984) and Moser and Sumida (1996), classified as preflexion, flexion, or postflexion developmental stage, counted, and their abundance standardized to numbers under 10 m2 of sea surface (Smith and Richardson 1979).
To identify the time of reproduction and the spawning areas of S. ovale in the Gulf of California, we could not count eggs, because the eggs are unknown. Instead, we used preflexion stage larvae. We assumed that preflexion stage larvae would be a good indicator of reproductive activity because another Paralichtydae species have a short incubation period of 72 h to 75 h at 17.5ºC in Paralichthys dentatus (Smith and Fahay 1970) and S. ovale has a short life cycle (aprox. 1 year) (Barba-Torres 1990).
For the comparative analysis of larval abundance, we calculated the larval index (IL) proposed by Smith and Richardson (1979) for the six regions. This index is given by the formula
IL= (sum Ci/NLi) * (NLi/Nti) * (U.A.)
Where:
Ci = total number of larvae in region i.NLi = number of positive stations in region i.
Nti = total number of stations in region i.
U.A.= Number of 10 m 2 areas in each region sampled.
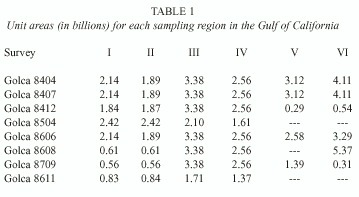
The perimeter of each station was considered as the intermediate point between the neighboring stations, which means that if the distance between stations is 20 nm, each station represents 400 nm 2 or 137*E6 U.A. The unit areas of the regions sampled for each cruise are shown in the Table 1.
Results
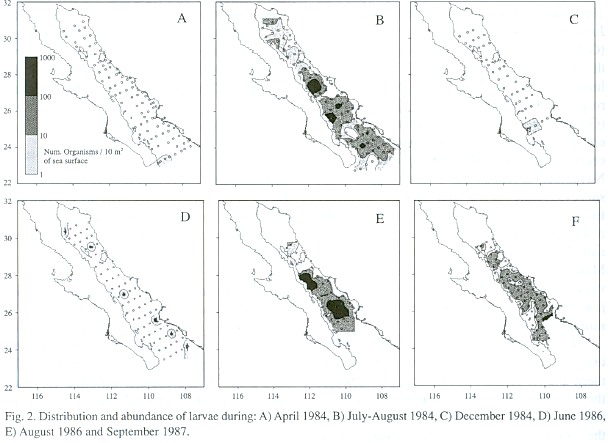
Larvae of Syacium ovale were present only in the southern portion of the Gulf during the cold periods of spring and fall (Golca-8404 and -8412); none was collected on cruises Golca-8504 and -8611 because these cruises we did not reach the southern Gulf (Fig. 2). At the beginning of summer (Golca-8606), larvae began to appear in the central region in low abundance and during the summer (Golca-8407, -8608 and -8709) they were found throughout the Gulf with higher abundance. The highest concentrations of larvae were found from the great islands to the south, especially off Santa Rosalía, Isla Carmen, Isla Sta. Catalina, and Topolobampo.
The larval index calculated for all the cruises are found in Table 2. The highest values corresponded to July-August 1984 (Golca-8407), September 1987 (Golca-8709), and August 1986 (Golca-8608). The proportion of larvae in each of the three developmental stages (Table 2) showed a high percentage of preflexion larvae in June 1986 (Golca-8606), July-August 1984 (Golca-8407), and September 1987 (Golca-8709), decreasing during August 1986 (Golca-8608). In April and December 1984 (Golca-8404 and -8412) we found the lowest larval indexes and the few specimens were in the postflexion stage.
We calculated the larval index for each region from the survey with the highest overall larval abundance (Golca-8407). The highest values were found in regions III to VI, but the central area of the Gulf (especially region III) was the most important (Table 3), at the head of the Gulf the highest values were found in the western section (region I). Although abundance was reduced at the head of the Gulf, region I had the highest proportion of preflexion larvae (Table 3).
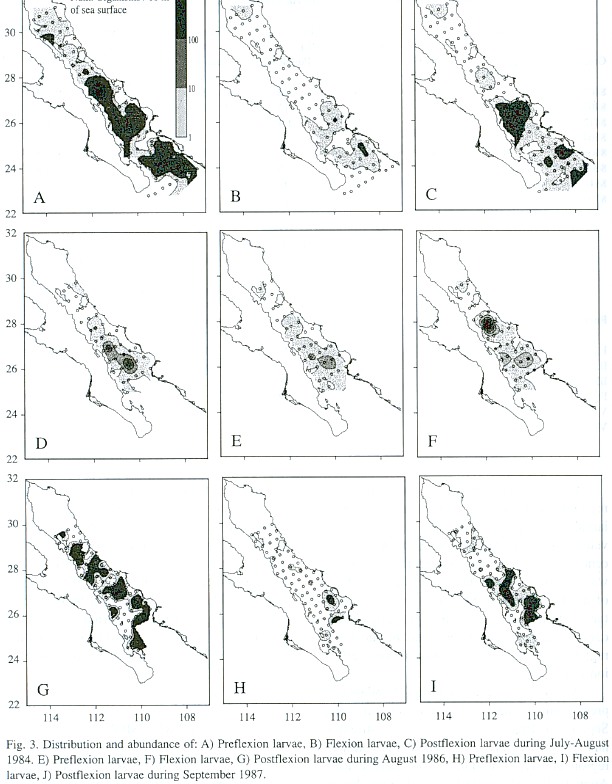
Awide distribution and high abundance of preflexion larvae were found in July and September (Fig. 3), but flexion and postflexion larvae were largely restricted to the southern region and were found in low numbers. During August 1986 (Fig. 3D-F), postflexion larvae had a higher abundance than preflexion and flexion larvae.
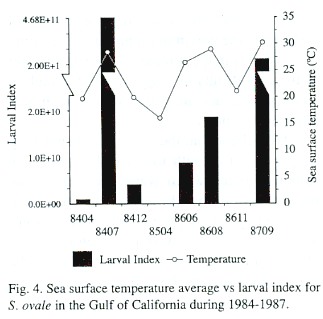
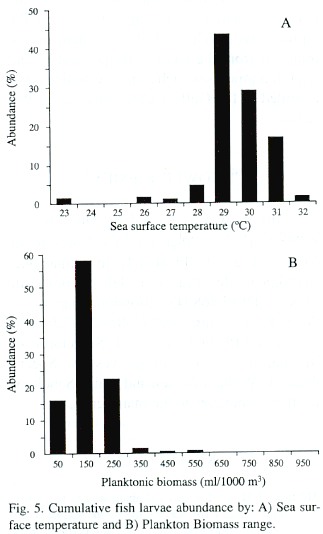
A direct relation between the larval index and surface seawater temperature was observed, with low temperatures associated with low larval index values (Fig. 4). The abundance of S. ovale larvae plotted against sst shows that though S. ovale larvae were captured between 23°C and 32°C, 73% of the specimens were found between 29°C and 30°C (Fig. 5A). The lowest planktonic biomass corresponded in general to periods of higher abundance of S. ovale, with about 70% of the larvae found between biomass values of 50 -250 mL/1 000 m 3 (Fig. 5B).
Discussion
Syacium ovale is a species of tropical affinity widely distributed along the coast of the Panamic province (Castro-Aguirre 1978), from the Gulf of California to the south of Perú (Castro-Aguirre et al. 1999). There is no detailed information about its reproductive cycle, but Amezcua (1985) recorded the presence of a high proportion of mature females in the Nayarit-Guerrero corridor, with highest abundance along the coast of Guerrero during the beginning of spring. Moser et al. (1994) and Moser and Sumida (1996) affirmed the presence of larval of S. ovale along the southern outer coast of the Baja California Peninsula during June-January, with the highest abundance between July and September. Ahlstrom (1972) and Moser et al. (1994) mentioned that during the summer of 1967 the larvae were abundant in the Eastern Tropical Pacific between Manzanillo, México and Ecuador, and although they were present during winter, their abundance was low.
The results in this work showed that in the Gulf of California larval S. ovale can be present during spring, but it is at the beginning of summer when an increase of abundance is observed. Larvae are most abundant from mid-to late summer, and abundance decreases during fall. This suggests that in the study area the adults that have matured during the spring start to spawn at the beginning of summer, have their highest reproductive activity during July-August, and finish at the end of summer or beginning of fall with isolated spawnings. This is consistent with the larval development stages: 100% of the specimens were in pre-flexion stage during June, 73% in July, 64% in September, and only 30% in August. A similar pattern was observed in S. gunteri in the Gulf of México, a species that has its principal reproductive activity from June to September (García-Abad et al. 1992).
During the spawning peak the greatest abundance of larvae is from the great islands to the south, and the proportion of preflexion larvae decreases from north to south in this area, suggesting that spawning occurs in a south-north direction as a consequence of the progressive warming of the water during spring and summer (Rosas-Cota 1977). This does not happen at the head of the Gulf, which has more temperate characteristics. There was a tendency for preflexion larvae to occur in high abundance in the region surrounding Bahía Concepción, which could be a preferred spawning area, two other areas of larval concentration, one south of Isla Carmen and one in the mouth of the Gulf, separated by low abundance areas, suggest the presence of physical processes that result in retention and concentration of larvae.
One mechanism that could be related to those nuclei of retention and concentration is the development during summer of cyclonic circulation cells which form eddies that cause convergence zones in the central and southern region of the Gulf (Emilson and Alatorre 1980).
Owen (1980, 1981), Hewitt (1981) and Fiedler (1986) have shown that similar eddies along the coasts of California and Baja California can act as mechanisms of transport and concentration of larvae and play an important role in recruitment to adult populations that spawn when these eddies are formed. Syacium ovale is a shallow water species that has not been recorded in > 70 m depth, which means that the larvae are transported from shallow spawning areas to deep areas (especially in the mouth of the Gulf of California); this should be a critical period in the life cycle. García-Abad et al. (1992) found that in the Gulf of México juvenile S. gunteri, which are benthonic, were found principally in deeper areas during March and June, suggesting that recruitment occurred there. If this happens with S. ovale, then we can speculate that adults spawn in shallow water, eggs and larvae are transported to deeper waters, maybe as a strategy to avoid the high predation in the coastal zone, and juveniles return later to the shallow water areas.
Temperature appears to play an important role in the distribution and abundance of larvae because they were captured in narrow sst intervals of temperature; almost 90% of the specimens occurred between 28 - 31°C, and 70% between 29 - 30 °C. The concentration of plankton biomass vs. larval abundance also indicates the preference of this species for spawns in environments with tropical characteristics, because the larvae were collected exclusively in areas with low plankton biomass.
We conclude that in the Gulf of California, the maximum reproductive activity of S. ovale is in the summer, primarily in water of tropical affinity as suggested by the highest larval abundance in warm waters with low plankton biomass.
We believe that the initiation of spawning progresses in a south to north direction is in response to the warm water invasion of the tropical current that flows into the study area. Accordingly, we propose that the high concentration of larvae observed in the central region of the Gulf of California is attributable to the northward shift of the reproductive activity from the southern to central region of the Gulf as the environmental conditions suitable for spawning are established, and that the highest concentrations at the center and south of the Gulf are related to a coupling between the reproductive cycle and the mechanisms of transport from the coast to deeper waters and retention processes such as the cyclonic eddies recorded in the Gulf of California during this season.
Acknowledgements
This study was funded by Dirección de Estudios Profesionales - I.P.N. through the research projects Plancton del Noroeste de México (Clave DEPI 86804), Investigaciones Ecológicas del Plancton del Noroeste de México (DEPI 868043), Bionomía Planctónica de la Parte Central del Golfo de California (Clave DEPI 903388), and Secretaria de Marina, by our use of its vessels. Special thanks to William Watson and Elaine Sandknop for their comments to the manuscript.
References
Ahlstrom, E.H. 1972. Kinds and abundance of fish larvae in the Eastern Tropical Pacific on the second multi-vessel EASTROPAC survey and observations on the annual cycle of larval abundance. Fish. Bull. 70 (4):1153-1242. [ Links ]
Ahlstrom, E.H., D.A. Hensley, H.G. Moser & B.Y.Sumida. 1984. Pleuronectiformes: Development, pp. 640- 670. In H.G. Moser, W.J. Richards, D.M. Cohen, M.P. Fahay, A.W. Kendall & S.L. Richardson (eds.). Ontogeny and systematic of fishes. Spec. Pub. Num. 1- Amer. Soc. of Icthyol. and Herpetol. Allen, Lawrence, Kansas. [ Links ]
Amezcua, L.F. 1985. Recursos potenciales de peces capturados con redes camaroneras en la costa del Pacífico de México, pp. 39-94. In A.A. Yañez-Arancibia, (ed.). Recursos pesqueros potenciales de México: La pesca acompañante del camarón. Prog. Univ. de Alim. Inst. Cienc. Mar y Limnol., Inst. Nal. Pesca. Univ. Nal. Autón. Méx. México, D.F. [ Links ]
Barba-Torres, J.F. 1990. Taxonomía, biología y ecología del lenguado Syacium ovale (Günter) en áreas del Pacífico mexicano 1982-1983 (Teleostei: Bothidae). Tesis de Maestría. Fac. de Ciencias. Univ. Nal. Autón. Méx, México, D.F. [ Links ]
Beers, J.R. 1976. Determination of zooplankton biomass, pp. 35-84. In H.F. Steedman, (ed.). Zooplankton fixation and preservation. Monographs on Oceanographic Methodology, UNESCO, Paris. [ Links ]
Brinton, E., A. Fleminger & D. Siegel-Causey. 1986. Temperate and tropical planktonic biotas of the Gulf of Callifornia. CalCOFI Rep. 27: 228-263. [ Links ]
Castro-Aguirre, J.L. 1978. Catálogo sistemático de los peces marinos que penetran a las aguas continentales de México con aspectos zoogeográficos y ecológicos. I.N.P. México, D.F. Serie Científica No. 19: 298 p. [ Links ]
Castro-Aguirre, J.L., H.S. Espinoza-Pérez & J.J. Shmitter-Soto. 1999. Ictiofauna estuarino lagunar y vicaria de México. México, D.F. LIMUSA. 711 p. [ Links ]
Coronado M., A. & F. Amezcua. 1988. Distribución y abundancia de los peces demersales de la costa de Guerrero en el Pacífico de México. An. Inst. Cienc. Mar. Limnol. Univ. Nal. Autón. México. 15(2): 67-94. [ Links ]
Emilsson I. & M.A. Alatorre. 1980. Recent investigations on the circulation in the outer part of the Gulf of California, pp. 1-24. In A. Ayala-Castañares, F.B. Phleger, R. Schwartzlose y A. Laguarda (eds.). Memorias Simposio El Golfo de California. Univ. Nal. Autón. Méx. México, D.F. [ Links ]
Fiedler, P.C. 1986. Offshore entrainment of anchovy spawning habitat, eggs and larvae by a displaced eddy in 1985. CalCOFI Rep. 27: 144-152. [ Links ]
García-Abad, M.C., A.Yañez-Arancibia, P. Sánchez-Gil & M. Tapia-García. 1992. Distribución, reproducción y alimentación de Syacium gunteri Ginsburg (Pices:Bothidae), en el Golfo de México. Rev. Biol. Trop. 39: 27-34. [ Links ]
Gilbert, J.Y. & W.E. Allen. 1943. The phytoplankton of the Gulf of California obtained by the E.W. Scripps in 1939 and 1940, J. Mar. Res. 5(2): 89-110. [ Links ]
Hensley, D.A. 1995. Paralichthyidae, pp.1349-1380. In Fischer, W., F.Krupp, W. Scneider, C. Sommer, K.E. Carpenter & V.H. Niem. (eds.). Guía FAO para la identificación de especies para los fines de la pesca. Vol. III. Vertebrados-Parte 2. O.N.U. FAO, Roma. [ Links ]
Hewitt, R. 1981. Eddies and speciation in the California current. CalCOFI Rep. 22: 96-98. [ Links ]
Kramer, D., M.J.Kalin, E.G. Stevens, J.R. Thrailkill & J.R. Zweifel. 1972. Collecting and processing data on fish eggs larvae in the California current region. NOAA Tech. Rep. NMFS Circ. 370, U.S. Dep. Of Comerce. Seatlle, WA. 38 p. [ Links ]
Moser, H.G., R.L. Charter, P.E. Smith, D.A. Ambrose, S.R. Charter, C.A. Meyer, E.M. Sandknop & W. Watson. 1994. Distributional atlas of fish larvae in the California current region: Taxa with less than 1000 total larvae, 1951 through 1984. CalCOFI Atlas 32. Scrpps Ins. Ocean. La Jolla California. 181 p. [ Links ]
Moser, H.G. & B.Y. Sumida. 1996. Paralichthyidae: Lefteye flounders and sanddabs, pp. 1325-1355. In H.G. Moser (ed.). The early stages of fishes in the California Current Region. CalCOFI Atlas No. 33. Allen, USA. [ Links ]
Owen, R.W. 1980. Eddies of the California current system: Physical and ecological characteristics, pp. 237-263. In D. Power (ed.). The California islands. Mus. Nat. Hist., St. Bárbara, California. [ Links ]
Owen, R. 1981. Fronts and Eddies in the sea: Mechanisms, interactions and biological effects, pp. 197-233. In A.R. Longhurst, (ed.). Analysis of marine ecosystems. Academic, London. [ Links ]
Rosas-Cota, A. 1977. Corrientes geostróficas en el Golfo de California en la superficie y a 200 m, durante las estaciones de invierno y verano. CalCOFI Rep. 19: 89-106. [ Links ]
Round, F.E. 1967. The phytoplankton of the Gulf of California. Its composition, distribution and contribution to the sediments. J. Exp. Mar. Biol. Ecol. 1(1): 76-97. [ Links ]
Smith, P.E., S. De la Campa & J.R. Thrailkill. 1976. Instituto Nacional de la Pesca and CalCOFI statistical regional divisions for ichthyoplankton surveys. SWFC Admn. Rep. LJ-76-31. p. 4. [ Links ]
Smith, P.E. & S.L. Richardson. 1979. Técnicas modelo para prospecciones de huevos y larvas de peces pelágicos. F.A.O. Doc. Tec. Pesca.175. París. 107 p. [ Links ]
Smith, W.G. & M. P. Fahay. 1970. Description of eggs and larvae of the summer flounder, Paralichthys dentatus. U.S. Bur. Sport. Fish. Wildlife, Res. Rep. 75, 21 p. [ Links ]
Van der Heiden, A.M. 1985. Taxonomía biología y evaluación de la ictiofauna demersal del Golfo de California, pp.149-200 In A.A. Yañez-Arancibia, (ed.). Recursos pesqueros potenciales de México: La pesca acompañante del camarón. Prog. Univ. de Alim. Inst. Cienc. Mar y Limnol., Inst. Nal. Pesca. Univ. Nal. Autón. Méx. México. [ Links ]
1 Dpto. Plancton y Ecología Marina. Centro Interdisciplinario de Ciencias Marinas-IPN. Apdo. Postal 592. La Paz, Baja California Sur, México. C.P. 23000. Fax: 122 5322; aceves651@prodigy.net.mx
2 Depto. Biología Marina. Universidad Autónoma de Baja California Sur. Apdo. Postal 19-B. La Paz, Baja California Sur, México. C.P. 23080.