Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.2 San José Jun. 2003
Larval, pre-juvenile and juvenile development of Diapterus peruvianus (Perciformes: Gerreidae)
Sylvia Patricia Adelheid Jiménez Rosenberg 1,*, Enrique González Navarro 2 & Ricardo Javier Saldierna Martínez 1,*
Received 27-VIII-2001. Corrected 27-IX-2002. Accepted 30-X-2002.
Abstract
The development of Diapterus peruvianus (Sauvage 1879) is based on 60 larvae collected in superficial tows made in Bahía Concepción, and on 16 prejuvenile and juvenile organisms collected in Bahía de La Paz, B. C. S., México, using a standard plankton net and a rectangular epibenthonic net, respectively. Larvae of D. peruvianus show three large blotches on the dorsum of the gut that can fuse together and give the appearance of one large continuous blotch. There are two to three pre-anal pigments and 16 post-anal pigments in the ventral midline; cephalic pigments are present from the postflexion stage, as well as a serrated preoperculum. The prejuvenile and juvenile organisms are distinguished by their body depth, the analfin formula, the serrated preoperculum and the base pigments in the dorsal and anal fins.
Key words: Diapterus peruvianus, Gerreidae, larvae, juvenile, development.Species of the family Gerreidae, commonly named "Mojarras", are distributed in tropical and subtropical seas and oceans, with 40 species included in about eight genera, four of which are present in Pacific American coastal waters (Deckert and Greenfield 1987, Nelson 1994).
This family is a commercially valuable resource in coastal waters, bays, lagoons and estuaries (Aguirre-León et al. 1982, Aguirre-León and Yáñez-Arancibia 1986). There are nine gerreid species on the Northwest Mexican coast, one of them is Diapterus peruvianus (Sauvage 1879) which is distributed in the Pacific Ocean from the Gulf of California to the coast of Perú.
Specimens of Diapterus Ranzani 1840 and Eugerres Jordan and Evermann 1972 are characterized by a smoothly serrated pre-operculum (Bussing 1995). Even when the specimens of these two genera are similar, Eugerres is distinguished by a smoothly serrated preorbital, while in Diapterus this characteristic is not present (Castro-Aguirre 1978, Aguirre-León et al. 1982, Aguirre-León and Yáñez-Arancibia 1986, Deckert and Greenfield 1987, Tapia-García and Ayala-Pérez 1996).
The descriptions of the early stages of species in the family Gerreidae are scattered. Among them are the descriptions of the larvae of Gerres sp. Cuvier 1839, by Leis and Rennis (1983), the initial ontogeny of Eugerres axillaris (Günther 1864), reared with laboratory methods (Ortiz-Galindo 1991), and the larval, pre-juvenile and juvenile development of Eucinostomus currani Yáñez-Arancibia, 1978 (Jiménez-Rosenberg et al., unpublished). This paper details the development of the larvae, pre-juvenile and juvenile organisms of D. peruvianus, and proposes that the pigment pattern in larvae and characteristics such as the serrated pre-operculum, the dorsal-anal fin formula and the body depth in pre-juvenile and juvenile stages are diagnostic features for its recognition among other species of the family.
Materials and methods
The larval specimens were collected in superficial tows made in Bahía Concepción, B. C. S. (26º33 to 26º53 N and 111º42 to 112º56 W), in 1990, 1991 and 1992, using a standard 50 cm diameter and 3.50 m long plankton net of 333 mm mesh.
The pre-juvenile and juvenile organisms were obtained in Bahía de La Paz, B. C. S. (24°07 to 24°11 N and 110°17 to 110°40 W) between 1992 and 1996 with a rectangular epibenthonic net containing a 70 x 40 cm mount and 1 mm mesh with a conventional planktonic net, 3.0 m long and 505 mm mesh, adapted in the center. Larvae and pre-juvenile organisms were preserved in 4% formalin buffered with sodium borate, juvenile specimens were preserved in 50% isopropyl. All larvae used in this study were deposited in the larval fish collection of the Plankton and Marine Ecology Department of the Interdisciplinary Center of Marine Sciences of the National Polytechnic Institute (Catalog number ICTIGEDipe-273). Pre-juvenile and juvenile organisms were deposited in the Ichthyology Collection of the Museum of Natural History of the Autonomous University of Baja California Sur (catalog number MNHUABCS-649).
Juvenile specimens of D. peruvianus were identified with the keys developed by Castro-Aguirre (1978) and Tapia-Garcia and Ayala-Pérez (1996). The diagnostic features are: serrate pre-operculum margin, second dorsal spine longer than the distance between the snout tip and the posterior orbital margin, second anal spine both strong and long; smooth pre-orbital, flanks without longitudinal stripes, rhomboid body (depth and compress) maximum body depth 1.70 to 2.00, slender dorsal spines and second spine long (reaches the third ray when depressed).
The basic characteristics used to join the identified juvenile series with the pre-juvenile and larval ones were the serrated pre-operculum and smooth pre-orbital, as well as the pigment pattern. Larval stages were divided into pre-flexion, flexion and post-flexion, according to Kendall et al. (1984), and for the statement of pre-juvenile and juvenile stages were used the criteria of Hubbs (1943).
On all organisms the following measurements were made on the left side of the body: notochord length (NL) (in the pre-flexion and flexion stages), standard length (SL) (in the post-flexion, pre-juvenile and juvenile stages), body depth (BD), snout-anus length (SnAL), head length (HL), snout length (SnL) and eye diameter (ED).
Results
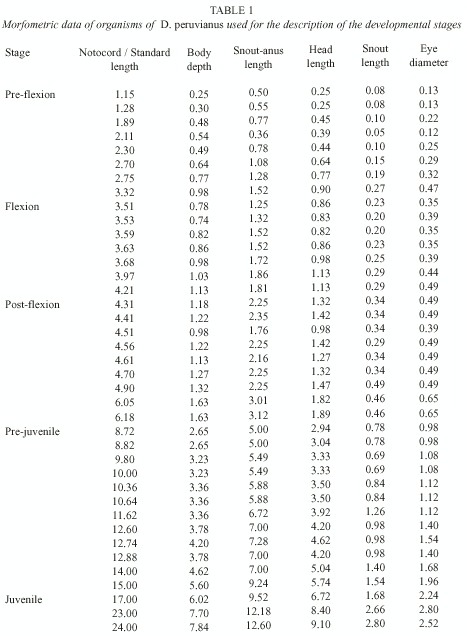
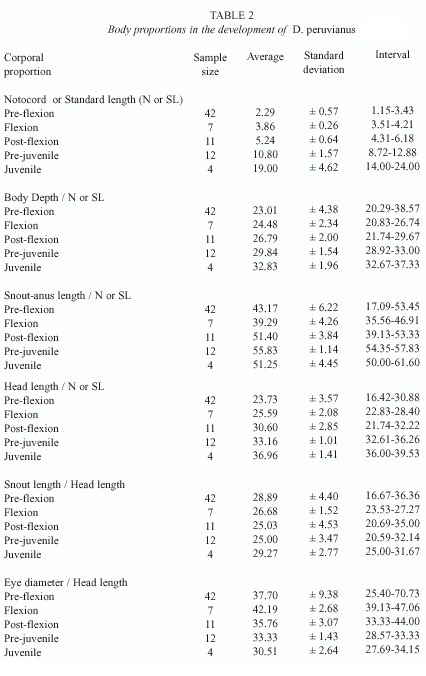
Morphology: A total of 76 organisms in different developmental stages were identified, 60 larvae collected in Bahía Concepción, B.C.S. and 16 pre-juvenile and juvenile collected in Bahía de La Paz, B.C.S. Of these, 39 organisms were used for the description of development, their measurements are shown in Table 1. The body proportions of all 76 organisms with reference to the SL, NL and HL are shown in Table 2.
The larvae are small and compressed. In the beginning of development the gut is a third of the SL, and it goes from the fifth myomer in the pre-flexion stage (1.15 mm NL to 3.50 mm NL) to the seventh one during the flexion stage (3.51 mm NL to 6.17 mm SL) and for part of the stage of post-flexion (6.18 mm SL to 8.71 mm SL). In the beginning of the pre-juvenile stage (8.72 mm SL to 16.99 mm SL) the anus is located in the ninth myomer, just before the insertion of the anal fin, near the mid-point of the body. The proportions for the BD and the HL remain constant during the larval development, increasing slightly during the pre-juvenile and juvenile (17.00 mm SL to 24.00 mm LS) stages. The SnAL length increases in proportion to the SL starting in the post-flexion stage. The SnL is constant during all of development with respect to the HL, while the DE decreases from the larval stage to the juvenile stage.
In the cephalic region, the beginning of the serration of the pre-operculum occurs at 6.18 mm SL, transforming into a smooth serrated pre-operculum by the juvenile stage. Scale development begins at 9.80 mm SL in the mid-region of the flanks of the body, increasing peripherally until the body is entirely covered by ctenoid scales at 14.00 mm SL.
During the post-flexion stage, eight branchial spines are observed in the lower branch of the first branchial arch, increasing during pre-juvenile development until 14 at the beginning of the juvenile stage.
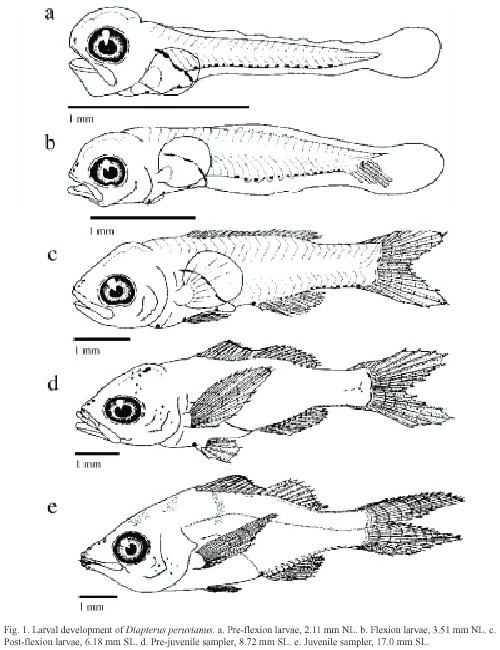
Pigmentation: From the pre-flexion stage reaching until the post-flexion (Fig. 1a-c), three large blotches are present on the dorsal margin of the gut, from above pectoral insertion to the anus. In the mid-ventral pre-anal line these are initially two pigments, one under the pectoral fin and one previous to the anus. Before the pre-flexion ends a third pigment appears in the isthmus, though in some organisms it appears in the flexion stage. Sixteen post-anal pigments in the mid-ventral line are arranged from the sixth myomer.
In the flexion stage (Fig. 1b) the number of post-anal pigments in the mid-ventral post-anal line is from 13 to 16, beginning in the ninth myomer. One or two of these pigments are located in what is the future base of the caudal fin.
In the post-flexion stage (Fig. 1c) seven pigments are on the base of the anal fin and eight are posterior to it, on the mid-ventral line. These fuse together, forming a continuous line in the end of the stage. Four large pigments are on the base of the caudal fin, which also tend to fuse together.
During the pre-juvenile stage (Fig. 1d) the cephalic pigments begin to appear; four sets of paired pigments appear first and during development the number of pigments increases. At 8.72 mm SL several pigments are observed in the maxilla, the operculum and the ocular edge. The distal portion of the dorsal spines is densely pigmented and internal pigments appear over the last caudal vertebrae and the hippural plate. At 12.60 mm SL, there are four to five lightly pigmented bands on both sides of the body that are subsequently covered by scales.
Heavy pigmentation appears at the base of the anal and caudal fins during the pre-juvenile stage, while silver ctenoid scales forming over the body cover the pre and post-anal pigments.
The pigmentation pattern shown in pre-juvenile organisms is maintained in the juvenile stage (Fig. 1e). The pelvic, pectoral and anal fins of juvenile acquire a yellowish tone, and the distal portion of the dorsal fin becomes densely pigmented. At the end of this stage silver ctenoid scales cover the body and two lines of pigment alongside the flanks distinguish the lateral line.
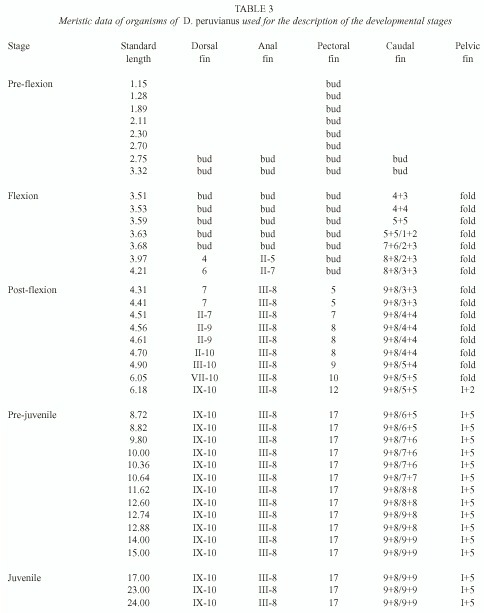
Fin development: Table 3 shows the fin development sequence of 39 organisms in different developmental stages. In pre-flexion larvae the body is surrounded by an embryonic fin from the anus to the first dorsal myomer, its fragmentation begins at 2.75 mm NL.
The caudal fin is the first one to begin its formation, developing principal fin rays from 3.51 mm NL to 4.31 mm SL. The secondary rays appear from 3.63 mm NL to 14.00 mm SL; in these sizes the bisection of the principal rays begins. The definitive formula for this fin is 9+8/9+9.
The dorsal and anal fins begin their development at 3.97 mm NL. The complete formation of the anal fin occurs at 4.31 mm SL. With a III-8 formula the last ray is a double one, the second spine is thicker than the first and third ones, and the third one is the longest. This fin is placed ventrally between the tenth and the 18 th myomer. The dorsal fin development ends at 6.18 mm SL, with a IX-10 formula and is placed dorsally between the fourth and 18 th myomer.
At 1.15 mm NL the primordial from the pectoral fins appear, but the first radial elements do not appear until 4.31 mm SL. At 8.72 mm SL, 17 complete rays are observed in this fin, ending its formation. Their bases are placed in both flanks of the body, in the first third of the gut.
The pelvic fins are the last ones to begin development. Their folds appear during the flexion stage, their first elements are observed at 6.18 mm SL and formation ends at 8.72 mm SL. With a I-5 formula, they are placed ventrally in the middle of the gut.
Discussion
Due to the dynamic changes of the morphological and meristic characteristics in the early development of fish, the interspecific and some times intergeneric differences are subtle (Powles and Markle 1984). In this case we are able to determine the larvae of the gerreid fishes, which can be confused with other percoid larvae. Larvae of Sciaenidae, Haemullidae and Mullidae are morphologically similar to those from Gerreidae and most of the time the adults also share the same niches and reproductive periods.
Sciaenidae contain at least 40 species in the Gulf of California. The few known larvae, compared with gerreid larvae, have spines in the operculum and pre-operculum from the pre-flexion stage, a big and bulbous head, a coiled and compact gut and the caudal region is narrower than in gerreid larvae (Moser 1996). In the post-flexion stage, the larvae are distinguished by their pigment patterns and the fin formula. There are 26 species of haemulids in the Gulf of California and its larvae (the known ones) are distinguished from the gerreid primarily by the number of myomers (26 in haemulids versus 24 in gerreids), by their cephalic spines, and by their pigment patterns and fin formula from the post-flexion stage.
Pigment patterns of Mullidae larvae, with three species in the Gulf of California, are the key in distinguishing them from the gerreid larvae; principally by the cephalic pigments (forming a constant triangular pattern) and by their internal pigments on the notochord (Watson 1996).
The description of the early development of D. peruvianus begins at 1.15 mm NL larvae, posterior to the hatching. Here the organisms show a functional snout, pigmented eyes and there is no vitelum sac present. The pigment pattern in larvae of D. peruvianus differs from that described for Gerres sp. (Lewis and Rennis 1983), E. axillaris (Ortiz-Galindo 1991) and Eucinostomus (Jiménez-Rosenberg 1998, P. Jiménez-Rosenberg et al., unpublished), because larvae of D. peruvianus present a larger number of post-anal pigments in the ventral mid-line. In addition, in D. peruvianus larvae the cephalic pigments appear at the end of larvae development and there is no pigmentation on the gut during development, as shown in Eucinostomus larvae (Jiménez-Rosenberg 1998, Jiménez-Rosenberg et al., unpublished). Larvae of D. peruvianus are also distinguished from those of Eucinostomus because Eucinostomus larvae have two to four long pigments posterior to the dorsal and anal fins, between the 18 th and 24 th myomers, and one dorsal pigment in the first myomer (from flexion to post-flexion stage) (Jiménez-Rosenberg 1998). In the post-flexion stage, in addition to the pigmentation patterns, larvae of D. peruvianus are distinguished from Eucinostomus and Gerres sp. larvae by the presence of a serrated pre-operculum, at the pre-juvenile stage by the anal fin formula (III-8 for D. peruvianus and III-7 for Eucinostomus and Gerres sp.) and by the branchial spines in the inferior branch of the first branchial arch (13 to 14 for D. peruvianus and seven to nine for Eucinostomus and Gerres sp.).
The relation of body depth to the standard length is useful for the distinction of juveniles of D. peruvianus (29.84 to 32.83% of SL). In Eucinostomus this relation is lower (Jiménez-Rosenberg 1998, P. Jiménez-Rosenberg et al., unpublished).
Larvae of D. peruvianus are more similar to those of E. axillaris, than the larvae of Eucinostomus or Gerres sp. They share characteristics like fin formula, the presence of a serrated pre-operculum and the values for body depth. However, from the pre-flexion stage they are distinguished by the pigment pattern. In E. axillaris this pattern consists of pigments in the caudal peduncle, from behind the anus to the tip of the notochord. E. axillaris is also distinguished by the presence of cephalic pigments during the larval stages. In the pre-juvenile and juvenile stages the presence of a serrated pre-orbital in E. axillaris is the most important characteristic to distinguish them from D. peruvianus.
Acknowledgments
We wish to thank the Public Education Ministry and the Interdisciplinary Center of Marine Sciences of the National Polytechnic Institute for the financial aid in the investigation projects "Biology and Ecology of commercially important species of ichtyoplankton in the Bay of La Paz, B.C.S." (UABCS-SEP-DGICSA-C92-01-045), "Zooplankton of La Paz Lagoon, B.C.S." (UABCS PRONAES No. 84-01-0127) and "Dynamics of the plankton of Conception Bay" (CICIMAR-IPN DEPI Code 903371). We thank the project directors, the scientific staff and the technical aid for the realization of this study. Also P. Jiménez-Rosenberg wishes to thank the Commission of Operation and Promotion of Academic Activities (COFAA) for the financial support.
Resumen
El desarrollo de Diapterus peruvianus se analizó con base en 60 larvas recolectadas en Bahía Concepción y 16 pre-juveniles y juveniles recolectados en la Ensenada de La Paz, B. C. S. México, usando respectivamente, una red estándar de plancton en arrastres superficiales y una red epi-bentónica para arrastres de plancton. Las larvas presentan desde la pre-flexión tres manchas alargadas sobre la superficie dorsal de la masa visceral, que pueden unirse y dar apariencia de pigmentación continua, observándose hasta 16 pigmentos post-anales en la línea media ventral y de dos a tres pigmentos pre-anales; la pigmentación cefálica así como la forma aserrada del pre-opérculo característica del género, aparecen a partir de la post-flexión. Los organismos pre-juveniles y juveniles se distinguen por la profundidad del cuerpo, la fórmula de la aleta anal, la fina forma aserrada del pre-opérculo y la pigmentación en la base de las aletas dorsal y anal.References
Aguirre-León, A. & A. Yáñez-Arancibia. 1986. Las mojarras de la Laguna de Términos: taxonomía, biología, ecología y dinámica trófica (Pisces: Gerreidae). An.Inst. Cienc. Mar Limnol. 13: 369-444. [ Links ]
Aguirre-León, A., A. Yáñez-Arancibia & F. Amezcua Linares. 1982. Taxonomía, diversidad, distribución y abundancia de las mojarras de la Laguna de Términos, Campeche (Pisces: Gerreidae). An. Inst. Cienc. Mar Limnol. 9: 213-250. [ Links ]
Bussing, W.A. 1995. Gerreidae, pp. 1114-1128. In W. Fisher, F. Krupp, W. Schneider, C. Sommer, K.E. Carpenter & V.H. Niem (eds.). Guía para la identificación de especies para los fines de la pesca. Pacífico Centro-Oriental. F A O. Roma. [ Links ]
Castro-Aguirre, J.L. 1978. Catálogo sistemático de peces marinos que penetran a las aguas continentales de México con aspectos zoogeográficos y ecológicos. Dir. Gral. Inst. Nal. Pesc. 19: 1-298. [ Links ]
Deckert, G.D. & D.W. Greenfield. 1987. A review of the western Atlantic species of the genera Diapterus and Eugerres (Pisces: Gerreidae). Copeia 1: 182-194. [ Links ]
Hubbs, C.L. 1943. Terminology of early stages of fishes. Copeia 1: 260. [ Links ]
Jiménez-Rosenberg, S.P.A. 1998. Descripción del desarrollo larvario de Eucinostomus gracillis y larvario y juvenil de Eucinostomus dowii y Diapterus peruvianus. Tesis de Maestría. Centro Interdisciplinario de Ciencias Marinas, La Paz, México. [ Links ]
Kendall, A.W. Jr., E.H. Ahlstrom & H.G. Moser. 1984. Early life history stages of fishes and their characters, pp. 11-23. In H.G. Moser, W.J. Richards, D.M. Cohen, M.P. Fahay, A.W. Kendall, Jr. & S.L. Richardson (eds.). Ontogeny and systematics of fishes. Amer. Soc. Ichthy. Herpet. Florida. [ Links ]
Leis, J.M. & D.S. Rennis. 1983. The larvae of Indo-Pacific coral reef fishes. New South Wales Univ., Sydney and Univ. Hawaii Press, Honolulu. 269 p. [ Links ]
Moser, H.G. 1996. Introduction, pp. 1-72. In H.G. Moser (ed.). The early stages of fishes in the California Current region. Atlas 33. Allen, Kansas. [ Links ]
Nelson, J.S. 1994. Fishes of the World. John Wiley & Sons, New York. 600 p. [ Links ]
Ortiz-Galindo, J.L. 1991. Ontogenia inicial de la mojarra rayada Eugerres axillaris Günther 1864. Tesis de Maestría. Centro Interdisciplinario de Ciencias Marinas, La Paz, México. [ Links ]
Powles, H. & D.F. Markle. 1984. Identification of larvae, pp. 31-33. In H.G. Moser, W.J. Richards, D.M.Cohen, M.P. Fahay, A.W. Kendall, Jr. & S.L. Richardson (eds.). Ontogeny and systematics of fishes. Amer. Soc. Ichthy. Herpet., Florida. [ Links ]
Tapia-García, M. & G. Ayala-Pérez. 1996. Clave para la determinación de las especies de mojarras en México (Pisces:Gerreidae). Rev. Biol. Trop. 44/45: 519-526. [ Links ]
Watson, W. 1996. Mullidae: Goatfishes, pp. 1034-1037. In H.G. Moser (ed.). The early stages of fishes in the California Current region. Atlas 33. Allen, Kansas. [ Links ]
1 Departamento de Plancton y Ecología Marina, Centro Interdisciplinario de Ciencias Marinas-IPN. Av. Instituto Politécnico Nacional, s/n Col. Playa Palo de Sta. Rita, La Paz, Baja California Sur, México. Tel./Fax: 52 (612) 12 2 53 44; adelheid@balandra.uabcs.mx
2 Departamento de Biología Marina, Universidad Autónoma de Baja California Sur, A.P. 19-B, La Paz, Baja California Sur, México. Tel./Fax: 52 (612) 12 8 03 42.
* COFAA Grant Recipient.