Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.2 San José Jun. 2003
Combined effect of concentrations of algal food (Chlorella vulgaris) and salt (sodium chloride) on the population growth of Brachionus calyciflorus and Brachionus patulus (Rotifera)
Víctor M. Peredo-Álvarez 1, S.S.S. Sarma 1,3 & S. Nandini 2
Received 02-XI-2000. Corrected 08-XI-2001. Accepted 24-XI-2001.
Abstract
Salinity is an important variable influencing the density and diversity of rotifers. Studies on salt tolerance of rotifers have so far concentrated on euryhaline species while very little information is available on noneuryhaline taxa. In the present work, we have evaluated the combined effects of Chlorella vulgaris and sodium chloride on the population growth of two freshwater rotifers B. calyciflorus and B. patulus. A 24 hr acute tolerance test using NaCl revealed that B. calyciflorus was more resistant (LC50 = 3.75 ± 0.04 g l-1 ) than B. patulus (2.14 ± 0.09 g l-1 ). The maximal population density (mean±standard error) for B. calyciflorus in the control at 4.5 X10 6 cells ml-1 (algal level) was 80 ±5 ind. ml-1 , which was nearly a fifth of the one for B. patulus (397 ± 7 ind. ml-1 ) under comparable conditions. Data on population growth revealed that regardless of salt concentration, the density of B. calyciflorus increased with increasing food levels, while for B. patulus, this trend was evident only in the controls. Regardless of salt concentration and algal food level, the day of maximal population density was lower (4 ± 0.5 days) for B. calyciflorus than for B. patulus (11 ±1 day). The highest rates of population increase (r values) for B. calyciflorus and B. patulus were 0.429 ± 0.012 and 0.367 ± 0.004, respectively, recorded at 4.5 X106 cells ml-1 of Chlorella in the controls. The protective role of algae in reducing the effect of salt stress was more evident in B. calyciflorus than B. patulus.
Key words: Rotifera, Population growth, Salt stress, Brachionus patulus, Brachionus calyciflorus.
Rotifers, in natural waters, experience extremes of variations with respect to both abiotic (e.g. temperature, salinity) and biotic factors (e.g., food availability, predation) (Nogrady et al. 1993). Studies on the effect of food concentration and temperature on the population growth of rotifers have been well-documented (Edmondson 1965). Among abiotic factors salinity is an important variable strongly influencing the density and diversity of freshwater zooplankton (Green 1993, Zhao et al. 1996). Rotifers, generally being less halotolerant, are restricted to freshwaters (Nogrady et al. 1993). Of the nearly 2000 taxa of rotifers, only about 150 species are considered to be marine (Koste 1978). Various mechanisms have been suggested to explain the poor survival of freshwater rotifers to salt stress. Some of them are a) failure of osmoregulation b) energetic costs associated with osmoregulation, c) reduced swimming rate, d) possible effect of salt on algal survival, e) low hatching success or even heavy mortality of neonates, even though some reproduction may have occurred in the population and f) biochemical changes (Epp and Lewis 1984, Yamasaki and Hirata 1985, Frolov et al. 1991, Oeie and Olsen 1993).
Salinity tolerance studies on rotifers have concentrated on selected euryhaline species such as Brachionus plicatilis and Brachionus rotundiformis, because of their role as starter food in mariculture for larval stages of fish and crustaceans (Snell 1986, James and Abu-Rezeq 1990). A number of freshwater rotifers such as Brachionus calyciflorus, B. rubens and B. patulus are also used in freshwater aquaculture or even in rearing brackishwater crustaceans (Sarma 1991). It is therefore necessary to understand the extent to which freshwater rotifers of the genus Brachionus can survive under different levels of salt stress.
Anthropogenic causes such as agricultural activities and natural changes such as seasonal evaporation of freshwater bodies can contribute to increase the salinity in many aquatic ecosystems (Dash et al. 1995, Williams et al. 1998). Under these circumstances, it is not known whether changes in the abundances of certain rotifer species in natural waterbodies are result of diminution of food supply or increased salinity levels or both.The aim of the present work is to study the combined effects of food levels and salt (sodium chloride) concentrations on the population growth of two freshwater rotifer species Brachionus calyciflorus and Brachionus patulus under laboratory conditions.
Materials and methods
We used two laboratory-cultured rotifer species Brachionus calyciflorus Pallas and Brachionus patulus (Müller) (synonyms: Platyias patulus and Plationus patulus), originally isolated from lake Chapultepec (Mexico City) and the waterbody Presa Santa Elena (State of Mexico), respectively. Starting with a single parthenogenetic female from each species, clonal populations were established in large aquaria (40 l) using the single-celled green alga Chlorella vulgaris as exclusive food and reconstituted moderately hardwater as the medium (EPA medium, Anonymous 1985). The EPA medium was prepared by dissolving 96 mg NaHCO3 , 60 mg CaSO4, 60 mg MgSO4 and 4 mg KCl in one litre of distilled water. Rotifers in mass cultures were fed daily with Chlorella (centrifuged and resuspended in EPA medium) at a density of 1 X106 to 2 X106 cells ml and the medium was changed every alternate day. Normally the mass cultures of both the rotifers had an average density of 40 to 50 ind. ml-1 ; at higher densities they were harvested.
For regular feeding in rotifers mass culture tanks as well as in experiments, we used Chlorella vulgaris, cultured on Bolds basal medium (Borowitzka and Borowitzka 1988). Algae in log phase of their growth were harvested, centrifuged at 3 000 rpm for 5 minutes, rinsed with distilled water and resuspended in EPA medium. The stock algal density was estimated using a haemocytometer. From this stock we prepared three densities, viz. 0.5 X106, 1.5 X106 and 4.5 X106 cells ml-1 using EPA medium. Standard grade (99.9% purity) sodium chloride was used in the experiments. A stock solution (nominal concentration) of 10 g l-1 was prepared using distilled water. From this stock solution, we prepared seven salinity levels (0,0.5, 1.0, 2.0, 3.0, 4.0 and 5.0 g l-1 ) using EPA medium. While preparing various combinations of algal-sodium chloride mixtures, we considered the effect of mutual dilution and adjustments were made accordingly.
In order to detect the range of salt concentration that would permit population growth of rotifers for a reasonable period of time, it was necessary to conduct acute tolerance tests for a 24 h period. For both species, we used neonates obtained from hatching parthenogenetic eggs. For both, B. calyciflorus and B. patulus, we used 6 concentrations of sodium chloride (0, 1.0, 2.0, 3.0, 4.0 and 5.0 g l-1 ). For each concentration we maintained 3 replicates, each one with 50 individuals. In order to keep rotifers active, we added Chlorella at a density of 0.5 X106 cells ml-1 .
After 24 h of inoculation, we counted the number of rotifers alive in each replicate. The data were used to derive median lethal concentration (LC50) using probit method (Finney 1971).
Based on the results of the acute tolerance tests, we chose five salt concentrations (0, 0.5, 1.0, 2.0 and 3.0 g l-1 ) for B. calyciflorus and four (0, 0.5, 1.0, 2.0 g l-1 ) for B. patulus. For B. calyciflorus, the experimental design consisted of a total of 45 (= 5 salt concentrations X 3 algal levels X 3 replicates) transparent test jars (50 ml capacity) containing 50 ml of EPA medium with one of the selected combinations of Chlorella-sodium chloride mixture. Into each of these test jars, we introduced a mixed population (young and non-egg bearing adults) of B. calyciflorus at an initial density of 5 ind. ml-1 using finely drawn Pasteur pipette under a stereomicroscope at a magnification of 30 X. The test jars were maintained at 25 ± 2ºC, pH 7.2 to 7.5 under continuous but diffused fluorescent illumination. Following inoculation, the density of live individuals of B. calyciflorus was estimated daily using total counts or 2-3 aliquots of 1 ml each. Males were rarely encountered. Following the counting, rotifers were transferred (using 50 µm mesh) to new jars containing appropriate salt concentration and algal food density. We discontinued the experiment after 14 days, the time when B. calyciflorus began to decline in most replicates.
The experimental design for B. patulus was similar to that used for B. calyciflorus. In this case however, we continued the experiments for 20 days after then we observed a declining trend in all test jars.
Based on the data collected, we calculated the rate of population increase (r) using the exponential growth equation: r = (ln Nt - ln No )/t, where, No = initial population density, Nt = density of population after time t (days) (Krebs 1985). The r was obtained, as far as possible, from a mean of 4-5 values during the exponential phase of the population growth for each rotifer species.
Results
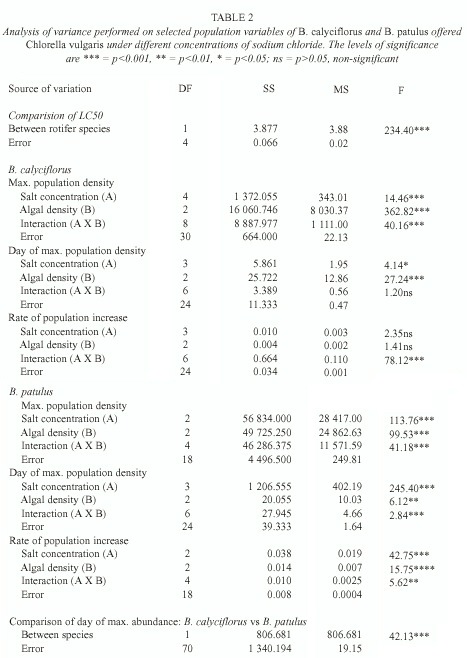
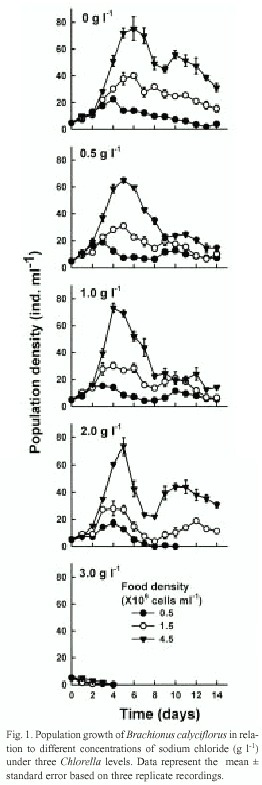
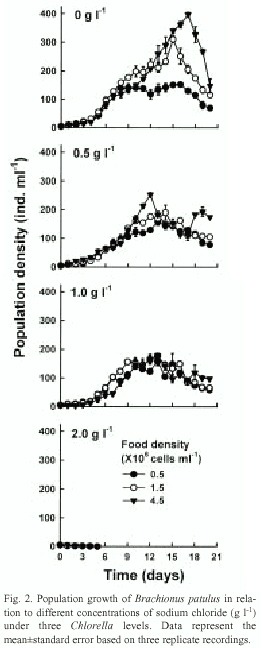
Data on the median lethal concentration of sodium chloride for B. calyciflorus and B. patulus are presented in Table 1. B. calyciflorus was more resistant to salt concentrations than B. patulus. The differences in the LC50 values for both the species were statistically significant (p<0.001, F-test, Table 2). Population growth curves of B. calyciflorus and B. patulus in relation to different concentrations of sodium chloride and algal food levels are presented in figures 1 and 2, respectively. Regardless of salt concentration, B. calyciflorus showed increased population density with increasing food levels, while for B. patulus, this trend was evident only in the controls. The maximal population density (mean±standard error) for B. calyciflorus in the control at 4.5 X106 cells ml-1 algal level was 80±5 ind. ml-1, which was nearly one-fifth of the one for B. patulus (397 ±7 ind. ml-1) under comparable conditions (Fig. 3). B. calyciflorus reached the maximal population density in a shorter time (4±0.5 days) than B. patulus (11±1 days) regardless of salt concentration and algal food level.
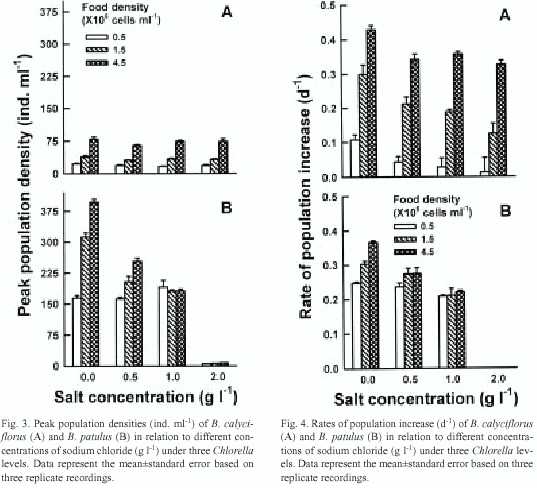
The rate of population increase per day (r) (mean ±standard error) for B. calyciflorus varied from 0.013±0.001 (at 0.5 X106 cells ml-1 of food level and 2.0 g l-1 of sodium chloride) to 0.429± 0.028 (at 4.5 X106 cells ml-1 of food level in the control). The highest r value (0.367±0.004) for B. patulus was recorded at 4.5 X106 cells ml-1 of food level in the control and the lowest was (0.209±0.003) observed at 0.5 X106 cells ml-1 of Chlorella at a salt concentration of 1.0 g l-1 (Fig. 4).
Maximal population density, day of peak population density and the rate of population increase were all significantly affected by the concentrations of sodium chloride, food level as well their interaction (p<0.05, ANOVA) for B. patulus. However, for B. calyciflorus the interaction between salt concentration and algal food level on the day of maximal population abundance was not significant (p>0.05). Similarly, the rate of population increase for B. calyciflorus was not significantly affected by salt concentration or algal food density (Table 2).
Discussion
It is evident that both the rotifer species were negatively affected by salt concentrations of 4 g l-1 as demonstrated in the acute tolerance tests, and 2 g l-1 when exposed chronically. In general, our results agree with field observations, where most members of the family Brachionidae are nearly absent when the salinity exceeds 2 g l-1 (Greenwald and Hurlbert 1993). Relatively higher salt tolerance (> 5g l-1) of B. calyciflorus in some laboratory studies using rotifers obtained from hatching resting eggs (Snell et al. 1991) is probably due to strain differences or origin of the test population.Generally, neonates hatched from resting eggs are more resistant than those from parthenogenetic eggs (Sarma 2000). In our case, the rotifer population was obtained from parthenogenetic eggs.
Regardless of salt concentration and food levels, the growth curves obtained in the present study are typical for planktonic rotifers (Walz 1995). An increase in density with increasing algal food level has been well-documented for several rotifer genera of Brachionidae e.g., Anuraeopsis (Dumont et al. 1995), Brachionus (Halbach and Halbach-Keup 1974), Keratella (Walz 1983) and Notholca (May 1980). In the present study, B. calyciflorus and B. patulus have also increased in population abundance with increasing availability of Chlorella. Nevertheless, the magnitude of rotifer increase was influenced by the concentration of sodium chloride (Figs. 1-2).For single species studies, maximal population density, day of maximal abundance and the rate of population increase are important variables which are sensitive to changes in the medium (Sarma et al. 1998). Usually an increase in the available food, results in an increase in the population growth rate (Dumont et al. 1995). This is evident in the present work (Fig. 3). However, some factors can modify this relation such as temperature, salinity or both (Oltra and Todoli 1997). Effect of temperature and salinity on the life history variables of B. plicatilis has been reviewed by Miracle and Serra (1989). According to them, the intensity of salt stress has a greater influence on the fecundity of rotifers when compared to temperature variation. However, these conclusions are based on the euryhaline B. plicatilis, which inhabits freshwater environments and can survive even in hypersaline conditions (Walker 1981). In the present work, both rotifer species showed nearly a similar response to salinity, i.e. up to a certain level of sodium chloride (2 g l-1 in case of B. calyciflorus and 1 g l-1 for B. patulus); although they continue to grow but beyond this, they suddenly crashed in all the replicates.
Information published so far does not support the hypothesis that death of the algae under the range of salt concentrations used in this study could have contributed to the low population growth of the species tested (He et al. 1993). Reduced swimming speed of rotifers as a result of stress could probably cause reduced food intake (Lee and Macko 1981, Korstad et al. 1995). This is probably responsible for low population growth of B. calyciflorus and B. patulus under 3 g l-1 and 2 g l-1 of sodium chloride, respectively. The protective role of algae in reducing the effect of salt stress was more evident in B. calyciflorus. Thus, if the sodium chloride had a uniform effect up to 2 g l-1 , this would have caused more or less similar density of B. calyciflorus either at 0.5 X106 or 4.5 X106 cells ml-1 of Chlorella. Nevertheless, under salt concentrations up to 2.0 g l-1 , B. calyciflorus showed population growth rates dependent on food level. Conversely, due to relatively low tolerance to salt stress, B. patulus showed no food level-dependent population growth at 1.0 g l -1 . The absence of population growth at 3.0 g l-1 for B. calyciflorus and 2.0 g l-1 for B. patulus even under 4.5 X10 6 cells ml-1 of Chlorella suggested that food density could protect rotifers from the salt stress up to a certain limit, beyond which the effect of salinity is independent of food supply. In conclusion, our data show that both B. calyciflorus and B. patulus have a narrow range of tolerance to salt concentration and that the algal food had a protective role in reducing the effect of sodium chloride but only up to a concentration of 2.0 g l-1.
Acknowledgements
This investigation was supported by a Project from CONACyT (C01-41786). SSSS and SN thank the National System of Investigators (SNI-18723 and 20520).
Resumen
La salinidad es una variable importante que tiene influencia sobre la densidad y la diversidad de los rotíferos. Los estudios de rotíferos sobre tolerancia a la sal que se tienen hasta ahora se han concentrado en especies eurihalinas, sin embargo, hay muy poca información sobre taxas no eurihalinos. En el presente trabajo, se evaluaron los efectos combinados de las concentraciones de Chlorella vulgaris y cloruro de sodio sobre el crecimiento poblacional de dos rotíferos de agua dulce, B. calyciflorus y B. patulus. Una prueba de toxicidad aguda de 24 h utilizando cloruro de sodio reveló que B. calyciflorus fue más resistente (CL50 = 3.75 ± 0.04 g l-1) que B. patulus (2.14 ± 0.09 g l-1). La máxima densidad de población (media ± error estándar) de B. calyciflorus en el lote control, utilizando una concentración 4.5 X106 células ml-1 de alga fue de 80 ± 5 ind. ml-1, casi una quinta parte de B. patulus (397 ±7 ind. ml-1) sobre condiciones comparables. Datos sobre el crecimiento poblacional revelaron que cualquier concentración de sal carece de efecto, la densidad de B. calyciflorus se incrementa cuando aumentan los niveles de alimento. Sin embargo para B. patulus esta tendencia fue evidente únicamente en los controles. Independientemente de las concentraciones de sal y los niveles de alimento, el día de abundancia máxima fue menor para B. calyciflorus (4 ± 0.5 días) que para B. patulus (11 ±1 días). Los valores de la máxima tasa de crecimiento poblacional (r) fueron para B. calyciflorus y B. patulus de 0.429 ± 0.012 y 0.367 ± 0.004, respectivamente en lotes control con 4.5 X10 6 células ml-1 de Chlorella. El papel de protección de alga para reducir el efecto del estrés de la sal fue más evidente en B. calyciflorus que en B. patulus.
References
Anonymous. 1985. Methods of measuring the acute toxicity of effluents to freshwater and marine organisms. US Environment Protection Agency EPA/600/4- 85/013. [ Links ]
Borowitzka, M.A. & L.J. Borowitzka. 1988. Microalgal biotechnology; Cambridge University: London, England, 480 p. [ Links ]
Dash, R.C., P.K. Mohapatra & R. C. Mohanty. 1995. Salt induced changes in the growth of Chlorococcum humicolo and Scenedesmus bijugatus under nutrient limited cultures. Bull. Environ. Contam. Toxicol. 54:695-702. [ Links ]
Dumont, H.J., S.S.S. Sarma & A.J. Ali. 1995. Laboratory studies on the population dynamics of Anuraeopsis fissa (Rotifera) in relation to food density. Freshwater Biol. 33: 39-46. [ Links ]
Edmondson, W.T. 1965. Reproductive rate of planktonic rotifers as related to food and temperature in nature. Ecol. Monogr. 35: 61 - 111. [ Links ]
Epp, R.W. & W.M. Lewis Jr. 1984. Cost and speed of locomotion for rotifers. Oecologia 61: 289-292. [ Links ]
Finney, D.J. 1971. Probit analysis. Cambridge University Press, 3rd edn, London, 333 p. [ Links ]
Frolov, A.V., S L. Pankov, K.N. Geradze & S.A. Pankova. 1991. Influence of salinity on the biochemical composition of the rotifer Brachionus plicatilis Müller. Aspects of adaptation. Comp. Biochem. Physiol. A. Comp. Physiol. 99: 541-550. [ Links ]
Green, J. 1993. Zooplankton associations in East African lakes spanning a wide salinity range. Hydrobiologia 267: 249-256. [ Links ]
Greenwald, G.M. & S.H. Hurlbert. 1993. Microcosm analysis of salinity effects on coastal lagoon plankton assemblages. Hydrobiologia 267: 307-335. [ Links ]
Halbach, U. & G. Halbach-Keup. 1974. Quantitative beziehungen zwischen phytoplankton und der populationsdynamik des rotators Brachionus calyciflorus Pallas. Befunde aus laboratoriumsexperimenten und freilanduntersuchungen. Arch. Hydrobiol. 73: 273-309. [ Links ]
He, Z., K. Qin, Y. Wang & W. Zhao. 1993. Biological resources in inland saline waters from southern Shanxi, China Part 1. Lake Xiaochi. J. Dalian Fish. Coll. 8: 1-15. [ Links ]
James, C.M. & T. Abu Rezeq. 1990. Efficiency of rotifer chemostats in relation to salinity regimes for producing rotifers for aquaculture. J. Aquacult. Trop. 5:103-116. [ Links ]
Korstad, J., A. Neyts, T. Danielsen, I. Overrein & Y. Olsen. 1995. Use of swimming speed and egg ratio as predictors of the status of rotifer cultures in aquaculture. Hydrobiologia 313/314: 395-398. [ Links ]
Koste, W. 1978. Rotatoria. Die Rädertiere Mitteleuropas. Ein Bestimmungswerk begründet von Max Voigt. Bornträger, Stuttgart. Vol. 1, Textband 673 p. Vol. 2,Tafelband 234 p. [ Links ]
Krebs, C.J. 1985. Ecology. The experimental analysis of distribution and abundance. Harper and Row, New York. 789 p. [ Links ]
Lee, W.Y. & S.A. Macko. 1981. Toxic effects of cembranolides derived from octocorals on the rotifer Brachionus plicatilis and the amphipod Parhyale hawaiensis. J. Exp. Mar. Biol. Ecol. 54: 91-96. [ Links ]
May, L. 1980. Studies on the grazing rate of Notholca squamula Müller on Asterionella formosa Hass. at diferent temperatures. Hydrobiologia 73: 79 - 81. [ Links ]
Miracle, M.R. & M. Serra. 1989. Salinity and temperature influence in rotifer life history characteristics. Hydrobiologia 186/187: 81-102. [ Links ]
Nogrady, T., R.L. Wallace & T.W. Snell. 1993. Rotifera. Vol. 1. Biology, ecology and systematics. SBP Academic, The Hague, 142 p. [ Links ]
Oeie, G. & Y. Olsen. 1993. Influence of rapid changes insalinity and temperature on the mobility of the rotifer Brachionus plicatilis. Hydrobiologia 255/256: 81-86. [ Links ]
Oltra, R. & R. Todoli. 1997. Effects of temperature, salinity and food level on the life history traits of the marine rotifer Synchaeta cecilia valentina, n. subsp. J. Plankton Res. 19: 693-702. [ Links ]
Sarma, S.S.S. 1991. Rotifers and aquaculture (Review). Environ. Ecol. 9: 414-428. [ Links ]
Sarma, S.S.S. 2000. The use of rotifers for ecotoxicological studies in Mexico, pp. 8-11. In E. Ríos-Jara et al. (eds). Estudios sobre plancton en México y el Caribe. Sociedad Mexicana Planctología, Universidad de Guadalajara, México. [ Links ]
Sarma, S.S.S., R.A.A. Stevenson & S. Nandini. 1998 Influence of food (Chlorella vulgaris) concentration and temperature on the population dynamics of Brachionus calyciflorus Pallas (Rotifera). Ciencia Ergo Sum 5: 77-81. [ Links ]
Snell, T.W. 1986. Effect of temperature, salinity and food level on sexual and asexual reproduction in Brachionus plicatilis (Rotifera). Mar. Biol. 92: 157-162. [ Links ]
Snell, T.W., B.D. Moffat, C. Janssen & G. Persoone. 1991. Acute toxicity tests using rotifers. IV. Effects of cyst age, temperature, and salinity on the sensitivity of Brachionus calyciflorus. Ecotoxicol. Environ. Saf. 21: 308-317. [ Links ]
Walker, K.F. 1981. A synopsis of ecological information on the saline lake rotifer Brachionus plicatilis, Müller, 1786. Hydrobiologia 81: 150 - 167. [ Links ]
Walz, N. 1983. Individual culture and experimental population dynamics of Keratella cochlearis (Rotatoria). Hydrobiologia 107: 35 - 43. [ Links ]
Walz, N. 1995. Rotifer populations in plankton communities: Energetics and life history strategies. Experientia 51: 437 - 453. [ Links ]
Williams, W.D., P. De Deckker & R.J. Shiel. 1998. The limnology of Lake Torrens, an episodic salt lake of central Australia, with particular reference to unique events in 1989. Hydrobiologia 384: 101-110. [ Links ]
Yamasaki, S. & H. Hirata. 1985. Effects of salinity on food conversion rate of rotifer Brachionus plicatilis. Mem. Fac. Fish. Kagoshima Univ. 34: 37-43. [ Links ]
Zhao, W., H. Jiang, & Z. He. 1996. Planktonic crustaceans of inland saline waters in Sanbei District, Northern China. J. Dalian Fish. Coll. 11: 1-13. [ Links ]
1. Laboratorio de Zoología Acuática, División de Investigación y Posgrado, Edificio UMF, Universidad Nacional Autónoma de México, Campus Iztacala, AP 314, CP 54090, Tlalnepantla, Edo. de México. México. FAX: +52 5 623 1256. e-mail: sarma@servidor.unam.mx
2. División de Investigación y Posgrado, Universidad Nacional Autónoma de México, Campus Iztacala, AP 314, CP 54090, Tlalnepantla, Edo. de México.
3. Corresponding author.