Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.1 San José Mar. 2003
Abstract
Sex ratio, size at maturity, maturity stages, fecundity and egg diameter of Oreochromis niloticus from Coatetelco Lake, Morelos State, Mexico, were studied from January to December 1993. Sex ratio (male:female) was approximately 1:1.02. Length at maturity was 117 mm (males) and 120 mm (females). The fecundity ranged between 104 and 709 eggs, with egg diameter from 1 000 to 3 000 µm. The gonadosomatic and hepatosomatic index indicate that the species breeds during summer and winter.
Key words: Oreochromis niloticus, fecundity, gonadosomatic index, sex ratio, Coatetelco lake, Mexico.
Tilapias (Cichlidae) constitute a group of native fishes from Africa, that have been introduced into various other countries. The Niles tilapia Oreochromis niloticus (Linnaeus, 1757) is one of the most widespread and important fishes in tropical freshwater aquaculture. There is a growing consensus that tilapias can become the worlds most important warm water cultured fishes. The main advantage of tilapia is its relatively low cost of production, mainly for fry and feed, and the quality of its flesh. The attributes which make Niles tilapia so suitable for fish farming are its general hardiness, ease of breeding, rapid growth rate, ability to efficiently convert organic and domestic wastes into high quality protein, and good taste (Wohlfarth and Hulata 1983, Yi et al. 1996). In spite of these qualities, during 1997 the extensive aquaculture in Mexico produced 83 132 t of tilapias (Anonymous 1998). This figure only represents about 5.29 % of total production from fish caught in lakes and from fish farming.
Knowledge on their biology is essential for rational utilization of stocks. Studies on breeding season and factors associated with it are needed to protect new recruits and predict recruitment variability. Other advantages in favour of economic culturing of O. niloticus are its herbivorous nature and its mouth-brooding habits. The main reason that tilapias make a relatively small contribution to fisheries production in most countries, in spite of their desirable traits, is their early sexual maturity.Tilapias reproduce when they are only a few months old, often below market weight. Early sexual maturity may also have a negative influence on growth rate (Morales 1991). Actually, O. niloticus is ecologically and commercially the most important species in Mexico aquatic systems. The fish is a maternal mouth-brooder.
Various aspects of the biology of Cichlids of commercial importance have been studied by Fryer and Iles (1972), Babiker and Ibrahim (1979), Arredondo-Figueroa and Guzmán-Arroyo (1986), Gómez et al. (1993), Morales (1991), Morales (1992), Castrejón et al. (1995), Palacios (1995), Ramos-Cruz (1995) and Fawole and Arawomo (2000). There are however, unpublished accounts on the biology of these species from Coatetelco Lake, Mexico. The purpose of this study was to analyze the relationships among fecundity-length, fecundity-weight, oocyte diameter, and the reproductive cycle of O. niloticus.
Materials and methods
Samples from the commercial catch at Coatetelco Lake, located in Morelos State (18°45 N, 99°20 W, altitude 1100 m), were taken approximately at monthly periods from January to December 1993. Fish were caught by a static gill net with 5.08 cm of mesh size. The specimens were collected by the same fisherman in the same place and date. A total of 319 fish were analyzed: 161 females and 158 males. Fish were measured for total (TL) and standard lengths (SL) to the nearest 1 mm (from the tip of the snout to the end of the caudal fin), weighed (total weight) to the nearest 0.1 g and dissected to determine sex; the gonadic maturity stage was recorded following Holden and Raitt (1975) procedures. The gonads and liver were removed and weighted to the nearest 0.01 g. The sexual proportion male:female was obtained. The mean length at first reproduction, or mean length at sexual maturity was obtained as the length at which 50 percent of all individuals are sexually mature (King 1995). Gonadosomatic index (GSI) for each fish was calculated as the weight of the gonads relative to the total body weight, expressed as a percentage (de Vlaming et al. 1982). Hepatosomatic index (HSI) was calculated for females as the weight of the liver relative to the total body weight, expressed as a percentage. The monthly frequency of the various gonad stages, and mean monthly GSI and HSI were plotted by month to determine the reproductive cycle and the breeding season of the fish. Ripe female fish (85) were used for fecundity estimations. Fecundity was estimated using the total number of oocytes contained in the ovary of the fish, and diameters of the oocytes were measured with a dissecting microscope equipped with an ocular micrometer. The variation of fecundity with fish body length was estimated (Bagenal 1978). A mean fecundity from all samples was calculated using a "direct summation" procedure (Shoesmith 1990).
Catalogue number of voucher specimens: IBUNAM-P 8832.
Results
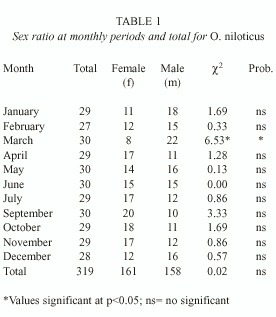
Sex ratio
The 319 fishes ranged from 117 to 206 mm in total length, and 31.2 to 167.0 g in body weight with 161 females (50.4 %) and 158 males (49.6 %). Females were more numerous from July to November and the males from January to June, except April (Table 1). The sex ratio was 1:1.02 (males: females), and did not differ significantly of the sex ratio 1:1 (X2 = 0.02; p=0.05).
Maturity stages
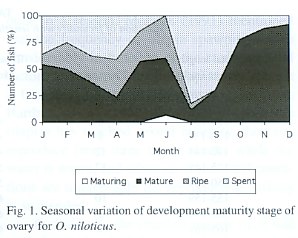
The increase from the immature condition to the ripe condition through well-defined intermediate stages of maturation and ripening, and the percentage of each stage of gonadal development is illustrated in Fig. 1. According with the females gonadic maturation stages, 0.6% of the total fishes were maturing (II), 51.6% were mature (III), 14.3% were ripe (IV), and 33.5% were spent (V). Therefore, the 47.8% of the fishes were in reproductive process. In the males gonadic maturation stages were: 7.0% of the total fishes were maturing (II), 29.0% were mature (III), 5.0% were ripe (IV), and 59.0% were spent (V). Therefore, the 64.0% of the fishes were in reproductive process
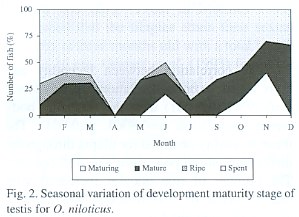
Fig. 1 shows that for the female in February, April and June the highest proportion of the average gonadal ripe stage (IV) was present. On the other hand, the fishes in spent stage (V) increased from January to April and in July. This percentage decreased progressively towards December. Similar results were obtained for the male (Fig. 2).
Size at first maturity
The relation between body length and gonadal development was examined in 311 fishes from randomly selected catches. Young males and females attain maturity while they are in their first year of life. The smallest mature female was 120 mm long and the smallest mature male was 117 mm long. The smallest ripe female was 141 mm in total length and 50 g of total weight, while the smallest ripe male was 144 mm long. It appears that females attain maturity earlier in life with a size around 3 mm larger than males. None of the fish examined was immature beyond a body length of about 150 mm.
Fecundity
The total lengths of the specimen examined ranged from 125 mm to 209 mm while the total weight ranged from 41.4 g to 167.0 g. In small ripe females, fecundity ranged from 104 to 373 oocytes/fish, and the mean fecundity decreased progressively with fish length between 125 to 144 mm long body. In larger fish, fecundity increased (609 to 709 oocytes/fish) with total length (205 to 209 mm) (Table 2). The relationship of fecundity with fish total length was analyzed for all the examined fish; it can be expressed by the following equation where F= fecundity (oocytes/fish) and TL= total length (mm):
F = 0.4701 TL 2.4808
Regression analysis of fecundity versus body weight, where F= fecundity (oocytes/fish) and TW= Total weight (g) was:
Log F = 1.1421 + 0.8085 Log TW
The correlation between fecundity and body length expressed relationship was lower (r=0.7930, p<0.001) than the fecundity-body weight correlation (r=0.8064, p<0.001). The mean fecundity obtained for tilapia through the direct summation procedure was 465 oocytes. The number of ripe oocytes increased with ovarian weight in small fishes and the diameter of oocytes contained in the ovaries ranged of less than 1 000 µm for fish in gonadal stage II; between 1 000 and 2 000 µm for fish in gonadal stage III, and between 2 000 and 3 000 µm for fish in stage of gonadal stage IV.
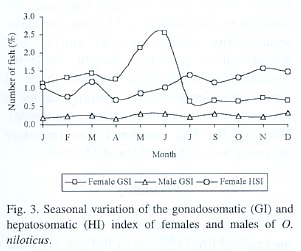
Gonadosomatic and hepatosomatic index
The gonadosomatic index increases with the progressive development of the gonads in both males and females until gonads are ripe and, the index then declines sharply in spawning and spent fish. The increase in weight from immature to the ripe condition through intermediate stages of maturation is illustrated in Fig. 1 and Fig. 2. In relation with reproduction, the maximum value of the gonadosomatic index for females was obtained in June and January (Fig. 3) and, during December and June for the males. An opposite pattern between gonad and liver weight means for females was detected.
Discussion
According to sizes obtained in Oreochromis niloticus the individuals have been considered as small fishes, due to that were caught by static gill-net with mesh size of 5.08 cm. Therefore, is necessary change the mesh size of the gillnet (probably to eight or nine cm) with the intention to obtain more production in weight and size. Ramos-Cruz (1995) and Palacios (1995) reported that the total average length for O. aureus using gillnet with mesh size of 9 cm is 20.9 cm. This is the reason why the former recommendation is made.
The sex ratio for O. niloticus in Coatetelco lake indicate that male and females are approximately nearly equal in numbers and the deviation from the expected 1:1 (male:female) was not significant. Fawole and Arawomo (2000) also indicated that for every female, there is a male specimen in Sarotherodon galilaeus. Babiker and Ibrahim (1979) reported for T. nilotica that the two sexes are present in an almost 1:1 ratio in populations of middleage fish. Nikolsky (1963) cited that the sex ratio varies considerably from species to species, but in the majority of cases it is close to one, and may vary from year to year in the same population. It is important to stand out the fact that the captures in some time are composed by more males, which could be favourable to the fishery, because this can serve as a regulatory mechanism for the sex ratio. This may be due to the passive nature gill nets or because the nets not were set close to the brooding grounds. Ramos-Cruz (1995) mentioned that the sex ratio for O. aureus was of 2.6:1 males:females, which considers normal. Also, Fryer and Iles (1972) point out that in the African lakes it is common that in the populations of cichlids the males dominate because they generally present more growth than the females, without this representing a risk situation for the fishery.
In this study the sex ratio was different in each one of the samples. This variation in the sex ratio can occur because once the fertilization of the eggs has concluded, the males possibly emigrate from the areas of spawning toward feeding areas located in the shallow part of the lake (where they are captured). The females possibly go toward submerged vegetation and rocky areas to avoid the fishermen and to carry out the incubation and protection of the offspring.
In spite of the variation in the gonadal stages males and females, the periodicity of spawning for O. niloticus at Coatetelco lake, indicate that the fish spawn at least twice in the breeding season. Castrejón et al. (1995) for O. niloticus and Babiker and Ibrahim (1979) for T. nilotica found that females breed more than once in a season. Morales (1991) mentioned that the frequency of tilapia spawning varies considerably in function of the environmental factors and that in Mexico tilapia may present a frequency of up to 10 spawning for year. Bardach et al. (1986) reports that when the tilapias has reached sexual maturity, they can reproduce from three to six weeks while the water is warm, and when environmental conditions are unfavorable the reproductive activity is suspended.
In this study the smallest mature male had a total length of 11.7 cm while the smallest mature female had a total length of 12.0 cm. Uchida and King (1962, in Morales 1991) mentioned that the tilapias attain their sexual maturity at three months old with a total length of 8 to 16 cm. Gómez et al. (1993) found for O. mossambicus that the total length of the smallest mature male and female were between six and eight cm, and three months of age. Ramos-Cruz (1995) indicates that O. aureus reaches the sexual maturity at 22 cm of total length at six months old. Fawole and Arawomo (2000) cited for S. galilaeus that males in Opa reservoir matures at about the same size as the female (12.5 and 11.7 cm respectively). This difference arises because the sexual maturity is a function of the size and may be influenced by the abundance and seasonal availability of food, the temperature, the photoperiod and by other environmental factors in different localities (Nikolsky 1963, Babiker and Ibrahim 1979).
Arredondo-Figueroa and Guzmán-Arroyo (1986) mentioned that Oreochromis spp is a mouth brooder with small gonads of less than 700 eggs. In this study, we reported a fecundity range from 104 to 709 eggs. This result is lower than that observed by other workers for O. niloticus. Fryer and Iles (1972) obtained a fecundity of 3 706 eggs from a gigantic female specimen with total length of 57 cm of T. nilotica. The maximum numbers of eggs counted in the ripe ovaries of any mouth-brooding cichlid are 4 300 in a 25 cm specimen of the T. aurea. Babiker and Ibrahim (1979) reported a fecundity range of 300 - 2 800 eggs for T. nilotica whose body length ranged from 11.0 and 32.0 cm. Palacios (1995) cited a fecundity range of 162 - 954 eggs for O. aureus whose body length ranged from 18.4 cm and 30.2 cm in Adolfo López Mateos dam, in Mexico. Moyle and Cech (2000) mentioned that in mouth-brooding cichlids, the fecundity is often considerably higher because the parents assure the survival of the offspring, and in consequence less mortality. In addition, the variation in fecundity may be attributed to differential abundance of food within the members of the population, and Bagenal (1978) cited that fish species exhibit wide fluctuations in fecundity among fish of the same species, size and age.
On criteria of length, females reach maturity before males (12.0 and 11.7 cm, respectively). Small females produce only a few eggs but fecundity is directly correlated with body length (r=0.7930) and body weight (r=0.8064). Even when expressed in terms of body length, the fecundity by O. niloticus, of the order of exponential (2.4808) of length, is much lower than that of most economically exploited species, as mentioned by Babiker and Ibrahim (1979) for T. nilotica. Similar values for O.mossambicus (Gómez et al. 1993) and S. galilaeus (Fawole and Arawomo 2000) were obtained. This is most probably a result of the restrictions imposed by the mouth-brooding habit of this species and the limited space available for rearing of the spawn in the mouth cavity (Babiker and Ibrahim 1979).
The occurrence of eggs of varying sizes is also an indication of multiple spawning by this species and the egg diameter varied in fish of the same length or weight. In this study the eggs ranged between 2.0 and 3.0 mm. Fawole and Arawomo (2000) mentioned for S. galilaeus an egg diameter of 2.49 ± 1.81 mm. Fryer and Iles (1972) reported that T. galilaeus had a maximum egg diameter of 2.2 mm; furthermore, they mentioned that egg size varies considerably from species to species among the mouth brooding forms. These differences in egg and brood size must have some biological significance and an attempt has been made to explain them, at least in part, in terms of the habitat in which the various species live. There appears to be a relationship between the risk of being eaten and the size of the egg produced. However, the degree to which an animal is able to separate the growth and reproductive phases must depend on the constancy and pre-dictability of the environment.
In spite of the periodicity of oviposition and spawning, the generation and development of gonads seemed to proceed without interruption as indicated by the high frequency of occurrences of all stages of gonadal maturation. Babiker and Ibrahim (1979) cited that it is possible that in this species and in the majority of teleosts oogenesis proceeds uninterruptedly or cyclically throughout reproductive life. Mature ovaries were available all the year round and this is an indication that the fish breeds throughout the year. The GSI for the males was always lower than those from females. This is associated with relatively heavier female gonads. These observations suggest, that, in spite that the pattern of variation in the mean gonad weight, the evidence found in this study corroborates the summer and winter spawning season for this species.
The reproductive season for this species, showed that the liver weight is minimal in spring. Salgado-Ugarte (1995) mentioned that the poor somatic condition during the spawning season is a common observation in many species of fish, and give and indication that the somatic growth is limited due to the development of gonads, furthermore, the decrease in condition has been ascribed to a depletion of body reserves during gonad maturation.
The data and information presented here indicate that the GSI may perhaps not yield a totally accurate indication of gonadal activity. García-Berthou and Moreno-Amich (1993) cited that the use of the multivariate analysis of covariance (MANCOVA) could be applied most appropriate the use of this measurement. This method have the advantages of eliminates the effect of the individual size, the increment in the variability when comparing variables by means of a ratio and the anomalous characteristics of estimating mean true values of the ratio.
Acknowledgements
We thank everyone who kindly assisted in several parts of the work, especially Mercedes Garduño Paredes and Juan Avelar Esquivel for collecting, processing and providing measurements of O. niloticus. The authors acknowledge Benny Weiss Steider for his help with the manuscript. This work was supported by the Facultad de Estudios Superiores Zaragoza, U.N.A.M. and Secretaría de Desarrollo Rural del Estado de Morelos, Mexico.
Resumen
En este estudio se analizaron algunos aspectos reproductivos de la mojarra-tilapia Oreochromis niloticus L. en el lago de Coatetelco, Morelos. La información esta basada en los datos biológicos de 319 organismos obtenidos de enero a diciembre de 1993 de la captura comercial. Las hembras (161) representaron el 51% y los machos (158) el 49 % del total de la población. La proporción de sexo fue de 1:1.02 (X = 0.02; p=0.05). Los peces alcanzan la primera madurez sexual a los 117 mm y 120 mm de longitud total (hembras y machos respectivamente). El número de óvulos presentes en las gónadas osciló entre 104 y 709 con diámetro de 1 000 a 3 000 µm, lo cual hace que esta especie tenga baja fecundidad pero alta fertilidad por los hábitos de reproducción que presenta la especie. Se aplicó el indice gonadosomático y hepatosomático con los cuales se detectaron dos períodos (verano e invierno) de máxima actividad reproductiva.
References
Anonymous. 1998. Anuario Estadístico de Pesca 1997. Dirección de Estadística y Registro Pesquero. Secretaría de Pesca. 235 p. [ Links ]
Arredondo-Figueroa, J.L & M. Guzmán-Arroyo. 1986. Actual situación taxonómica de las especies de la Tribu Tilappini (Pisces: Cichlidae) introducidas en México. An. Inst. Biol. U.N.A.M., Serie Zool. 56: 555-572. [ Links ]
Babiker, M.M. & H. Ibrahim. 1979. Studies on the biology of reproduction in the cichlid Tilapia nilotica (L): Gonadal maduration and fecundity. J. Fish. Biol. 14: 437-447. [ Links ]
Bardach, E. J., J. H. Ryther & W.O. Mclarney. 1986. Acuacultura. Crianza y cultivo de organismos marinos y de agua dulce. AGT Editor, S.A.: 288-316. [ Links ]
Bagenal, T. 1978. Aspects of fish fecundity: 75-101. In Shelby, D.G. (Ed.). Ecology of freshwater fish production. Blackwell Scientific Publication. Oxford, London. [ Links ]
Castrejón, M.T., R. Palacios & A. García-Alarcón. 1995. Patrón reproductivo de la tilapia del Nilo Oreochromis niloticu s, Programa Resúmenes. XIII Congreso Nacional de Zoología. Nov. 1995. Morelia, Mich.:24.
de Vlaming, V., G. Grossman & F. Chapman. 1982. On the use the gonosomatic index. Comp. Biochem. Physiol., 73: 31-39. [ Links ]
Erickson, L.D., J. E. Hightower & G.D. Grossman. 1985. The relative gonadal index: An alternative index for quantification of reproductive condition. Comp. Biochem. Physiol. 81: 117-120.
Fawole, O.O. & G.A.O. Arawomo. 2000. Fecundity of Sarotherodon galilaeus (Pisces:Cichlidae) in the Opa reservoir, Ile-Ife, Nigeria. Rev. Biol. Trop., 48: 201-204.
Fryer, G. & T.D. Iles. 1972. The Cichlid Fishes of the Great Lakes of Africa. Their Biology and Evolution. Oliver and Boyd, Edinburgh. 641 p. [ Links ]
García-Berthou, E. & R. Moreno-Amich. 1993. Multivariate analysis of covariance in morphometric studies of the reproductive cycle. Can. J. Fish. Aquat. Sci. 50: 1394-1399.
Gómez-Márquez, J.L., R.M.A. Castillo, M.J.G. Fabila & V.A.D. Zamora. 1993. Reproducción de la tilapia en la laguna El Rodeo, Estado de Morelos, México. Tópicos de Investigación y Posgrado. III: 16-22.
Holden, M.J. & D.F.S. Raitt (eds.). 1975. Manual de ciencia pesquera. parte 2. Métodos para investigar los recursos y su aplicación. Doc. Tec. FAO. Pesca, 115, 1: 211 p. [ Links ]
King, M. 1995. Fisheries biology, assessment and management. Fishing News Books, Blackwell Science, Ltd., 341 p. [ Links ]
Morales, D. A. 1991. La Tilapia en México. Biología, Cultivo y Pesquerías. AG Editor, S.A. 190 p. [ Links ]
Morales, B.E. 1992. Evaluación de algunos aspectos biológico-pesqueros de la tilapia Oreochromis aureus (Steindachner, 1864) en la presa Vicente Guerrero, Las Adjuntas, Tamaulipas. Tesis de Licenciatura, ENEP-Iztacala, UNAM. 28 p.
Moyle, B. Peter & J.J. Cech, Jr. 2000. Fishes: An Introduction to Ichthyology. Fourth edition. Prentice Hall: 123-144.
Nikolsky, D.V. 1963. The ecology of fishe s. Part II. Academic Press, New York: 352 p.
Palacios, S.S.E. 1995. Estudio biológico pesquero de la tilapia Oreochromis aureus (Steindachner, 1864) en la presa Adolfo López Mateos (El Infiernillo), Michoacán-Guerrero, México. Tesis de Licenciatura, Facultad de Ciencias, UNAM. 81 p.
Ramos-Cruz, S. 1995. Reproducción y crecimiento de la mojarra tilapia (Oreochromis aureus) en la Presa Benito Juárez, Oaxaca, México, en 1993. INP-SEMARNAP. México, Ciencia Pesquera No. 11: 54-61. [ Links ]
Shoesmith E. 1990, A comparision of methods for estimating mean fecundity. J. Fish Biology 36: 29-37. [ Links ]
Salgado-Ugarte, I.H. 1995. Nonparametric methods for fisheries data analysis and their application in conjunction with other statistical techniques to study biological data of the Japanese sea bass Lateolabrax japonicus in Tokyo Bay. Ph. D. in Aquatic Bioscience Thesis, Laboratory of Fisheries Biology, University of Tokyo, Japan. 389 p.
SPSS Inc. 1993. SPSS for Windows. Release 6.0. SPSS Inc, Chicago III USA.
Wohlfarth G.W. & G. Hulata. 1983. Applied of Tilapias. ICLARM Studies and Reviews 6, 26 p. International Center for Living Aquatic Resources Management, Manila, Philippines.
Yi, Y, C.K. Lin & J.S. Diana. 1996. Influence of Nile tilapia (Oreochromis niloticus) stocking density on their growth and yield in cages and ponds containing the cages. Aquaculture, 146: 205-215.
1. Laboratorio de Limnología, F.E.S. Zaragoza, U.N.A.M. Av. 5 de Mayo y Fuerte de Loreto, Col. Ejército de Oriente, Iztapalapa. C.P. 09230, México, D.F., Fax: 5773 6336; lgomez@servidor.unam.mx .
2. Instituto de Limnología. Universidad de Guadalajara. A.P. 310, Chapala (45900) Jalisco; mguzman44@prodigy.net.mx .














