Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.1 San José Mar. 2003
Abstract
In this paper we use allozyme analyses to demonstrate that individuals in Anthopleura krebsi aggregates are monoclonal. Additionally, sympatric samples of the red and the green colour-morphs of A. krebsi from Pernambuco, Brazil were genetically compared and no significant differences were observed between them (gene identity= 0.992), indicating that they do not belong to different biological species. All individuals within aggregates of the green colour-morph were found to be identical over the five polymorphic loci analysed. Such results would be extremely unlikely (P<10 -11 ) if the individuals analysed had been generated through sexual reproduction, thus confirming the presence of asexual reproduction in this species.
Key words: Cnidaria, allozymes, clones, fission, molecular systematics.
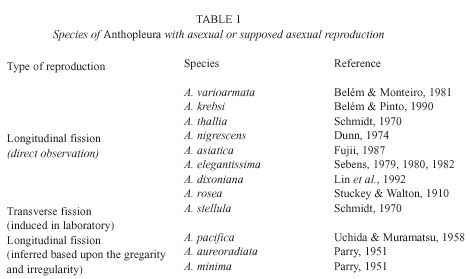
Sea anemones are polyp animals of Actiniaria, an order of skeletonless Anthozoa. Longitudinal fission has traditionally been acknowledged as the most common type of asexual reproduction in sea anemones of the family Actiniidae (Shick 1991). This type of reproduction has important ecological consequences, since it often leads to the production of geographically restricted clones, which can out-compete exclusively sexually reproducing species (Jackson 1985). This type of reproduction is reported to occur in eight out of the 42 species of the widely distributed sea anemone genus Anthopleura Duchassaing & Michelotti, 1860 (Table 1). Other three species of this genus have been inferred to reproduce asexually based upon their aggregation habit and the irregularity of some characters like number of mesenteries and siphonoglyphs. Based on the existence of aggregations, of regenerating individuals, and on the complete absence of gonads in anemones from some localities, Anthopleura krebsi has been presumed to be able to reproduce asexually (Carlgreen and Hedgepeth 1952, Belém and Pinto 1990, Gomes and Mayal 1997). Although gregarity is indeed very common among sea anemones that reproduce asexually (Hand 1955, Schmidt 1970, Shick and Lamb 1977), it is not a clear proof of asexual reproduction, since some species, like Bunodosoma caissarum Corrêa, 1964 reproduce exclusively sexually but can form very large aggregations (Belém 1987, Russo et al. 1994). Also, the observation of fission or other forms of asexual reproduction in aquaria demonstrates the potential of the species to reproduce asexually, but it doesnt indicate how common the process may be under natural conditions. The best approach for the confirmation of the presence of asexual reproduction in natural populations is the study of composite genotypes using genetic markers (Jackson 1985, Avise 1994).
Genetic markers have been used successfully to determine the asexual origin of broods of several species of sea anemones (Gashout and Ormond 1979, Carter and Thorp 1979, Schaefer 1981, Monteiro et al. 1998), and to confirm the asexual reproduction of Anthopleura legantissima Brandt, 1835 (Sebens 1982, McFadden 1997). The rationale behind the usage of molecular markers to study asexual reproduction is that it is extremely unlikely that two sexually produced individuals will be identical over a large number of polymorphic loci. Putative clonemates are, thus, those individuals in the population analysed that have identical multiloci genotypes when the cumulative probability (P) of that identity is very small (usually a conservative threshold value of P <10 -5 is used for each comparison; Monteiro et al. 1998).
In A. krebsi two colour morphs are very common: the so-called "red" and "green" varieties (Bigger 1980, Belém and Pinto 1990). The two types occur sympatrically on the same position in the intertidal area. Bigger (1980) studied the aggressive behaviour of A. krebsi from Florida with specimens inferred to be clonemates based upon their colour and proximity in aggregates. No aggression was observed between individuals from the same putative clone, neither between individuals supposedly from different clones of the "red" colour-morph. However, anemones from different clones of the green A. krebsi presented some aggressive behaviour against each other and invariably attacked anemones of the red morph. The constant antagonism between the anemones of the two colour-morphs, compared to the small aggression between clones of the red morph or some of the clones of the green morph, could be an indication that all anemones of the red morph were members of one single clone, whereas the green anemones belonged to different clones, or that, instead of being between individuals of the same species, the aggression was interspecific like that observed between the clonal and solitary forms of A. elegantissima studied by Francis (1979) and McFadden et al. (1997). In this case, the two colour-morphs should be raised to specific status.
The aims of this paper were, thus, to verify whether individuals in A. krebsi aggregates were produced asexually, and to use molecular markers to determine if sympatric samples of the red and the green morphs of A. krebsi belonged to different biological species. The technique chosen was the analysis of allozymes, which has been extremely useful for the study of reproductive biology in sea anemones and for clarifying taxonomic problems (McCommas and Lester 1980, Bucklin and Hedgecock 1982, Solé-Cava et al. 1985, Shaw et al. 1987, McFadden et al. 1997, Monteiro et al. 1997, 1998, Perrin et al. 1999).
Materials and methods
a) Collection of samples: Twenty anemones of the green morph and twenty-one samples of the red morph of Anthopleura krebsi were collected at Carneiros Beach (8º45 S, 35º05 W), Pernambuco State, Brazil, in January 1999. Care was taken to collect individuals that were at least 2 meters apart (as in Monteiro et al. 1997), to avoid the collection of clone-mates. The anemones were usually found in horizontal crevices on the upper area of the intertidal zone on the shore side of a beach rock (sensu Guilcher 1985). Red A. krebsi were normally found in the upper crevices while individuals of the green morph were found in the lower crevices.
Additionally, we collected all the individuals (n=7 and n=14, respectively) of two closely packet groups of the green variety of A. krebsi that were about 2 meters distant from each other. These individuals would normally be inferred to be "clone-mates", based on their proximity and their homogeneity in colour and size.
All anemones were transported alive in seawater and maintained in an aquarium until electrophoresis, which occurred no later than one week after collection.
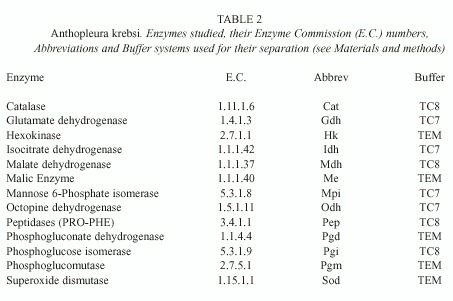
b) Electrophoresis: Samples were ground with a glass rod in a cold solution (1:1 vol/vol) of 0.1% NADP in 0.02 M Tris-HCl (pH 8.0). After homogenisation, the samples were inserted, on paper wicks, in a 13% hydrolysed starch gel, using three different buffer systems: 0.01 M Tris, 0.01M EDTA, 0.10 M Maleate, pH 7.4 ("TEM", Hillis et al. 1996); 0.25 M Tris, 0.06 M Citrate, pH 8.0 ("TC8", Ward and Beardmore 1977) and 0.135 M Tris, 0.043 M Citrate, pH 7.0 ("TC7", Shaw and Prasad 1970). Gels were run at a constant power of 3 Watts, during 6 (TC8), 7 (TC7) or 12 (TEM) hours. Stains for seventeen enzymes were tested, of which thirteen yielded reproducible results (Table 2). Genotype frequencies were used to estimate gene frequencies, and unbiased heterozygosity (h) and gene identity (I) levels (Nei 1978) using the BIOSYS 1.6 programme (Swofford and Selander 1981).
The electrophoretic work was divided into two phases: first, the 20 samples of each colour morph were analysed and compared. Second, the genotypes of the putative clone-mates were determined for the more polymorphic enzymes, to verify whether they indeed belonged to monoclonal colonies. For that, we used the composite probability of complete identity over all polymorphic loci analysed, using the gene frequencies of the green morph estimated in the first part of the work (as in Monteiro et al. 1998).
Results
Comparison between colour morphs:
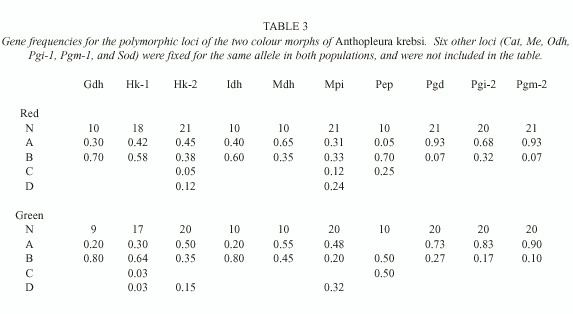
Gene frequencies of the two colour morphs of Anthopleura krebsi studied are given in Table 3. Levels of gene variation were high in the two morphs (h=0.282 and 0.283 for the red and green morphs, respectively), as usually found for sea anemones both from intertidal and sub-tidal
areas (Solé-Cava and Thorpe 1991). Genotype frequencies did not deviate significantly from Hardy-Weinberg expectations at any of the loci analysed (Fishers exact test, P>0.05, after Bonferronis transformation, Lessios 1992). A pair-wise comparison of the genotypes of the anemones of each colour morph demonstrated that each anemone collected within each colour morph was genetically unique. On the other hand, no diagnostic loci (sensu Ayala 1983) could be found between the two colour morphs, and a high level of gene identity (Nei 1978) was observed between them (I=0.992).
Comparison between putative clone-mates:
The genotypes of the individuals within each of the two putative clones analysed were identical over the five polymorphic loci analysed (Clone 1: Hk-2=AB, Mpi=AD, Pgd=AB, Pgi-2=AA, Pgm-2=AA; Clone 2: Hk-2=AA, Mpi=AB, Pgd=AB, Pgi-2=AA, Pgm-2=AA). The estimated compound probability of observing that complete genotype identity by chance alone, when sampling sexually produced individuals, was extremely small (binomial calculations; P<10 -11 for clone 1 and P<10 -28 for clone 2).
Discussion
The main results of this work were the genetic demonstration of asexual reproduction in Anthopleura krebsi and the high genetic similarity between its green and red colour morphs.
The genetic study of the two groups of individuals supposed to be clones, demonstrates that they originated through asexual reproduction. Given the high level of gene variation observed in A. krebsi (h=0.283), if the aggregations were the result of sexual reproduction they should have individuals with different genotypes. The complete genotype identity of the anemones within each clone, thus, clearly shows that they are clone-mates.
Populations of sea anemones that have facultative asexual reproduction are usually found, in the intertidal zone, to consist of locally distributed clones (e.g. Shick et al. 1979, Ayre 1983, Shaw 1991, McFadden et al. 1997). The population of A. krebsi studied here was found in the upper intertidal zone, where stress conditions include long periods of air exposure and high variation in air temperature (Gomes et al. 1998). The two clones studied were genetically distinct, regardless of the relatively short distance (2m) between them. Also, no putative clone-mates – defined in this study as individuals with identical multilocus genotypes – were found among the 41 individuals analysed. Since, during the fieldwork, care was taken to avoid collecting samples closer than 2 meters from each other, it can be said that clones in this species are unlikely to be spread over areas larger than 2 meters, at least in the region studied. Therefore, it appears that A. krebsi may have a clonal structure similar to that of Anthothoe albocincta, whose populations seem to consist predominantly of spatially restricted clones (Billingham and Ayre 1997) resulting from the local increase of population size through longitudinal fission, supplemented by the production of widely dispersed colonists through sexual reproduction.
Sympatric samples of different morphs should, if conspecific, be freely interbreeding. This means that, within sampling errors, they should have the same gene frequencies at each gene locus. They should be different only at the loci coding for the morphological difference used to separate them in the first place, and perhaps some closely linked loci. Usually, conspecific populations of marine invertebrates have gene identity values above 0.90 and rarely as low as 0.85, whilst between congeneric species the usual range is about 0.30 to 0.85 (Knowlton 1993, 2000, Thorpe and Solé-Cava 1994, Solé-Cava and Boury-Esnault 1999).
The sympatric populations of the red and green morphs of A. krebsi showed a high level of gene identity (I=0.992). This result and the absence of diagnostic loci, indicates that there is no restriction to gene flow between the two colour morphs of A. krebs i, i.e. we dont have any reason to believe, with the data at hand, that they do not belong to the same species.
Colour has been considered a unreliable character for sea anemone taxonomy (Stephenson 1928). However, in many cases it has proven to be a good first evidence of interspecific differences: several colour varieties of sea anemones were found to belong, in fact, to different species (Carter and Thorpe 1981, Haylor et al. 1984, Solé-Cava and Thorpe 1987, Perrin et al. 1999).
Contrastingly, no genetic differences were observed (McFadden et al. 1997) between colour morphs of A. elegantissima or in A. albocincta (Hutton, 1878) (Billingham and Ayre 1997). Also, colour morphs of Adamsia carcioniopados ("pink" and "white" mesenteric filaments), of Anemonia viridis ("green" and "red" columns) and of Metridium senile ("brown" and "white" columns) from Britain (A.M. Solé-Cava, unpublished results) and of Actinia bermudensis ("yellow", "green" and "red" columns) from Brazil (C. Russo, unpublished results) did not display any major genetic differences. There appears to be a strong bias in the literature for molecular systematics papers describing the detection of genetic isolation between colour morphs, in detriment of those indicating that they were conspecific. Molecular systematics has, indeed, demonstrated that, many times, the variation treated by taxonomists as phenotypic plasticity or intraspecific polymorphism was in fact due to interspecific differences (reviewed in Knowlton 2000). However, because of the publication bias in the field, this trend has been exaggerated, to a point that some may prematurely conclude that most of the described colour morphs in marine invertebrates do belong to different species. We believe that the best way to obtain a more balanced view of this problem will be through the publication not only of molecular systematics papers demonstrating hidden species, but also of those presenting genetic evidence of conspecificity between colour morphs.
Acknowledgements
This work was supported by grants from CNPq, FAPERJ and CAPES.
Resumen
En el presente trabajo se utilizó análisis de aloenzimas para demostrar que los individuos en los agregados de la especie Anthopleura krebsi son monoclonales. Además, muestras simpátricas de las variedades roja y verde fueron comparadas genéticamente y ninguna diferencia significativa fue observada entre ellas (Identidad génica= 0.992), indicando que ellas no pertenecen a especies biológicamente distintas. Todos los individuos dentro de un agregado de la variedad roja resultaron ser idénticos para los cinco loci polimórficos analizados. Tal resultado sería extremadamente improbable (p<10 -11 ) si los individuos analizados hubiesen sido generados a través de reproducción sexual, confirmando así la presencia de reproducción asexual en esta especie.
References
Avise, J.C. 1994. Molecular Markers, Natural History and Evolution. Chapman, London. 511 p. [ Links ]
Ayala, F.J. 1983. Enzymes as taxonomic characters. pp. 3- 26. In G.S. Oxford & D. Rollinson (eds.). Protein Polymorphism: Adaptive and Taxonomic Significance. Academic, London. [ Links ]
Ayre, D.J. 1983. The effects of asexual reproduction and intergenotypic aggression on the genotypic structure of populations of the sea anemone Actinia tenebrosa. Oecologia 57: 158-165. [ Links ]
Belém, M.J.C. & D.C. Monteiro. 1981. Fauna de Cnidários do Rio de Janeiro. III. Anthopleura var-ioarmata Watzl, 1922 (Actiniaria, Endomyaria), uma nova ocorrência de Actiniidae. Seminários de Biologia Marinha - Acad. Bras. Ci. pp. 193-203.
Belém, M.J.C. 1987. Anatomy and biology of Bunodosoma caissarum Corrêa, 1964 (Cnidaria, Anthozoa, Actiniidae) 1. Systematic position and morphological and microanatomical revision. An. Acad. Bras. Cienc. 59: 275-275. [ Links ]
Belém, M.J.C. & S.M. Pinto. 1990. Morphological and microanatomical study of Anthopleura krebsi Duchassaing and Michelotti, 1860 (Cnidaria, Anthozoa, Actiniidae), a new record in Brazil. An. Acad. Bras. Cienc. 62: 183-192.
Bigger, C.H. 1980. Interspecific and intraspecific acrorhagial aggressive behavior among sea anemones: a recognition of self and not-self. Biol. Bull. 159: 117-134.
Billingham, M.R. & D.J. Ayre. 1997. Asexual reproduction and genetic determination of colour patterns within populations of the subtidal sea anemone Anthothoe albocincta. Mar. Ecol. Prog. Ser. 156: 121-130.
Bucklin, A. & D. Hedgecock. 1982. Biochemical genetic evidence for a third species of Metridium (Coelenterata: Actiniaria). Mar. Biol. 66: 1-7. [ Links ]
Carlgreen, O. & J.W. Hedgepeth. 1952. Actiniaria, Zoantharia and Ceriantharia from shallow water in the North Western Gulf of Mexico. Pub. Inst. Mar. Sci. Univ. Texas 2: 141-172. [ Links ]
Carter, M.A. & C.H. Thorp. 1979. The reproduction of Actinia equina L. Var. mesembryanthemum. J. Mar. Biol. Ass. U. K. 59: 989-1001. [ Links ]
Carter, M.A. & J.P. Thorpe. 1981. Reproductive, genetic and ecological evidence that Actinia equina var. mesembrianthemum and var. fragacea are not conspecific. J. Mar. Biol. Ass. U. K. 61: 79-93.
Dunn, D.F. 1974. Redescription of Anthopleura nigrescens (Coelenterata, Actiniaria) from Hawaii. Pac. Sci. 28(4): 377-382. [ Links ]
Francis, L. 1979. Contrast between solitary and clonal lifestyles in the sea anemone Anthopleura elegantissima. Amer. Zool. 19: 669-681. [ Links ]
Fujii, H. 1987. The predominance of clones in populations of the sea anemone Anthopleura asiatica (Uchida). Biol. Bull. 172: 202-221. [ Links ]
Gashout, S.E. & R.F.G. Ormond. 1979. Evidence for parthenogenetic reproduction in the sea anemone Actinia equina L. J. Mar. Biol. Ass. U.K. 59: 975-987. [ Links ]
Gomes, P.B. & E.M. Mayal. 1997. Levantamento preliminar das anêmonas-do-mar (cnidaria, actiniaria) da costa de Pernambuco, Brasil. Biociências 5: 45-52.
Gomes, P.B., M.J.C. Belém & E. Schlenz. 1998. Distribution, abundance and adaptations of three species of Actiniidae (Cnidaria, Actiniaria) on an intertidal beach rock in Carneiros Beach, Pernambuco, Brazil. Misc. Zool. 21(2): 65-72. [ Links ]
Guilcher, A. 1985. Coral Reef Geomorphology. I-XIII. Wiley, New York. 228 p. [ Links ]
Hand, C. 1955. The sea anemones of Central California. pt. 2. The Endomyarian and Mesomyarian anemones. Was. J. Biol. 13: 37-99. [ Links ]
Haylor, G.S., J.P. Thorpe & M.A. Carter. 1984. Genetic and ecological differentiation between sympatric colour morphs of the common intertidal sea anemone Actinia equina. Mar. Ecol. Prog. Ser. 16: 281-289. [ Links ]
Hillis, D.M., S. Moritz & B.K. Mable. 1996. Molecular Systematics. Sinauer, Massachusetts. 588 p. [ Links ]
Jackson, J.B.C. 1985. Distribution and ecology of clonal and aclonal benthic invertebrates, pp. 297-353. In J.B.C. Jackson, L.W. Buss & R.E. Cook (eds.). Population biology and evolution of clonal organisms. Yale University, London.
Knowlton, N. 1993. Sibling species in the sea. Annu. Rev. Ecol. Syst. 24: 189-216.
Knowlton, N. 2000. Molecular genetic analyses of species boundaries in the sea, pp. 73-90. In A.M. Solé-Cava, C.A.M. Russo & J.P. Thorpe (eds.). Marine Genetics. Kluwer, Dordretch, The Netherlands.
Lessios, H.A. 1992. Testing electrophoretic data for agreement with Hardy-Weinberg expectations. Mar. Biol. 112: 517-523. [ Links ]
Lin, J., C.P. Chen & I.M. Chen. 1992. Sexual and asexual reproduction of Anthopleura dixoniana (Anthozoa: Actiniaria): periodicity and regulation. Mar. Biol. 112: 91-98. [ Links ]
McCommas, S.A. & L.J. Lester. 1980. Electrophoretic evaluation of the taxonomic status of two species of sea anemone. Biochem. Syst. Ecol. 8: 289-292. [ Links ]
McFadden, C.S. 1997. Contributions of sexual and asexual reproduction to population structure in the clonal soft coral, Alcyonium rudyi. Evolution 51: 112-126. [ Links ]
McFadden, C.S., R.K. Grosberg, B.B. Cameron, D.P. Karlton & D. Secord. 1997. Genetic relationships within and between clonal and solitary forms of the sea anemone Anthopleura elegantissima revisited: Evidence for the existence of two species. Mar. Biol. 128: 127-139.
Monteiro, F.A., A.M. Solé-Cava & J.P. Thorpe. 1997. Extensive genetic divergence between populations of the common intertidal sea anemone Actinia equina from Britain, the Mediterranean and the Cape Verde Islands. Mar. Biol. 129: 425-433.
Monteiro, F.A., C.A.M. Russo & A.M. Solé-Cava. 1998. Genetic evidence for the asexual origin of small individuals found in the coelenteron of the sea anemone Actinia bermudensis McMurrich. Bull. Mar. Sci. 63:257-264. [ Links ]
Nei, M. 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89: 583-590. [ Links ]
Parry, G. 1951. The Actiniaria od New Zealand, part I. Records of the Canterbury Museum 6: 83-119. [ Links ]
Perrin, M.C., J.P. Thorpe & A.M. Solé-Cava. 1999. Actinia equina: a genetic role model and reproductive enigma. Oceanogr. Mar. Biol. Annu. Rev. 37: 129-152. [ Links ]
Russo, C.A.M., A.M. Solé-Cava & J.P. Thorpe. 1994. Population structure and genetic variation in two tropical sea anemones (Cnidaria, Actinidae) with different reproductive strategies. Mar. Biol. 119: 267-276. [ Links ]
Schaefer, W. 1981. Reproduction and sexuality of Cereus pedunculatus and Actinia equina (Anthozoa, Actiniaria). Helgol. Meer. 34: 451-461. [ Links ]
Schmidt, H. 1970. Anthopleura stellula (Actiniaria: Actiniidae) and its reproduction by transverse fission. Mar. Biol. 5: 245-255.
Sebens, K. P. 1979. The energetics of asexual reproduction and colony formation in benthic marine invertebrates. Amer. Zool. 683-697. [ Links ]
Sebens, K.P. 1980. The regulation of asexual reproduction and indeterminate body size n the sea anemone Anthopleura elegantissima (Brandt). Biol. Bull. Mar. Biol. Lab. 158: 370-382. [ Links ]
Sebens, K.P. 1982. Asexual reproduction in Anthopleura elegantissima (Anthozoa: Actiniaria): seasonality and spatial extent of clones. Ecology 63: 434-444. [ Links ]
Shaw, P.W. 1991. Effects of asexual reproduction on population structure of Sargatia elegans (Anthozoa: Actiniaria). Hydrobiologia 216/217: 519-525. [ Links ]
Shaw, C.R. & R. Prasad. 1970. Starch gel electrophoresis of enzymes- A compilation of recipes. Biochem. Genet. 4: 297-320. [ Links ]
Shaw, P.W., J.A. Beardmore & J.S. Ryland. 1987. Sagartia troglodytes (Anthozoa: Actiniaria) consists of two species. Mar. Ecol. Prog. Ser. 41: 21-28. [ Links ]
Shick, J.M. 1991. A functional biology of sea anemones. Chapman, London. 395 p. [ Links ]
Shick, J.M., R.J. Hoffman & A.N. Lamb. 1979. Asexual reproduction, population structure, and genotype-environment interactions in sea anemones. Amer. Zool. 19: 699-713. [ Links ]
Shick, J.M. & A.N. Lamb. 1977. Asexual reproduction and genetic population structure in the colonizing sea anemone Haliplanella luciae. Biol. Bull. 153: 604-617.
Solé-Cava, A.M. & N. Boury-Esnault. 1999. Levels of inter and intraspecific differentiation in marine sponges. Mem. Queensland Mus. 44: 591-602.
Solé-Cava, A.M. & J.P. Thorpe. 1987. Further genetic evidence for the reproductive isolation of green sea anemone Actinia prasina Gosse from common intertidal beadlet anemone Actinia equina (L.). Mar. Ecol. Prog. Ser. 38: 225-229.
Solé-Cava, A.M. & J.P. Thorpe. 1991. High levels of genetic variation in natural populations of marine lower invertebrates. Biol. J. Linn. Soc. 44: 65-80. [ Links ]
Solé-Cava, A.M., J.P. Thorpe & J.G. Kaye. 1985. Reproductive isolation with little genetic divergence between Urticina (= Tealia) felina and U. eques (Anthozoa: Actiniaria). Mar. Biol. 85: 279-284.
Stephenson, T.A. 1928. The British Sea anemone. Ray Soc. 113: 1-148. [ Links ]
Stuckey, F.G.A. & C.L. Walton. 1910. Notes on a collection of sea-anemones. Transactons of the New Zealand Institute 42: 541-543. [ Links ]
Swofford, D.L. & R.B. Selander. 1981. BIOSYS-1, a FORTRAN programme for the comprehensive analysis of electrophoretic data in population genetics and systematics. J. Hered. 72: 281-283.
Uchida, T. & S. Muramatsu. 1958. Notes on some japanese sea-anemones. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 14: 111-119. [ Links ]
Thorpe, J.P. & A.M. Solé-Cava. 1994. The use of allozyme electrophoresis in invertebrate systematics. Zool. Scripta 23: 3-18. [ Links ]
Ward, R.D. & J.A. Beardmore. 1977. Protein variation in the plaice (Pleuronectes platessa). Genet. Res. 30: 45-62. [ Links ]
1. LAMAMEBEN, Departamento de Zoologia-CCB, Universidade Federal de Pernambuco, Av. Prof. Moraes Rego 1235, Cidade Universitária, Recife-Pe, 50670-901, Brazil. bragagomes@hotmail.com
2. Laboratorio de Biología de Cnidarios, Depto. Cs. Marinas, FCEyN, Funes, 3250 (7600), Mar del Plata - Argentina. CONICET Research.
3. Molecular Biodiversity Lab. Departamento de Genética, Instituto de Biologia, Bloco A, CCS, Universidade Federal do Rio de Janeiro, Ilha do Fundão, CEP 21941-590, Rio de Janeiro, RJ, Brazil and Port Erin Marine Laboratory, University of Liverpool, Isle of Man, IM9 6JA, UK.














