Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.1 San José Mar. 2003
Abstract
We visited 51 lakes in Costa Rica as part of a broad-based survey to document their physical and chemical characteristics and how these relate to the mode of formation and geographical distribution of the lakes. The four oxbow lakes were low in elevation and tended to be turbid, high in conductivity and CO2 , but low in dissolved O2 ; one of these, L. Gandoca, had a hypolimnion essentially composed of sea water. These were similar to the four wetland lakes, but the latter instead had low conductivities and pH, and turbidity was often due to tannins rather than suspended sediments. The thirteen artificial lakes formed a very heterogenous group, whose features varied depending on local factors. The thirteen lakes dammed by landslides, lava flows, or lahars occurred in areas with steep slopes, and were more likely to be stratified than most other types of lakes. The eight lakes that occupy volcanic craters tended to be deep, stratified, clear, and cool; two of these, L. Hule and L. Río Cuarto, appeared to be oligomictic (tending toward meromictic). The nine glacial lakes, all located above 3440 m elevation near Cerro Chirripó, were clear, cold, dilute, and are probably polymictic. Cluster analysis resulted in three significant groups of lakes. Cluster 1 included four calcium-rich lakes (average 48 mg l-1 ), Cluster 2 included fourteen lakes with more Si than Ca+2 and higher Cl - than the other clusters, and Cluster 3 included the remaining thirty-three lakes that were generally less concentrated. Each cluster included lakes of various origins located in different geographical regions; these data indicate that, apart from the high-altitude glacial lakes and lakes in the Miravalles area, similarity in lake chemistry is independent of lake distribution.
Key words: Costa Rica, limnology, lakes, tropical, lake ecology, lake biogeography.
Costa Rica has long been a mecca for tropical biology, yet most visiting biologists are probably unaware of the number and diversity of its lakes. Only in the past two decades has much attention been directed toward its lakes. In 1985, the Instituto Geográfico Nacional prepared an unpublished list of all lakes shown on its 1:50 000 scale topographic maps. The surprisingly high total of 652 lakes underestimated the total number of lakes, because those smaller than about 0.1 hectares were often not mapped. However, by the time the list was prepared, many of the floodplain lakes shown on the maps had already been drained for agriculture. Other lakes have been drained or filled subsequently, such that the total number of lakes in Costa Rica today is probably lower than reported by IGN – but still very high for a country of its size.
A very complete, recent overview of limnological research in Costa Rica was compiled by Umaña et al. (1999). Much of the early aquatic biology was concerned with disease vectors and water as a resource. Most publications on Costa Rican lakes have focused on a single lake (or on a small number of similar lakes) and how they have changed over time (see review in Umaña et al. 1999). Bumby (1982) and later Horn and Haberyan (1993) published the first surveys that included data from large numbers of lakes of differing origin and geographical setting.
This paper reports first-time observations of the physical and chemical properties of 43 lakes, along with additional data on 8 lakes we have previously visited (Horn and Haberyan 1993). These observations supplement and expand our recent limnological summary of the country (Umaña et al. 1999), providing baseline data against which future changes in lake conditions can be assessed. The limnological observations reported here also improve our understanding of the ecology of modern lake biota and therefore assist our interpretation of past lake conditions from diatoms and other microfossils in lake sediments (Chavez and Haberyan 1997, Haberyan and Horn 1999a, Zeeb et al. 1996, Wujek et al. 1998).
Materials and methods
Most of these data were collected during July 1997, with supplemental visits in March 1998 and March 1999. In general, water samples were collected near the middle of the lakes, but occasionally external constraints (time, weather, lake smallness, or crocodiles) caused us to sample at the edge.
Lake name, location, elevation, and area were mainly extracted from the 1:50 000 scale topographic maps published by the Instituto Geográfico Nacional de Costa Rica. For smaller lakes not shown on topographic maps, we estimated sizes in the field, and gave informal names based on local villages or families. For glacial lakes in Chirripó National Park we calculated areas from 1:14 000 scale aerial photography (Horn et al. 1999).
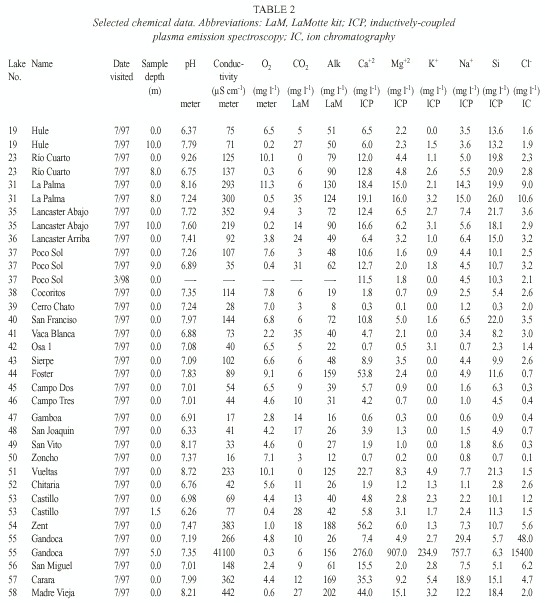
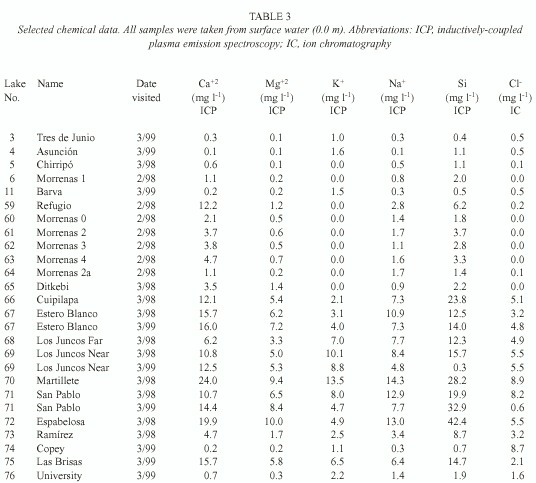
We used field meters for temperature and oxygen (YSI model 55), pH (Oakton pH Wand), and conductivity (Hanna HI 8733); transparency was measured with a Secchi disk. Samples collected in 1997 were filtered through pre-ashed Whatman GF/C filters, and usually analysed immediately for carbon dioxide and within five hours for alkalinity using LaMotte field tests; samples collected during February-March 1998 and March 1999 were not filtered. Air-free samples were returned for additional analysis, including chloride (using ion chromatography, IC), calcium, magnesium, potassium, sodium, and silicon (using inductively-coupled plasma emission spectroscopy, ICP).
To group the lakes, we performed a k-means cluster analysis with six chemical variables from surface water in all lakes (Ca+2, Mg+2, K+ , Na+ , Si, and Cl - ). ANOVA was used to check for differences among clusters, using variables including elevation, surface area, maximum depth, relative depth (i.e. maximum depth relative to mean lake diameter), conductivity, pH, and dissolved oxygen. Missing data were excluded on a case-deletion basis, thus the total sample size for each test varied depending on data availability. The results were used to assess the importance of geography in lake similarities.
Results
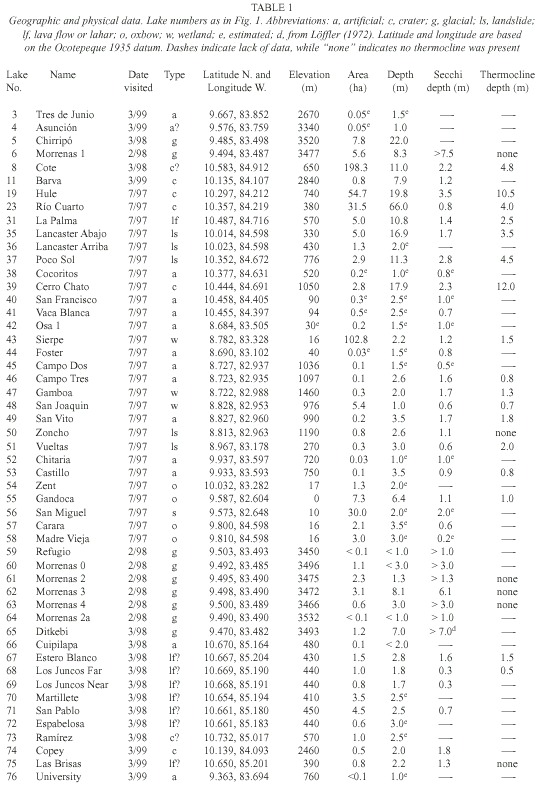
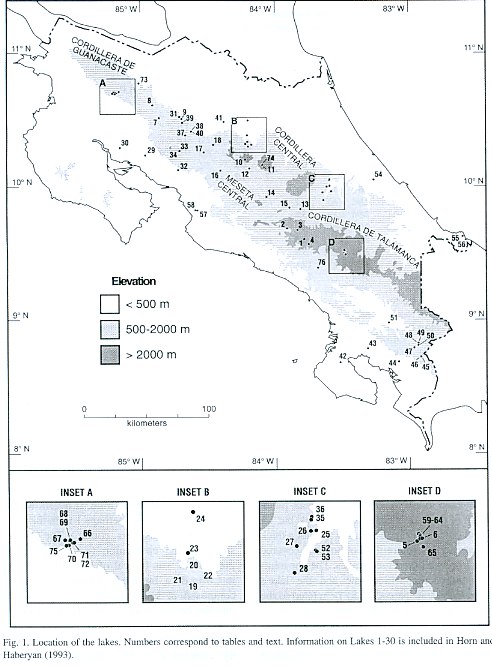
For purposes of discussion, we group the lakes by mode of formation. Because of the abundance of crater lakes, we also group these by region. Physical data are summarized in Table 1, chemical data are summarized in Tables 2 and 3, and location is given in Figure 1. Lake numbers expand upon those in Horn and Haberyan (1993).
Oxbow Lakes
Laguna Carara (Lake 57) is a turbid oxbow lake beside its parent river, the Río Tárcoles, in the Carara Biological Reserve in the central Pacific lowlands of Costa Rica. According to reserve staff, the lake often receives overflow from the Rió Tárcoles in the rainy season, and so reverts to an alternate river channel; consequently, its sediments are probably removed annually. At the time of our visit, the lake was not receiving overflow from the river. Its elevated CO2 content (12 mg l-1 ) and low O2 content (Table 3) suggest an elevated organic content from the surrounding forest and floodplain, although the color of the lake seemed primarily due to suspended sediment. The lake is home to crocodiles (Herrera 1992), which has deterred sampling. Floating vegetation is scarce, presumably due to frequent flushing by the Río Tárcoles.
Laguna Madre Vieja (58) lies immediately across the Río Tárcoles from L. Carara, but probably owes its origin to to the damming of a tributary stream by Río Tárcoles alluvium rather than to the cutoff of an old meander. Most major ions are more abundant in L. Madre Vieja than in L. Carara, and its surface is carpeted with water lilies; these observations suggest that L. Madre Vieja is not flushed with waters from the R. Tárcoles.
Laguna Zent (54) is an oxbow lake formed by the Río Chirripó just downstream of its emergence onto the Atlantic coastal plain. Much of the lake is covered with lilies, but we found open water in the shallows under a thick forest canopy, where the bottom was covered with very well preserved leaves. The apparently slow decomposition rate and the lakes remarkable transparency may be related to the likely input of pesticides from the extensive local banana plantations. Such pesticide inputs may also limit autochthonous productivity and thereby explain the unusual concentrations of CO2 (18 mg l-1 ) and O2 (1.0 mg l-1).
Laguna Gandoca (55) is the most remarkable oxbow lake in our survey. It is an oxbow of the Río Gandoca that, according to the 1:50 000 map, opens to the Caribbean, but according to local residents has been blocked by a sandbar for some time. It overflows the sandbar to a depth of perhaps 1 m in the rainy season (S. Vargas, pers. comm.). The composition of the epilimnion (e.g. conductivity 266 µS cm-1 ) is typical of low-altitude lakes in Costa Rica, but the hypolimnion is largely composed of sea water that has percolated into the basin (conductivity 41,100 µS cm-1).
Consequently, stratification is very stable (approximately 1000 g-cm cm-2 ). This stability, coupled with the minimal exposure to wind (due to both the surrounding rain forest and to the small fetch the lake is narrow and strongly curved) suggest that the lake may well be meromictic.
Wetland Lakes
The four lakes in this group have varied origins, but we group them because they are heavily colonized by aquatic macrophytes. In general, their mode of formation is not clear.
Laguna Gamboa (47) is a deep, tannin-stained lake in a large herbaceous marsh. Remnant premontane rainforest occurs on two hills bordering the marsh. The lakes color, its low O2 concentrations, and its high CO2 levels suggest that much of the carbon is allochthonous.
Laguna San Joaquin (48) is a small area of open water (0.07 ha) in a much larger marsh (5.37 ha), largely colonized by Najas sp. Like other wetland waters, it is low in dissolved minerals and O2, but elevated in CO2 . We observed a slight decrease in temperature near the bottom.
Pantano San Miguel (56) lies near sea level on the Caribbean coast. Local residents indicated that the swamp recently contained open water, but at the time of our visit (July 1997) its waters were completely covered by water lilies and other rooted vegetation. The area of standing water probably varies strongly with the season. Like other swamp waters, O2 concentrations are low and CO2 concentrations are high, but San Miguel is also influenced by the nearby ocean (within 5 km), as reflected in its elevated conductivity (148 µS cm-1 ) and ionic content (Table 3).
Laguna Sierpe (43), on the Pacific coast, is a large, shallow expanse of water surrounded by floating mat vegetation, including cane, a cord grass, and others. Its waters are warm and tannin-stained. The lake lies in a broad valley very near sea level which may have originally been an arm of the ocean, now colonized by swamp vegetation and freshened by rainwater and stream inputs. Its bottom is probably very flat at 2.2 m deep, but despite the great exposure of the lake, we found a slight change in temperature at 1.5 m.
Artificial Lakes
Laguna Asunción (4) is a tiny, high-elevation pond very close to the highest point along the route of the Inter-American Highway across the Cordillera de Talamanca. The pond is visible on a photograph taken in 1949 (Fig. 2A in Horn 1989), but may owe its origin to road grading activities already taking place at that time. Although small and shallow, the lake seems to persist even through extreme dry years. The lake is fringed by tropical páramo vegetation dominated by dwarf bamboo (Chusquea subtessellat a) and evergreen shrubs in the Ericaceae, Hypericaceae, and Compositae families (Horn 1989). Its waters are very low in dissolved minerals and in pH. The major difference in samples from July 1991 (rainy season) and March 1999 (dry season) is a much lower concentration of Cl - in the more recent sample.
Laguna Tres de Junio (3) probably also owes its origin to the construction of the Inter-American Highway, the roadbed of which blocked its drainage. The shores of the lake are fringed with Sphagnum and by shrubby vegetation that resembles the páramos of the higher peaks. Weber (1959) referred to this páramo like vegetation below the treeline as "paramillo." Values for most dissolved ions were reduced in the most recent sample (March 1999) relative to July 1991, especially Cl- and Ca+2 . Laguna Chitaria (52) is a tannin-stained lake that probably also owes its origin to highway construction. Local residents claim that it holds water year round, despite its shallow depth. Its edges are densely vegetated with sedges and grasses.
Laguna Castillo (53) is located near L. Chitaria. In the rainy season, an "island" of floating cane covers the majority of the lake surface, leaving open water only near the shore. In addition, a thick layer of dead cane covers the bottom, making it impossible to collect a grab sample and difficult to estimate the true depth of the lake (we measured 3.5 m of water near the shore; the center of the lake is probably deeper). Partly because of its lack of stream inflows, we suspect that lake level falls dramatically in the dry season, perhaps sufficiently to become a swamp without open water.
Laguna Cocoritos (38) is a small lake dammed for watering livestock. Its most distinctive feature is the orange flocculent layer, at least 20 cm thick, that covers the bottom. Its alkalinity is low (19 mg l-1) relative to its conductivity (114 µS cm-1), and its size suggests it may dry out completely in the dry season.
Laguna Vaca Blanca (41) resembles L. Castillo in having a central "island" of vegetation surrounded by a moat of open water. It was apparently a natural wetland that was flooded by construction of a dam shortly before our visit in July 1997. The vegetation still influences the lakes composition quite strongly, especially the low O2 and high CO2 levels.
Laguna San Francisco (40) is an ornamental pond near L. Vaca Blanca, with some-what elevated ion content (conductivity 144 µS cm-1 ). Among its notable features are the near-equivalency of O2 and CO2 levels.
Laguna San Vito (49) is located near the Laguna San Joaquin, and was apparently formed by road construction. It is relatively deep for its size, and stratified. Its pH is among the highest we have encountered (8.17), and its waters are very dilute. Much of the bottom is covered with Najas sp.
Laguna Campo Dos (45) and Laguna Campo Tres (46) are a few km apart and are quite similar in morphology and chemical composition, although L. Campo Tres is deeper and was stratified at the time of our visit. Laguna Osa 1 (42) is a farm pond on the Osa Penninsula; it may dry out during the dry season, but during our visit was clear and very warm, and its bottom was covered by macrophytes. Its waters are uncharacteristically dilute (conductivity 40 µS cm-1 ) for a small, seasonal pond.
Laguna Foster (44) formed between a hillside and a railroad bed, and is long and narrow. Its waters are rich in Ca+2 (53.8 mg l-1 ) although its conductivity is only moderate (89 µS cm-1 ).
Laguna Cuipilapa (66) is a shallow artificial lake on the lower slopes of Volcán Miravalles in northwestern Costa Rica. Its chemical composition resembles nearby natural lakes.
Laguna University (76) is a small pond on the San Isidro branch campus of the Universidad Nacional. It was originally described to us as a natural pond, but field inspection suggested it probably owes its origin to road construction. Its waters are typical of the more dilute lakes in the country.
Lava-dammed and Lahar-dammed Lakes
Laguna La Palma (31) is located on the north slope of Volcán Arenal, some 2 km from the summit. Alvarado (2000) mapped the lake as being dammed by a lava flow between 1525 and 1650 A.D.. It has no surface outlet and is surprisingly concentrated (surface conductivity 293 µS cm-1). It is deep enough (10.8 m) to stratify (at 2.5 m), but its surface and deep waters are quite similar in content except for deep water enrichment in CO2 (35 mg l -1 deep vs 6 mg l -1 surface) and H+ (pH 7.24 deep vs 8.16 surface), and depletion of O2 (0.5 vs 11.3 mg l-1).
Laguna Estero Blanco (67) is broad and shallow, and data from our two visits differ only slightly. Its chemistry is typical of the six other "Miravalles lakes," a group of natural lakes on the lower south slope of Volcán Miravalles (Inset A in Fig.1) whose descriptions follow. These lakes occupy depressions within a ~20 km2 area of undulating terrain formed by lava and lahar flows and by the deposition of material produced by the explosion and erosion of earlier volcanic structures (Alvarado 2000). The lakes are surrounded mainly by pasture, with remnant patches of tropical dry forest. Despite the pronounced seasonal aridity of this part of Costa Rica, these lakes hold water year-round, making them important sites for paleoenvironmental research.
Laguna Martillete (70) is colonized by water hyacinth but had large areas of open water in March 1998. Its waters are the most concentrated in most chemical species of any Miravalles lake; only Espabelosa contained a greater concentration of Mg+ and of Si.
Laguna Los Juncos Far (68) and Laguna Los Juncos Near (69) form a single lake during the wet season, when lake levels rise by a meter or more. Their waters bore a distinct orange tinge. We visited both in March of 1998, and found their waters to be similar in most chemicals; however, Los Juncos Far contained a greater concentration of potassium (7.0 vs 0.1 mg l-1 ). A repeat visit to Los Juncos Near in March of 1999 revealed remarkable differences, specifically much more K+ but less Na+ and much less Si.
Laguna San Pablo (71) is a broad, shallow lake with two large (>100 m2 ) floating islands of vegetation that are pushed back and forth across the lake by strong dry-season winds. Its waters are typical of Miravalles lakes. Relative to our first visit (March 1998), data from one year later indicate markedly increased concentration of Ca+2 and Si, while K+ , and especially Cl- were noticeably more dilute. These data are not consistent with the changes we observed in Los Juncos Near (see above), so lake-specific factors seem more likely than regional ones. In March 1999 the lake showed slight decline in temperature and dissolved oxygen with depth.
Laguna Espabelosa (72) is fringed with water hyacinth and other aquatic plants; during the wet season it receives some overflow from Laguna San Pablo. Its waters are somewhat more concentrated in most chemicals than those of San Pablo, particularly Ca+ , Na+ , Si, and Cl- .
Laguna Las Brisas (75) was very low and entirely covered in water hyacinth and other aquatic plants when we visited in March 1998, but the following March, its water level was higher and part of the surface was open. We measured a maximum depth of 2.15 m along the edge of the central mat of aquatic vegetation; depths under the vegetation were possibly greater. Its chemical composition is typical of Miravalles lakes.
Landslide Lakes
Laguna Lancaster Arriba (36) probably formed in a slump rotation. When we cored it in March 1994, the lake was 5.9 m deep and at least half of its surface was open water; by July 1997, the entire surface had been covered by aquatic vegetation including hyacinths, ferns, and Lemna sp. Perhaps because of this shading, its O2 levels are low and its CO2 levels are elevated. The outflowing stream smelled strongly of sulfur, but ICP analyses of lake water revealed only moderate concentrations (0.7 mg l-1 total S).
Laguna Lancaster Abajo (35) is some 100 m lower in elevation than L. Lancaster Arriba, and may also have formed due to slumping. It is probably stratified for much of the year due to its relative depth and sheltered location, in addition to cool stream water inputs. The concentrations of most ions we measured are comparable between surface and deep waters, but the conductivity of surface water is over 60% greater than deepwater.
Laguna Poco Sol (37) probably originated due to a slump or other type of landslide. Alvarado (1989) suggested that the lake might be an explosion crater, but this was based on a false report of a lake depth difficult to explain in any other way. We measured a maximum depth of 11.5 m, which is relatively deep for the lakes area but falls considerably short of the early report of a 70 m depth. The lake bottom is relatively flat, and slightly deeper adjacent to the hill that was the likely source of the landslide or slump. Laguna Poco Sol has been stratified during all visits, including the cool, dry season (e.g. Armoudlian and De Moraes 1994) when we found the thermocline at 8 m (March 1998) compared to 3 m in the rainy season (July 1997). The lakes sheltered location, depth, and cool stream inputs enhance this stratification.
Laguna Zoncho (50) is a small natural lake that formed by faulting or large-scale slumping, or by damming behind slumped debris. Submerged aquatic vegetation covers much of its bottom. The lake is very fresh (conductivity 16 µS cm-1, alkalinity 12 mg l-1 ). Sediment cores from the lake preserve micro-fossil evidence of prehistoric agriculture and forest clearance in the watershed (Clement and Horn 2001).
Laguna Vueltas (51) is known as L. Chocuacos by local residents, but we adopt Laguna Vueltas from the topographic map and to avoid confusion with another lake named L. Chocuaco. It was apparently formed by slump rotation from the adjacent steep slope. At the time of our visit in July 1997, L. Vueltas was warm and turbid. Its productivity is apparently high, based on elevated O2 (10.1 mg l-1 ) and depleted CO2 (0 mg l-1 ) levels. The lake has been stocked with tilapia for recreational fishing.
Crater Lakes of the Cordillera Central
These lakes range in elevation from 380 m to 2 840 m, but are similar in morphology; most of them occupy a steep-sided crater, at least in part. The lakes are described in detail in Haberyan and Horn (1999b), but we review their limnology here for completeness.
Laguna Barva (11) lies in a volcanic crater at 2 840 m elevation and was unstratified at the time of our visits, although oxygen concentrations declined slightly with depth. Despite its small watershed and relatively great depth, its waters were rather turbid (Secchi depth 1.2 m). Like many volcanic crater lakes, however, it is rather fresh (conductivity 60 µS cm-1 , hardness 3.7 mg l-1 CaCO 3 , Horn and Haberyan 1993).
Our visit in March of 1999 revealed surprising differences in some ions. Each of the ten chemical species we detected in 1991 was less common in 1998, including Ca+2 (0.9 mg l -1 in 1991 to 0.2 mg l -1 in 1998), Na+ (7.4 to 0.3 mg l -1), and SO4-2 (4.8 to 0.4 mg l-1); the decrease among each of the ten chemical species ranged from a factor of 1.3 to a factor of 13. These differences cannot be attributed to seasonality, because the more concentrated July samples were actually collected during the rainy season, while the more dilute March samples were collected near the end of the dry season. Consequently, these changes in ionic concentrations may reflect important changes in the lake.
Laguna Copey (74) is a shallow lake located a few km from Laguna Barva. We visited the lake under heavy clouds and mist that never afforded a clear view of the lake or its surroundings. We suspect that the lake occupies the unnamed crater, depicted without a lake, to the ENE of Laguna Barva on the 1:50 000 Barva topographic sheet. The lake bottom was covered with vegetation in some spots and rocks in others. Its waters are very similar to those of Laguna Barva but are more enriched in chloride.
Laguna Hule (19) is the largest of three lakes in an explosion crater or maar located at an elevation of 740 m on the northern slope of Volcán Poás (Horn 2001). The lake seems to be stratified for several years at a time, based on increased similarity of surfacewater and deep-water data in 1997 relative to 1991 (Haberyan and Horn 1999b), and based on the reports from local residents of occasional fish kills associated with turbid water and strong odors. Lake stratification is probably weakest in January, during the cool, windy season.
Such a turnover event occurred in January of 1997 and explains the strong decrease in deepwater CO2 levels between our visits of July 1991 (65 mg l-1 ) and July 1997 (27 mg l-1 ).
There is only slight danger to local residents from degassed CO2 , however, because of periodic turnover and because the hypolimnion of the lake (about 2.5 x 106 m3 , Gocke 1996) could hold up to about 6.2 x 103 m3 of the gas. This volume of gas is sufficient to fill the crater by about 2 m, but is far below the volume released by Lake Nyos, Cameroon, in 1986 (approximately 0.9 to 1.2 x 109 m3 , Kling et al. 1987).
Much of the CO2 in Laguna Hule may originate from subsurface springs. Anomalously old radiocarbon dates on surface sediments of the lake (Horn 2001) suggest the presence of isotopically-dead CO2 of volcanic origin, which could enter the lake through volcanic springs (Olsson 1986).
Laguna Río Cuarto (23) is a second maar lake located about 7 km north of L. Hule, at 380 m elevation along the same N-S trending fracture zone (Alvarado 2000). Laguna Río Cuarto also seems to have turned over in January 1997; at that time, local residents report it was red in color; its waters were still unusually turbid in the following July (Secchi depth 0.8 m, versus 6.1 m in July 1991), and there were reduced differences in surface water and deep water chemistry. In addition, we observed centimeter-sized flakes of unknown composition that dissolved during overnight storage, but ICP revealed undetectable concentrations of most metal ions (Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn). These data indicate that Laguna Río Cuarto, like Laguna Hule, is oligomictic rather than meromictic (Haberyan and Horn 1999b).
Crater Lakes in the Cordillera de Guanacaste
Laguna Cerro Chato (39) occupies the crater of Volcán Chato, near the active Volcán Arenal in northwestern Costa Rica. L. Cerro Chato lies at a much lower altitude than other volcanic crater lakes in Costa Rica, and in a much windier part of the country. However, it was weakly stratified (1.4º C gradient) around 12 m deep, perhaps because its surface lies farther below the crater rim. Judging by the difficulty in obtaining dredge samples, the bottom is strewn with large rocks. Previous visitors have noted extreme variations in pH in space and time in the lake (Umaña and Jiménez 1995), doubtlessly influenced by the lakes extremely low alkalinity (8 mg l-1).
Lago Cote (8) owes its origin either to volcanic or tectonic forces (Alvarado 2000); it has a flat bottom at 9-11 m depth. Although we found no thermocline in March of 1998, we did note a strong decrease in oxygen concentrations below about 7 m, suggesting that the lake had been poorly mixed.
Laguna Ramírez (73) is apparently of volcanic origin, located on the lower slope of Volcán Tenorio. It occupies a small closed depression at 570 m, which presumably was formed by an explosion of steam or volcanic ejecta. Remnant forest exists on one side of the lake, but much of its basin has been cleared and used for cattle grazing. Its waters are less concentrated in most chemicals we measured than the nearby Miravalles lakes.
Glacial Lakes
About 50 glacial lakes and ponds appear on the 1:50 000-scale topographic maps of Costa Rica, all above 3 440 m in elevation in a 15 km2 area surrounding Cerro Chirripó. The lakes occupy glacial depressions formed in bedrock, in till deposits, or a combination of the two. More than half generally hold water year round. We have sampled six of these lakes (numbers 59 to 65) once and two more of them (number 5 Chirripó and number 6 Morrenas 1) twice. Bedrock in most of the lake basins consists principally of granodiorite and other plutonic rocks of the Miocene-age Talamancan intrusive series; the exception is the basin of Lago Ditkebi, which appears to be quarried in pyroclastic deposits (Drummond et al. 1995, Calvo 1987). All of these lakes are very clear and dilute. Under normal conditions they are cold (10-11°C; Löffler 1972, Gocke et al. 1981, Jones et al. 1993), but we found temperatures to be noticeably warmer during the extreme El Niño conditions of February-March 1998 (Horn et al. 1999). Because of their exposure and reduced variation in seasonal temperature, these lakes probably turn over on a daily basis.
Lago Chirripó (5) is a tarn in the bedrock cirque floor at the head of the Valle de los Lagos immediately to the west of the Cerro Chirripó glacial horn. Its alternate name, Laguna Grande de Chirripó, describes it well as it is the largest and deepest lake on the Chirripó massif. Our chemical data indicate a fresher lake in February 1998 than in July 1991 by a factor of at least two, even though more rainfall typically falls in July (Horn et al. 1999). The reasons for this dilution are not clear, but may have involved reduced influx of sea-salt cloud condensation nuclei in the dry season, perhaps exacerbated by the El Niño of 1998 (K. Orvis, pers. comm.).
Lago Ditkebi (65) was mapped as a dry, closed contour on the Dúrika topographic map, but in fact the lake is permanent, natural, and rather deep. Our chemical data, the first available for this lake, are typical of lakes on the Chirripó massif; perhaps the most noteworthy feature is the slightly elevated concentration of magnesium, which may be a consequence of the differing bedrock geology of this site.
Lago de las Morrenas 1 (61) is the largest of the "Morrenas lakes," which are in the repeatedly-glaciated, upper Valle de las Morrenas of the Río Chirripó Atlanticó (Inset D in Fig. 1) (Orvis and Horn 2000). In February 1998, it was unstratified and transparent to the bottom, in keeping with prior observations by ourselves and others (Horn et al. 1999). As at Lago Chirripó, our 1998 water samples were more dilute in most ions (range 52 to 75% of the 1991 sample concentrations), except for Mg, which was 133% as abundant in 1998 compared to 1991.
We previously (Horn and Haberyan 1993) referred to this lake as simply "Lago de las Morrenas," but we have since sampled five other lakes in this valley. We now number the main chain of lakes from "Lago de las Morrenas 0" through "Lago de las Morrenas 4;" this scheme conserves the designation of Lago de las Morrenas #1 of Jones et al. (1993) for the largest lake in the valley.
For subsidiary lakes, we add a letter (a,b,c, etc) to the number of the lake that they discharge into (e.g., L. Morrenas 2a drains into L. Morrenas 2).
We describe these lakes and their geomorphic setting in greater detail in Horn et al. (1999) and summarize them below. Our results are consistent with past studies in all respects save temperature and water levels. These appear to be strongly affected by El Niño, which reduces the local cloud cover so that lakes are warmer and experience more evaporation. Otherwise these lakes resemble high-altitude lakes worldwide in their transparency, weak stratification, and low concentration of dissolved salts.
Lago de las Morrenas 0 (60) is the highest permanent lake in the valley; it is a shallow lake that occupies a basin behind a bedrock prominence at the base of the best-developed subcirque at the head of the valley. The lake is a popular bathing site for tapir, and we observed their tracks in the shallows.
Lago de las Morrenas 2 (62) is linked to L. Morrenas 1 by a shallow, boulder-strewn stream that was essentially dry during our February 1998 field work. L. Morrenas 2 is shallow for its size; we found a maximum depth of 1.3 m. Jones et al. (1993) reported a surface water temperature of 11ºC in August 1986. At 14:41 hours on 25 February 1998, we measured temperatures up to 7º C higher. The water temperatures were the highest we measured in the valley, probably because the shallowness of the lake favors warming of the bottom, which in turn warms the water column.
Lago de las Morrenas 2a (64) is a small pond dammed by a recessional moraine above L. Morrenas 2. The lake is less than 1 m deep, and transparent to the bottom. Its waters are among the most dilute on Cerro Chirripó.
Lago de las Morrenas 3 (62) is approximately the same depth as L. Morrenas 1, and it was also unstratified. Chemical results are consistent with those of the other lakes.
Lago de las Morrenas 4 (63) was second in temperature only to L. Morrenas 2, probably also because of its shallowness (maximum depth 3 m). Chemistry is similar to that of other lakes in the valley.
Laguna del Refugio (59) is one of four small ponds located downslope from the string of larger lakes in the Valle de las Morrenas. It is about 25 m NNW of the abandoned "refugio" (shelter) that sits just beyond the large bedrock step and terminal moraine below Morrenas 3 and Morrenas 4. The pond is probably less than 1 m deep, and was probably used for washing when the shelter was in use; this seems to have influenced the lakes chemistry, which included the highest values of most ions (especially Ca+2 , Na+ , and Si) we measured in these glacial lakes.
Statistical Comparison of Lakes
The statistical tests we used make few assumptions about the nature of the data. This is important since these lakes are widespread in the country and were sampled on different occasions. The use of statistical tests here is exploratory and descriptive; we do not intend to give an estimate of error about the conclusions or to make predictions based on these data.
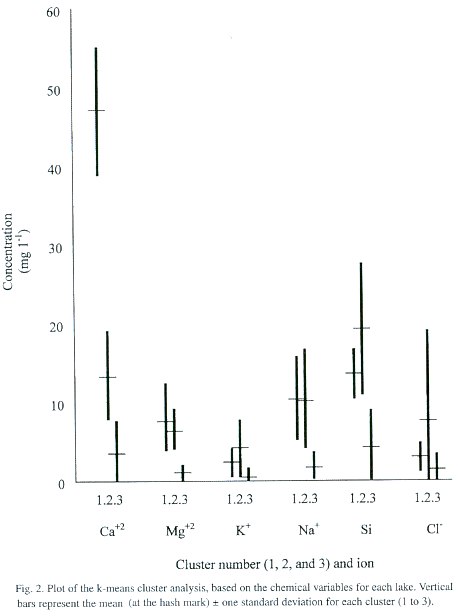
The cluster analysis produced three clusters (Fig. 2). Cluster 1 is characterized by a high Ca+2 content relative to the other ions examined, followed by Si and Na + . This cluster included Lagunas Foster, Zent, Carara, and Madre Vieja. Cluster 2 lakes have lower values of Ca +2 than Si, but similar values of Na+ , Mg+2 and K+ , and higher Cl - levels than the Cluster 1. To Cluster 2 belonged fourteen lakes: Río Cuarto, La Palma, Lancaster Abajo, Lancaster Arriba, Vueltas, Gandoca, Cuipilapa, Estero Blanco, Los Juncos Far, Los Juncos Near, Martillete, San Pablo, Espabelosa, and Las Brisas. Cluster 3 included the remaining lakes, and was characterized by the lowest chemical concentrations. In this cluster Si was slightly higher than Ca+2 as well.
Of the eight ANOVA tests, only five gave statistically-significant differences among the clusters. These included elevation (F=10.30, p=0.0002), conductivity (F=20.72, p=0.0000), pH (F=13.02, p=0.0001), dissolved oxygen (F=5.95, p=0.0070) and alkalinity (F=58.95, p=0.0000). Some variables produced significant results because of low variability within each group, including pH and, to a lesser degree, dissolved oxygen. In other cases the differences are based in part on variations between the clusters; for example, the elevations of Cluster 1 lakes are below 50 m, while lakes in Cluster 3 included all eighteen lakes above 1 000 m (as well as some lakes in the low plains). In addition to generally-higher elevations, Cluster 3 lakes also show low pH, conductivity and alkalinity. This confirms our previous perception that lakes at higher elevation tend to be more dilute (Horn and Haberyan 1993).
Discussion
Because of its varied topography, Costa Rica supports many lakes; in this survey, artificial lakes were the most common. The diversity of their settings and of their uses results in diverse lake characteristics. The highest lakes in the country, those on Cerro Chirripó, are rather uniform in chemistry and resemble high-altitude lakes worldwide in their clarity. Landslide lakes are more common in the middle elevations, and their formation no doubt reflects copious seasonal rainfall and its effect on slope stability; they tend to be deeper than most other lake types. Oxbow lakes are, of course, typically found in areas of low relief, and tend to be very warm and turbid.
Although they share a similar origin, lakes within most of our groups show noticeable variation in water chemistry. That is, lake chemistry is less influenced by mode of formation than by modern environmental factors. This is confirmed by the statistical analyses, because each ANOVA cluster included lakes that are geographically far apart and often in different climatic settings (e.g. humid Carribean and drier central Pacific). Other factors, such as lake size, watershed condition, and especially elevation are more important. However, lakes in two locales in particular tended to cluster together, namely the Chirripó lakes and the Miravalles lakes. While mode of formation seems poorly related to lake chemistry overall, it does maintain strong influence on lake stratification because of its relationship to lake morphology and elevation.
As a group, these fifty-one lakes have pH values close to neutral (range 6.26 to 8.72) and, with the exception of one seawater-based hypolimnion (in L. Gandoca), relatively low conductivities (16 to 442 µS cm -1 ). Consequently, Costa Rican lakes can be characterized as circumneutral and fresh, despite the great variation in mode of origin and in local climate (e.g. annual rainfall in the country varies from less than 150 to over 600 cm yr -1, and mean annual temperatures vary from ~5º C to ~30º C: Coen 1983).
These results agree well with other reports on water quality of Costa Rica. Bumby (1982) relatedplant distribution to water chemistry, and reported that most of her sites had similar chemistry, except for a few sites where sulfate was more abundant than bicarbonate. An examination of the water chemistry data from the 80 sampling points monitored by the national electrical company (Anonymous 1987) also shows that, with a few exceptions, most locations have similar water chemistry despite their location within the country. Part of the reason for this is the broadly similar geological origin of the different regions in the country: most of the bedrock is igneous (either volcanic or intrusive). There are a few places where ancient marine sediments have been exposed, but these are very localized. Other special conditions may result from geother-mally-influenced springs, which may show high acidity, high sulfur content, or high phosphate content (Pringle et al. 1993), but such streams are very localized as well.
In general, therefore, nearby lakes tend to be as different in water chemistry as lakes that are well separated; the exceptions are the glacial lakes and the Miravalles lakes. Lakes within most of our origin-groups are as different as lakes in different groups, so that intra-group variations seem at least as strong as intergroup variations.
Acknowledgements
We thank our many dedicated field assistants for their perserverance, especially Brandon League, Kim Raia, Esteban Estrada, Lisa Kennedy, Martin Arford, and Jennifer Krstolic. We also thank Sergio Chávez and Maureen Sanchez for critical field and logistical support; and Kenneth Orvis for field collaboration on Cerro Chirripó and assistance with air photo analysis. We are grateful to the Costa Rican government and private landowners for permission to sample lakes, and for their hospitality. This manuscript was improved by the efforts of two anonymous reviewers. This research was supported by grants from the National Geographic Society, The A.W. Mellon Foundation, the National Science Foundation (SES-9111588), the University of Tennessee, and Northwest Missouri State University; these agencies are gratefully acknowledged.
Resumen
Se visitaron 51 lagos en Costa Rica como parte de un sondeo de lagos más amplio, con el fin de documentar sus carácteristicas físicas y químicas y las relaciones entre estas carácteristicas y el modo de formación y distribución geográfico de los lagos. Los cuatro lagos en U tienden a ser de baja altitud y turbios, así como a tener alta conductividad y CO2 , pero baja concentración de O2 ; uno de estos, L. Gandoca, tuvo un hipolimnion compuesto esencialmente por agua de mar. Estos lagos fueron similares a los cuatro lagos de humedales, pero estos últimos presentaron en cambio baja conductividad y pH, y la turbidez se debió a menudo a taninos en vez de sedimentos en suspensión. Los trece lagos artificiales forman un grupo heterogéneo, cuyos rasgos variaron dependiendo de factores locales. Los siete lagos formados por represamiento debido a derrumbes, flujos de lava o lahares ocurren generalmente en áreas de pendientes pronunciadas, y son más probables de estar estratificados que la mayoría de los otros tipos de lagos. Los ocho agos que ocupan cráteres volcánicos tienden a ser profundos, estratificados, de aguas claras y de baja temperatura; dos de estos, L. Hule y L. Río Cuarto, parecen ser oligomícticos (con tendencia a la meromixis). Los nueve lagos glaciales, todos localizados arriba de los 3 440 m de altitud cerca del Cerro Chirripó, fueron de aguas claras, fríos, diluidos y son probablemente polimícticos. Un análisis de conglomerados dio como resultado tres grupos distintos. El Grupo 1 incluyó cuatro lagos ricos en calcio (promedio de 48 mg l-1 ), el Grupo 2 incluyó catorce lagos con más Si que Ca+2 y una concentración de Cl – más alta que los otros grupos, y el Grupo 3 incluyó los restantes treinta y tres lagos que fueron en general menos concentrados. Los lagos en cada grupo tienen varios orígenes y están distribuidos en diferentes regiones geográficos; estos datos indican que, aparte de los lagos glaciales de gran altitud, la similitud en la química de los lagos es independiente de su distribución.
References
Alvarado, G. 1989. Los volcanes de Costa Rica. 1. edition. Editorial Universidad Estatal y Distancia, San José, Costa Rica. [ Links ]
Alvarado, G. 2000. Los volcanes de Costa Rica: Geología, historia y riqueza natural. Editorial Universidad Estatal y Distancia, San José, Costa Rica. [ Links ]
Anonymous. 1987. Boletín de calidad físico química del agua. Departamento de Hidrología, Instituto Costarricense de Electricidad. San José, Costa Rica. [ Links ]
Armoudlian, A. & C. De Moraes. 1994. Preliminary limnological study of Lake Pocosol. pp. 99-103. In B. Young & S. Sargent, Coursebook for Tropical Biology: an ecological approach, 94-1. Organization for Tropical Studies, San José, Costa Rica.
Bumby, M.J. 1982. Survey of aquatic macrophytes and chemical qualities of nineteen locations in Costa Rica. Brenesia 19/20: 487-536. [ Links ]
Calvo, G. 1987. Geología del macizo de Chirripó, Cordillera de Talamanca, Costa Rica. Campaña Geológica G-5216, Informe Final. Escuela Centroaméricana de Geología, Universidad de Costa Rica, San José, Costa Rica.
Chavez, L. & K.A. Haberyan. 1997. Diatom assemblages from the Camastro Diatomite, Costa Rica. Rev. Biol. Trop. 44: 857-860. [ Links ]
Clement, R.M. & S.P. Horn. 2001. PreColumbian land use history in Costa Rica: A 3000-year record of forest clearance, agriculture, and fires from Laguna Zoncho. The Holocene 11: 419-426. [ Links ]
Coen, E. 1983: Climate. In D. Janzen. (ed.). Costa Rican natural history. University of Chicago. pp. 35-46.
Drummond, M.S., M. Bordelon, J.Z. De Boer, M.J. Defant, H. Bellon & M.D. Feigenson. 1995. Igneous petrogenesis and tectonic setting of plutonic and volcanic rocks of the Cordillera de Talamanca, Costa Rica–Panama, Central American arc. Am. J. Sci. 295: 875-919.
Gocke, K. 1996. Basic morphometric and limnological properties of Laguna Hule, a caldera lake in Costa Rica. Rev. Biol. Trop. 44: 537-548. [ Links ]
Gocke, K., E. Lahman, G. Rojas & J. Romero. 1981. Morphometric and basic limnological data of Laguna Grande de Chirripó, Costa Rica. Rev. Biol. Trop.35(2): 277-285.
Haberyan, K.A. & S.P. Horn. 1999a. A 10,000-year diatom record from a glacial lake in Costa Rica. Mountain Research & Development 19:63-68.
Haberyan, K.A. & S.P. Horn. 1999b. Chemical and physical characteristics of seven volcanic lakes in Costa Rica. Brenesia 51: 85-95. [ Links ]
Herrera, S.W. 1992. Mapa-Guia de la naturaleza: Costa Rica: Nature Atlas - Guidebook. Incafo Costa Rica, San José, Costa Rica. [ Links ]
Horn, S.P. 1989. The Inter-American Highway and Human Disturbance of Páramo Vegetation in Costa Rica.Yearbook of the Conference of Latin Americanist Geographers 15: 13–22. [ Links ]
Horn, S.P. 2001. The age of the Hule explosion crater, Costa Rica, and the timing of subsequent tephra eruptions: evidence from lake sediments. Rev. Geol. Am. Central 24: 57–66. [ Links ]
Horn, S.P. & K.A. Haberyan. 1993. Costa Rican lakes: physical and chemical properties. Nat. Geog. Res. Explor. 9(1): 86-103. [ Links ]
Horn, S.P., K.H. Orvis & K.A. Haberyan. 1999. Investigación limnológica y geomorfológica de lagos glaciares del Parque Nacional Chirripó, Costa Rica. Rev. Informe Semestral, Inst. Geog. Nacional Costa Rica 35: 95-106.
Jones, J.R., K. Lohman & G. Umaña V. 1993. Water chemistry and trophic state of eight lakes in Costa Rica. Verh. internat. verein. Limnol. 25: 899-905. [ Links ]
Kling, G.W., M.A. Clark, H.R. Compton, J.D. Devine, W.C. Evans, A.M. Humphrey, E.J. Koenigsberg, J.P. Lockwood, M.L. Tuttle & G.N. Wagner. 1987. The 1986 Lake Nyos gas disaster in Cameroon, West Africa. Science 236: 169- 175.
Löffler, H. 1972. Contribution to the limnology of high mountain lakes in Central America. Internat. rev. ges. Hydrobiol. 57(3): 397-408. [ Links ]
Olsson, I. 1986. Radiometric dating. pp. 273-312. In B.E. Berglund. Handbook of Holocene palaeoecology and palaeohydrology. Wiley, New York.
Orvis, K.H. & S.P. Horn. 2000. Quaternary glaciers and climate on Cerro Chirripó, Costa Rica. Quat. Res. 54: 24-37. [ Links ]
Pringle, C.M., G.L. Rowe, F.J. Triska, J.F. Fernandez & J. West. 1993. Landscape linkages between geothermal activity and solute composition and ecological response in surface waters draining the Atlantic slope of Costa Rica. Limnol. Oceanogr. 38: 753-774.
Umaña, G., K.A. Haberyan & S.P. Horn. 1999. Limnology in Costa Rica. In B. Gopal & R.W. Wetzel (eds.). Limnology in Developing Countries 2: 33-62. [ Links ]
Umaña V., G. & C. Jiménez. 1995. The basic limnology of a low altitude tropical crater lake: Cerro Chato, Costa Rica. Rev. Biol. Trop. 43: 131-139. [ Links ]
Weber, H. 1959. Los páramos costarricense y su concatenación fitogeográfico con los Andes suramericanos. Instituto Geográfico Nacional, San José, Costa Rica.
1 Department of Biology, Northwest Missouri State University, Maryville, Missouri 64468, U.S.A., Fax (660) 562-1188; khaber@mail.nwmissouri.edu
2 Department of Geography, University of Tennessee, Knoxville, Tennessee 37996, U.S.A. E-mail: shorn@utk.edu .
3 Escuela de Biología, University of Costa Rica, San Jose, Costa Rica. E-mail: gumana@cariari.ucr.ac.cr .














