Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.51 n.1 San José Mar. 2003
Abstract
Flowering plant density can increase number of visits and fruit set in multi-flowering plants, however this aspect has not been studied on few flower species. We studied the effects of individual floral display and plant density on the fruit production of the epiphytic, moth-pollinated orchid, Ryncholaelia glauca, in an oak forest of Chavarrillo, Veracruz, Mexico. Species is nonautogamous, and produced one flower per flowering shoot each flowering season. We hypothesized that orchids with more flowering shoots and those on trees with clumps of conspecific should develop more fruits than isolated ones. R. glauca population flowers synchronously, and individual flowers last up to 18 days, with flowers closing rapidly after pollination. Individuals produced few flowers per year, although some plants developed flowers in both seasons and fewer of them developed fruits both years. There was no relationship between flower number per orchid, or per host tree, with the number of fruits developed per plant. Host trees with flowering and fruiting orchids were randomly dispersed and the pattern of distribution of flowering and fruiting plants was not related. Apparently, pollinators visit the flowers randomly, with no evidence of density dependence. The fruit set of R. glauca was as low as fruit set of multiflowered orchids moth pollinated, suggesting that fruit set on moth pollinated orchids could be independent of the number of flowers displayed.
Key words: Floral display, abundance, reproduction, Ryncholaelia glauca, orchid, fruit set, spatial distribution.
Inflorescence size and population density are important features influencing the reproductive success of animal-pollinated plants (Schmitt et al. 1987, House 1992, Kearns and Inouye 1993, Widén 1993, Kunin 1997, Groom 1998). Both, large inflorescence size and density of reproductive individuals, increase flower display, visitor frequency, and fruit set on multi-flowered non-orchid species (Pleasants and Zimmerman 1990, Kilnkhamer and de Jong 1993, Ågren 1996, Kunin 1993, 1997), and multi-flowered orchid species (Montalvo and Ackerman 1987, RicoGray and Thien 1987, Wolfe 1987, Firmage and Cole 1988, Calvo 1990b, Robertson and Wyatt 1990, Rodríguez-Robles et al. 1992, Sabat and Ackerman 1996). Despite their importance, the effects of these factors have not been studied on few-flowered species. Fruit production in plants with many flowers may depend on several reproductive characteristics as flower size, shape, color, rewards, breeding systems, and resources (Zimmerman and Pyke 1988), these characteristics could be even more critical in plants with few flowers. Fruit set should be higher on clumped of few flowering plants than on isolated ones, because pollinators may be more likely
to visit high density of flowers (enlarging the overall floral display, as many flowers they have together). We studied the effects of floral display and plant abundance on fruit set of the non-auto-gamous orchid Ryncholaelia glauca (Lindl.) Schltr. (Orchidaceae). We hypothesized that: 1) clumped plants should produce relatively more fruits than isolated plants, and 2) plants with several flowering shoots should develop more fruits than plants with one flowering shoot.
Materials and methods
Study area: Fieldwork was done in an oak-forested hill near Chavarrillo, Coatepec, Veracruz, Mexico (19°2424" N, 96°4819" W; altitude 900 - 1000 m). The climate is warm subhumid with a mean temperature of 24.5 °C. Total annual precipitation is 1 106.4 mm, mostly falling between June and September (García 1981). The forest grows on limestone soils with many superficial rocks. Tree height ranged between 6 to 20 m. The most common species in this area include Quercus oleoides Schlecht. and Cham., Quercus laurina Humb. and Bonpl., and Quercus peduncularis Nee (Fagaceae). Epiphytes are abundant, especially Cattleya aurantiaca (Batem. ex Lindl.) P.N. Don., Encyclia cochleata (L.) Lemee, Laelia anceps Lindl., Nageliella purpurea (Lindl.) L.O. Wms., Notylia barkeri Lindl., Oncidium maculatum Lindl., Oncidium stramineum Batem. ex Lindl., Ryncholaelia glauca (Lindl.) Schltr. (Orchidaceae), Tillandsia ionantha Planchon, Tillandsia schiedeana Steudel, Catopsis spp. (Bromeliaceae), and Pepperomia spp. (Piperaceae) (Castillo 1985, Flores-Palacios 1995).
Study species: Ryncholaelia glauca is a sympodial epiphytic herb with compressed oblong-fusiform pseudobulbs (Ames and Correll 1954). As other orchids (Zotz 1995), each terminal shoot develops one pseudobulb yearly. The new pseudobulb produces a single, large creamy-white flower 10-12 cm in diameter (A. Palacios-Rios and J.G. García-Franco pers. obs.). As a consequence, the number of flowers depends on the number of new shoots developed by the plant. Each flower produces 1.0 to 10.5 µl of nectar with 10.25 ± 3.02% total sugars on wt/wt basis (mean ± S.D., n = 5), individual flowers last from 15 to 18 days, and fruit development starts immediately after pollination (Flores-Palacios 1995). Plants bloom synchronously between January and February, and fruits take one year to develop. Flowers are non-autogamous but they are self-compatible (100%), and geitonogamy and xenogamy could exist (100% each) (Flores-Palacios 1995). The large white flowers are pollinated by hawkmoths (Sphingidae) (Dressler 1990) (phalaenophyly syndrome sensu Pjil and Dodson 1966).
Data collection: We mapped all trees in two study plots (each 0.15 ha). On each tree we counted and recorded the spatial location of all individual orchids (using x, y, z coordinates). Plants of R. glauca were usually found isolated with one rhizome line and few pseudobulbs; each of them was considered an individual. We also recorded plants with mixed rhizomes (clump) as a single individual. Throughout two reproductive seasons (1993 and 1994), we surveyed each individual every 2-3 days to record the number of flowers and fruits.
Data analysis: The effects of abundance and floral display on fruit production were analyzed at three levels: a) individual plant, b) clumped individuals on host trees (each tree supporting different orchid numbers), and c) spatial distribution (two and three dimensions) of the reproductive orchids.
a) Effect of individual floral display on the fruit production. We compared flower and fruit numbers developed per individual plant of R. glauca. We expected to find a positive relationship between the number of flowers and fruits. We used a non-parametric correlation procedure to test this hypothesis (Spearman rank correlation test, StatView 1996).
b) Effect of the total flower number of R. glauca per host tree on the fruit production. We related the number of reproductive orchids and the total number of flowers produced per tree, with the pooled fruit set recorded for each tree. Each tree was considered a unit. We expected to find a positive relationship between number of flowering plants and number of fruits on the host trees. Once again, we used a non-parametric correlation procedures to test our hypothesis (Spearman rank correlation test, StatView 1996).
c) Spatial distribution of reproductive orchids. The spatial pattern of host trees supporting reproductive orchids was obtained for each plot and season using the nearest-neighbor method (Clark and Evans 1954). If clumping in reproductive plants is an important factor determining fruit production, we would expect to record more orchid fruits on clumped host trees. The spatial pattern of flowering and fruiting individuals of R. glauca was tested using the Zorro Program (Romesburg 1989). This program, based on the nearest-neighbor distance, can test for three-dimensional spatial patterns. The program performs a conditional randomization test based on three hypotheses: Ho , the spatial distribution of two or more types of individuals is random; H1 , individuals of the same type are attracted to each other, and H2 , individuals of the same type repel each other. The program estimates p-values using Monte Carlo sample iterations. We used 10 000 iterations to estimate the p value. When p < 0.025, Ho is rejected in favor of H1; if p > 0.975, Ho is rejected in favor of H2 , and when 0.025 <p < 0.975, H o is accepted (Romesburg 1989). We expected an attraction relationship between the spatial pattern of flowering and fruiting plants of R. galuca (H1).
Results
General reproductive characteristics:
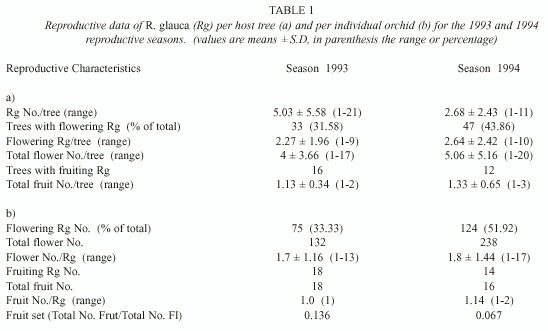
We recorded a total of 115 Q. peduncularis trees in our study plots, but only 56 of them (48.7%) supported plants of R. glauca. A total of 223 individuals of R. glauca were recorded. The number of flowering plants and number of flowers increased rapidly during the first twelve days since the start of the bloom season (Fig. 1). Seventy five plants flowered during the first study year (1993) producing 132 flowers, this was 33.63% of the total orchids considered. During the second year (1994) 123 individuals flowered (55.16% of the total) producing 238 flowers (Table 1). The number of flowering orchids and the total number of flowers produced were different between years = 9.91; p < 0.005; and
= 28.35; p <0.001, respectively). Twenty four plants (11.21%) failed to develop flowers in either season.
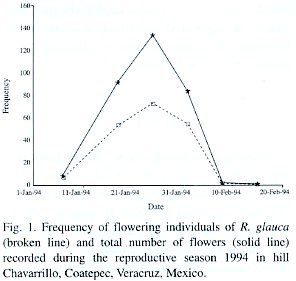
Reproductive plants did not develop the same flower and fruit number in both years. Eighty orchid plants flowered in only one year (16 in 1993, and 64 in 1994), and 59 individual plants flowered in both years (26.46% of the total orchids). In plants that flowered both years, 28 individuals (47.46%) produced the same number of flower both seasons, 24 plants (40.68%) increased their flower display and 7 orchids (11.86%) decreased their flower number in 1994.
Few plants developed fruits (Table 1). Most fruiting R. glauca produced one fruit per plant in 1993, but in 1994 we recorded two individuals with two fruits each (Table 1). Mean fruit number per plant was low (1.21 ± 0.5) and also the fruit set (0.136 and 0.672, respectively for each study season). Twelve individuals (66.67%) from those fruiting in 1993 flowered again in 1994, but only four of them developed fruits again in the second season (22.22%).
Effect of the total flower number of R. glauca per host tree on the fruit production:
Individuals of R. glauca produced few flowers during both seasons. In 1993, 49 flowering individuals (65.33%) developed only one flower, whereas the remaining plants (26) produced 2 to 13 flowers. In 1994, 58.54% of the total flowering individuals (72 plants) developed one flower, and the remaining plants (52) produced 2 to 17 flowers. Individuals with more than two flowers were probably mixed clumps of multiple individuals. Mean flower number per R. glauca individual was not different between years (Mann-Whitney U =4261, p = 0.369, Table 1).
Plants developed few fruits during both seasons (Table 1). Individual fruit production was not related to flower number per individual plant (rs 1993 = 0.120 and rs 1994 = 0.158, p > 0.05). There was no difference between fruit set of plants with one flower and fruit set of individuals with more than one flower ( = 0.18 in 1993 and
= 0.45 in 1994, p > 0.05).
Reproductive success of the R. glauca individuals per host tree: Host trees support 3.98 (± 4.5, range 1-21) orchids (reproductive and non-reproductive individuals). Almost half of the trees supported one flowering R. glauca in both seasons (48.5% in 1993 and 47% in 1994), while the remaining trees supported two or more flowering orchids (21-27.3% two plants, and 24.2-32% more than two orchids, respectively). The mean number of flowering R. glauca per tree and the total number of flowers per tree (Table 1) were not different between seasons (U = 728, p = 0.64; and U = 732.5, p = 0.67).
We recorded only 16 host trees in 1993 and 12 in 1994 with R. glauca fruiting plants. Fruit number per tree (Table 1) was not different between seasons (U = 83, p = 0.546). We found a weak positive correlation between the number of flowering individuals and the number of fruiting R. glauca per host tree in the first reproductive season (rs 1993 = 0.37, p = 0.036 and rs 1994 = 0.231, p = 0.117).
Spatial distribution of reproductive orchid plants: We found a random pattern of distribution for the host trees of R. glauca (plot A: R = 1.14, and plot B: R = 1.09, p < 0.05). Host trees with flowering orchids were randomly distributed in both seasons (plot A: R 1993 = 1.35 and R 1994 = 1.02; plot B: R 1993 = 0.95 and R 1994 = 1.01; p < 0.05). Also, host trees with fruiting plants of R. glauca showed a random spatial pattern (plot A: R 1993 = 1.01 and R 1994 = 0.46; plot B: R 1993 = 1.49 and R 1994 = 0.76; p < 0.05). Flowering and fruiting R. glauca plants were randomly distributed in both plots and seasons, as suggested by the randomization test (plot A: R 1993 = 154.14, p = 0.12 and R 1994 = 694.39, p = 0.59; plot B: R 1993 = 287.47, p = 0.483 and R 1994 = 150.95, p = 0.26).
Discussion
Ryncholaelia glauca is abundant at the studied forest; however only half or less of the studied population reproduces each year. Resources and previous reproductive efforts could influence new reproductive events in some orchid species (Montalvo and Ackerman 1987, Ackerman 1989, Ackerman and Montalvo 1990, Murren and Ellison 1996). Plant size (v.gr., total shoots and leaf number) should be a good indicator of potential reproductive capacity (v.gr., total flower number) (Murren and Ellison 1996), particularly in R. glauca where the number of inflorescences and flowers developed is constrained by the number of shoots and new pseudobulbs produced. We observed some large plants (clumps with many growing shoots and leaves) developing only one flower, and nearby small ones (with few growing shoots and leaves) developing two flowers. Large R. glauca plants with few flowers could be old ones. Also, we recorded some R. glauca individuals flowering and fruiting both study years. Apparently, in this species, previous reproductive effort does not limit the next one reproductive expression.
Individual floral display of R. glauca was low (1.8 flowers/plant). However flower life-time (15-18 days, from one quarter to half of the flowering season) increases each individual floral display and the flower population density. Flower display and density have been associated with female and male function (Kunin 1993, 1997, Murren and Ellison 1996). Therefore longer flower life span could increase pollen export and pollination possibilities. On multi-flowered plants, large inflorescence size could result in increasing pollinia removal (Sutherland 1986, Rodríguez-Robles et al. 1992) and high fruit set (Murren and Ellison 1996); though it is not a general pattern in orchids (Calvo 1990a). On the other hand, floral display and flower lifetime should influence pollinator behavior (Sih and Baltus 1987, Strauss and Conner 1996). Longer display period might be a result of scarcity of pollinators (Firmage and Cole 1988). Flower characteristics of R. glauca suggest a specialist pollinator (hawkmoth syndrome). Apparently, this type of relationship could be constant despite the number of flowers present, because specialists are efficient pollen vectors (Nilsson et al. 1987, Kunin 1993). Though we did not recorded pollinium removal, as on many orchid species, flowers of R. glauca close immediately after pollination even without pollinium removal (Flores-Palacios 1995). Infrequency of pollinators and low fruit set are common conditions for most orchid species (Ackerman and Montalvo 1990, Calvo 1990a, 1993, Dressler 1990, Sabat and Ackerman 1996). As other out-breeders species (Primack 1985), the extended flower life span in R. glauca, could increase individual pollination probabilities.
We found a weak relationship between individual floral display (flower number) and fruit set. Almost all flowering individuals of R. glauca developed one fruit over the two study years; only two plants developed two fruits each. Presence of conspecifics increased flower visitation rate on Nepeta cataria, but not all pollinator showed significant relationship (Sih and Baltus 1987). In nontropical areas hawkmoths show constancy when large patches of food plants are available,but inconstancy when resources are scarce (Nilsson et al. 1987). On the other hand, despite difference in total flowers of R. glauca produced each season, the mean number of flowering plants, orchid flower number, and fruits present per host tree were not different. These trees had a random spatial pattern, and the spatial pattern of flowering and fruiting orchid plants were not related. This suggests there is no density dependence effect on the flower visitors.
Pollinators do visit the flowering plants of R. glauca and host trees randomly, independently of flower number. In general, the fruit set recorded for the few-flowered R. glauca was similar to multi-flowered orchid species that have the same breeding system (Flores-Palacios 1995). It is particularly interesting the similarity of the fruit set of R. glauca with that of B. nodosa and E. ciliare, species with the same pollination syndrome (Shemske 1980, Ackerman and Montalvo 1990). Apparently, single and multi-flowering, hawkmoth pollinated species could have similar fruit set. However, plants with one or few flowers per year should have a critical reproductive reduction. A possible benefit of flower display reduction is achieved by a larger fruit size and seed number. Rhyncholaelia glauca produces large fruits (10-15 cm long). Moreover, reduction in flower display in not self-pollinated orchids could decrease geitonogamy and increase xenogamy. It has been noted that orchids offering rewards to pollinators have relatively higher fruit set (14.2-26.6%), while deceptive species lower fruit set (9%) (Rodríguez-Robles et al. 1992). Fruit set of R. glauca occurs between the range of both types of orchids mentioned. However, as in all plant species, flower number in R. glauca changes among years and sites (see Table 1), producing differences in reproductive success. In summary, fruit production of R. glauca was not related to individual floral display. Host trees with reproductive orchids were randomly distributed, and the spatial patterns of distribution of flowering and fruiting orchids were not associated. Finally, the fruit set of R. glauca is similar to that of multi-flowered orchid species, suggesting that the fitness of few flowered orchids with hawkmoth pollinated flowers could be equal to that of the former species.
Acknowledgements
We thank V. Rico-Gray and G. Dieringer for reviewed an early draft. Comments and constructive criticism from three anonymous reviewers improve this work. We also appreciate the suggestions by S. Koptur, J. Geiger, and J. Redwine especially to the English version. J. Navarro suggested the use of Zorro program, and R. Landgrave helped us to apply it. AFP presented early version as Bachelor thesis on the Fac. Biología, Universidad Veracruzana. The Instituto de Ecología A. C. (902-16) supported the study.
Resumen
La densidad de plantas floreciendo puede incrementar la tasa de visitas de polinizadores y la producción de frutos en plantas con inflorescencias multiflorales; sin embargo, este aspecto no ha sido estudiado en plantas con inflorescencias de una sola flor. En un encinar tropical de Chavarrillo, Veracruz, México, estudiamos el efecto de la densidad de plantas con flores y del despliegue floral en la producción de frutos de la orquídeas epífita, polinizada por polillas, Ryncholaelia glauca. La especie no es autógama y produce sólo una flor por eje de crecimiento, por año. La población de R. glauca florece sincronizadamente, las flores individuales duran más de 18 días y aquellas polinizadas se cierran rápidamente. Hipotetizamos que orquídeas con más ejes de crecimiento y aquellas en árboles con grupos de plantas floreciendo deben desarrollar más frutos que aquellas aisladas. Las plantas produjeron pocas flores por año, algunas plantas desarrollaron flores en ambos años y muy pocas desarrollaron frutos en ambos años. El número de frutos producidos por planta no estuvo relacionado con el número de flores por planta ni con el número de flores por árbol hospedero. Tanto los árboles con plantas con flores, como aquellos con plantas con frutos están distribuidos al azar y el patrón de distribución de plantas con flores y frutos no se relacionó. Aparentemente, los polinizadores visitan las flores al azar y no de forma densodependiente. La producción de frutos de R. glauca fue tan baja como la reportada para orquídeas multiflorales, polinizadas por polillas, lo que sugiere que la producción de frutos producidos en orquídeas polinizadas por polillas podría ser independiente del despliegue floral.
References
Ackerman, J.D. 1989. Limitations to sexual reproduction in Encyclia kruggi (Orchidaceae). Syst. Bot. 14:101-109. [ Links ]
Ackerman, J.D. & A. Montalvo. 1990. Short- and long-term limitations to fruit production in a tropical orchid. Ecology 71: 263-272. [ Links ]
Ågren, J. 1996. Population size, pollinator limitation, and seed set in the self-incompatible herb Lythrum salicari a. Ecology 77: 1779-1790. [ Links ]
Ames, O. & D.S. Correl. 1954. Orchids of Guatemala. Fieldiana Botany 26: 399-727. [ Links ]
Calvo, R.N. 1990a. Inflorescence size and fruit distribution among individuals in three orchid species. Amer. J. Bot. 77: 1378-1381. [ Links ]
Calvo, R.N. 1990b. Four-year growth and reproduction of Cyclopogon cranichoides (Orchidaceae) in south Florida. Amer. J. Bot. 77: 736-741. [ Links ]
Calvo R.N. 1993. Evolutionary demography of orchids: intensity and frequency of pollination and the cost of fruiting. Ecology 74: 1033-1042.
Castillo, G. 1985. Integración de paisajes en la región de Jalcomulco, Ver. B.Sc.thesis, Facultad de Biología, Universidad Veracruzana, Xalapa, Veracruz.
Clark, P.J. & F.C. Evans. 1954. Distance to nearest neighbor as a measure of spatial relationships in populations. Ecology 35: 445-453. [ Links ]
Dressler, R.L. 1990. The Orchids. Natural History and Classification. Harvard University Press. Cambridge. 332 p.
Firmage, D.H. & F.R. Cole. 1988. Reproductive success and inflorescence size of Calopogon tuberosus (Orchidaceae). Amer. J. Bot. 75: 1371-1377. [ Links ]
Flores-Palacios, A. 1995. Biología reproductiva de Rhyncholaelia glauca (Lindl.) Schltr. en un encinar del centro de Veracruz. B.Sc. thesis, Facultad de Biología, Universidad Veracruzana, Xalapa, Veracruz.
García, E. 1981. Modificaciones al Sistema de Clasificación Climática de Köeppen (para adaptarlo a las condiciones de la República Mexicana). 2a Edición. UNAM. México, D.F. 252 p.
Groom, M.J. 1998. Allee effects limit population viability of an annual plant. Amer. Nat. 136: 401-406. [ Links ]
House, S.M. 1992. Population density and fruit set in three dioecious tree species in Australian tropical rain forest. J. Ecol. 80: 57-69. [ Links ]
Kearns, C.A. & D.W. Ionuye. 1993. Techniques for pollination biologists. University Press of Colorado. Niwot, Colorado. 582 p. [ Links ]
Klinkhamer, P.G. L. & T. de Jong. 1993. Attractiveness to pollinators: a plant dilemma. Oikos 66: 108-114. [ Links ]
Kunin, W.E. 1993. Sex and the single mustard: population density and pollinator behavior effects on seed-set. Ecology 74: 2145-2160. [ Links ]
Kunin, W.E. 1997. Population size and density effects in pollination: pollinator foraging and plant reproductive success in experimental arrays of Brassica kaber. J. Ecol. 85: 225-234.
Montalvo, A.M. & J.D. Ackerman. 1987. Limitations to fruit production in Ionopsis utricularioides (Orchidaceae). Biotropica 19: 24-31.
Murren, J.C. & A.M. Ellison. 1996. Effects of habitat, plant size, and floral display on male and female reproductive success of the neotropical orchid Brassavola nodosa. Biotropica 28: 30-41. [ Links ]
Nilsson, L.A., L. Johnsson, L. Ralison & E. Randrianjohany. 1987. Angracoid orchids and hawk-moths in central Madagascar: Specialized pollinators systems and generalist foragers. Biotropica 19: 310-318.
Pijl, van der L. & C.H. Dodson. 1966. Orchid Flowers: Their Pollination and Evolution. University of Miami Press, Miami, Florida. [ Links ]
Pleasants, J.M. & M. Zimmerman. 1990. The effect of inflorescence size and pollinator visitation of Delphinium nelsonii and Aconitum columnianum. Collectanea Bot. 19: 21-39.
Primack, R.B. 1985. Longevity of individual flowers. Ann. Rev. Ecol. Syst. 16: 15-37. [ Links ]
Rico-Gray, V. & L.B. Thien. 1987. Some aspects of the reproductive biology of Schomburkia tibicinis Batem. (Orchidaceae) in Yucatan, Mexico. Brenesia 28: 13-24.
Robertson, J.L. & R. Wyatt. 1990. Reproductive biology of the yellow-fringed orchid Platanthera ciliaris. Amer. J. Bot. 77: 388-398. [ Links ]
Rodríguez-Robles, J.A., E.J. Meléndez & J.D. Ackerman. 1992. Effects of display size, flowering phenology, and nectar availability on effective visitation frequency in Comparettia falcata (Orchidaceae). Amer. J. Bot. 79: 1009-1017. [ Links ]
Romesburg, H.C. 1989. Zorro: A randomization test for spatial pattern. Computers & Geosciences 15: 1011-1017. [ Links ]
Sabat, A.M. & J.D. Ackerman. 1996. Fruit set in a deceptive orchid: effect of flowering, display size, and local floral abundance. Amer. J. Bot. 83: 1181-1186. [ Links ]
Schmitt, J., J. Eccleston & D.W. Ehrhardt. 1987. Density-dependent flowering phenology, outcrossing, and reproduction in Impatiens capensis. Oecologia 72:341-347. [ Links ]
Shemske, D.W. 1980. Evolution of floral display in the orchid Brassavola nodosa. Evolution 34: 489-493. [ Links ]
Sih, A, & M.S. Baltus. 1987. Patch size, pollinator behavior, and pollinator limitation in catnip. Ecology 68: 1679-1690. [ Links ]
Strauss, S.Y. & J.K. Conner. 1996. Foliar herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. Amer. Nat. 147: 1098-1107. [ Links ]
Sutherland, S. 1986. Floral sex ratios, fruit-set, and resource allocation in plants. Ecology 67: 991-1001. [ Links ]
Widén, B. 1993. Demographic and genetic effects on reproduction as related to population size in a rare, perennial herb, Senecio integrifolius (Asteraceae). Biol. J. Linnean Soc. 50: 179-195.
Wolfe, L.M. 1987. Inflorescence size an pollinaria removal in Asclepias curassavica and Epidendrum radicans. Biotropica 19: 86-89. [ Links ]
Zimmerman, M. & G.H. Pyke. 1988. Reproduction in Polemonium: assessing the factors limiting seed set. Amer. Nat. 131: 723-738. [ Links ]
Zotz, G. 1995. How fast an epiphyte grow? Selbyana 16:150-154. [ Links ]
1 Departamento de Ecología Vegetal, Instituto de Ecología A.C., Km 2.5 Antigua Carretera a Coatepec, Apartado Postal 63, Xalapa, Ver., 91000, México, Fax 52(228)8187809, floresa@ecologia.edu.mx ; franco@ecologia.edu.mx
* Corresponding author














