Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.50 n.2 San José Jun. 2002
Pia Parolin
Max-Planck-Institute for Limnology, Tropical Ecology, P.O. Box 165, 24302 Plön, Germany, pparolin@botanik.uni-hamburg.de
Recibido 26-VII-2001. Corregido 26-XI-2001. Aceptado 31-V-2002.
Abstract
Cecropia latiloba can be considered to be one of the most efficient colonizers of open areas in the nutrient-rich whitewater floodplains of the Amazon river. Its main strategy to be successful is the high tolerance towards waterlogging and submergence, and the fast vertical growth and reiteration capacity. This, and the tolerance of high irradiation and sediment deposition allow C. latiloba to form large monospecific stands on open sites, and thus the first closed canopy which represents the initial phase of a successional sequence which leads to highly diverse forests. This tree is extremely well adapted to the adverse growth conditions in Amazonian floodplains with prolongued periods of flooding and seedling submergence. The species occurs on the lowest levels in the flooding gradient. Although it belongs to the most often cited species under aspects of taxonomy, species distribution and general descriptions of the ecosystem, little has been published about its ecology. In the present paper the ecological, physiological and phenological characteristics of C. latiloba are described. It is an evergreen species which constantly produces new leaves. With flooding, leaf production is reduced but new leaves are flushed also with prolongued flooding. The peak of flowering and fruiting are in the flooded period. When mature, the fruits are dispersed mainly by water and fish. Seed germination occurs, without dormancy, within 5-13 days after water retreat. In the 7 months before the first flooded period seedlings reach 1 m of height, and height growth increases until a height of 15-20 m is achieved. Photosynthetic assimilation is high, with values of up to 21 mmol CO2m-2s-1 . C. latiloba is a very flood tolerant species, and waterlogged seedlings continuously produce new leaves and adventitiuos roots.
Key words: Cecropia latiloba, pioneer, inundation forest, floodplains, várzea, Amazon, Neotropics
Cecropia latiloba Miq. (Cecropiaceae) (Fig. 1) is a shade-intolerant pioneer tree which colonizes open areas in Amazonian floodplains. It grows mainly in the floodplains of the Amazon and its nutrient-rich whitewater affluents. It forms dense monospecific stands (Fig. 2) in disturbed places along the river, on landslides or on exposed riverbanks and does not occur in closed forest (Worbes et al. 1992, Prieto et al. 1995). It initializes the successional sequence which leads to highly diverse forests (Worbes et al. 1992) and is extremely well adapted to the difficult growth conditions in Amazonian floodplains with prolongued periods of flooding and seedling submergence. Although it belongs to the most often cited species under aspects of taxonomy, species distribution and general descriptions of the ecosystem (Worbes 1997), little is known about its ecology. The present paper aims at describing the ecological, physiological and phenological characteristics of C. latiloba.
Materials and methods
Study sites, climate and water regime in Amazonian floodplains: Amazonian flood-plains occur all along the Amazon River and its main tributaries (Junk et al. 1989). The white-water rivers which originate in the Andes carry a high sediment load, and the floodplains, which are very nutrient rich, are called seasonal várzea (Prance 1979). Seasonal igapós are nutrient-poor floodplains flooded periodically by blackwater rivers. In the flooded period, water covers the roots, stems and sometimes also canopies of the plants. The lack of oxygen in the rhizosphere influences phenological events and physiological activities of the plants (Crawford 1989, 1992). Especially seedlings have to cope with long periods of complete submersion, where they are subjected to lack of oxygen, mechanical disturbance by the water current, and to sediment deposition on the leaf surfaces (Parolin 2000a). Nevertheless, in these floodplains grow highly diverse forests with characteristic successional sequences (Worbes et al. 1992).
A vegetation zonation along the inundation gradient reflects the flood tolerance of the trees (Ferreira 1997). In the lowest parts, flooded up to nine months every year, only occur species which tolerate extended periods of flooding and whose seedlings tolerate total submersion. C. latiloba can be found along the whole inundation gradient, and is especially effective at low levels due to the high flood tolerance of the seedlings. It typically occurs in the "chavascal", low-lying areas with dense, often shrubby vegetation which are flooded for 6-8 months with water depths of 6-7 m (Ayres 1993).
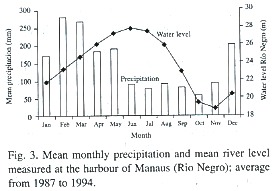
Field studies were performed at the Costa do Catalão (3° 10S, 59°54W, elevation 23- 24 m asl.) and Ilha de Marchantaria (3° 16S, 60° 03W, 24 m asl) around 20 km from the city of Manaus, Brazil, on sites with an average flood duration of 7 months. Mean monthly temperature ranges from 26.3 to 27.2°C. Rainfall ranges from 1 700 to 2 300 mm. The amount of rain is irregularly distributed throughout the year, with a dry season between June and November, and a rainy season from December to May. Variation of water level of the Amazon river near Manaus is markedly seasonal. The rising phase lies between December and June, and the receding period between July and November (Fig. 3). The amplitude of the river levels changes up to 10 m every year and the flooding lasts up to 230 days a year (Junk 1989). In the plains along the main river channel in Central Amazonia, sedimentation rates are usually about 50 cm a year, but they can reach up to one meter in particular years (Junk 1989).
Seeds, germination, biomass and seedling growth: Seed mass and germination were analysed at the Amazon Research Institute (INPA) in Manaus, Brazil, at an experimental site which was sunny in the morning and in the afternoon, and shady between 11:00 and 14:00 h. Seed mass of 50 mature seeds collected in the vicinity of Manaus and taken to the INPA in plastic bags was determined after oven drying to constant weight at 105°C. The seeds were preserved floating in water in plastic cups for 2-3 weeks. For germination, 25 seeds were tested, each seed was placed in one plastic cup (300 ml) with várzea soil from the floodplains. Germination defined as shoot emergence was checked daily. Germination rate was calculated by the amount of seeds germinated after 7 weeks in relation to the total initial seed number. Germination of submerged seeds was tested in an experiment with ten pairs of each species consisting of 1 plastic cup (300 ml) with 1 seed + well watered várzea soil and 1 cup with 1 seed + várzea soil + várzea water (seeds submerged). Seedling growth was determined under experimental conditions, at weekly intervals. Seedling mortality was determined on 50 marked seedlings naturally germinated in the field one month after establishment.
Structural characteristics of adult trees: Wood gravity and radial gradients were determined on 10 wood cores extracted with an increment borer (for calculations see Whitmore 1973, Parolin et al. 1998). Annual increments and age were measured on stem disks of felled trees (Worbes 1994). Tree height, diameter at breast height (dbh), crown size, crown density, and height of the first ramification, were measured on 3-10 trees in the field. The capacity for resprouting and vegetative propagation was observed on seedlings and adult trees in the field.
Morphological adaptations to flooding: The formation of morphological adaptations to flooding (adventitious roots, formation of lenticels and stem hypertrophy) was monitored qualitatively in the field on adult trees and on seedlings subjected to waterlogging and submergence under experimental conditions.
Physiological characteristics of the leaves: Leaf characteristics were measured at monthly intervals for 15 months on five adult individuals in the field. Photosynthetic CO2 -uptake, transpiration and stomatal conductance were measured between 9:00 and 12:00 a.m. with an infrared gas analyser (IRGA, ADC LCA-2, Analytical Development Co. Ltd., Hoddesdon, Herts, UK). Ten fully expanded, non-flooded leaves of five marked individuals were chosen for measurements at high quantum flux density, with photosynthetically active radiation (PAR) over 2 000 µmol m-2 s-1 producing maximal rates of photosynthesis (Amax). CO2 assimilation rate (A) and transpiration were calculated according to Coombs et al. (1985). Specific leaf weight was determined by calculating leaf dry weight (g) by leaf area (m2 ) (Medina 1983). Leaf area was measured with a "leaf area meter" (D T Area Meter, Delta-T Devices).
Phenology: Leaf production and loss, flowering, and fruiting were measured at intervals of two weeks for 15 consecutive months between April 1993 and June 1994 on 5 adult trees mainly on Costa do Catalão.
Results and discusion
Biology of Cecropia latiloba
Description: Cecropia latiloba (Miq.) (Cecropiaceae, synonym C. paraensis Huber, C. stenostachya Warburg) in the Brazilian Central Amazon is called imbaúba branca (synonym "embaúba branca") because of the white undersurfaces of the leaves. The common name in Perú is Cetico negro del bajo (Lamotte 1992) and in Venezuela it is Yagrumo (Hueck 1966). In Amazonian floodplains, C.llatiloba can reach a height of 20 m (Berg 1978) but generally forms a closed canopy at 15 m (Klinge et al. 1995). It is a fast-growing, short-lived tree with a maximum age of 20 years (Worbes et al. 1992) and often forms the first successional stage of trees in clearings by shading out other competing tree species with their huge digitate leaves. The large lobed, simple leaves have white pubescent undersurfaces, by which C. latiloba can be distinguished from the co-occurring C. membranacea. Mean leaf area is 1070 cm2 , with a diameter of 42-57 cm, and a mean specific leaf weight of 76 g m-2 (Parolin 1997). Young trees form leaves with up to 15 deep lobes, whereas adult trees have 9-12 less pronounced lobes (Lamotte 1992). The stipules are light green in young trees, and red to brownish in adult trees. The cotyledons are elliptic, with entire margins. As is typical for the genus, C. latiloba is dioecious (Berg 1978, Fleming and Williams 1990, Milton 1991). Cecropiaceae are distinct from Moraceae, to which they belonged before, and the other families of Urticales in inflorescence and floral characters, but in terms of karyomorphology they are not distinguishable (Oginuma and Tobe 1995). Flowers of both sexes are grouped on clustered spikes, and are minute and numerous. The male inflorescence consists of up to 40 spikes, while the female inflorescences have 2-4 spikes. The female spikes develop into multiple fruits, swollen drupes to 6-26 cm long with a diameter of 0.7-1.6 cm. They contain many minute fruits, each with one seed (achene). Mean dry mass of the fruits is 25.8 g (± 0.62), and mean dry seed mass is 0.002 g (±l0.0001) (Parolin 2000b). With C.lmembranacea and Salix humboldtiana these are the smallest seeds in trees of Amazonian floodplains.
The roots may form stilts, but not as pronounced as in C. membranacea. Branches are few, with bifurcation starting high on the stem. The bark is white, and the wood has low specific gravity (0.33 g cm-3 , Parolin et al. 1998) with irregular increment rings. It has circular rings around the trunk (Fig. 1) and a segmented, hollow pith (Fig. 4), i.e. this species possesses a biologically relatively inexpensive stem structure which is essential for this type of growth strategy to grow very fast by avoiding an energetically costly stem construction (Speck et al. 1998).
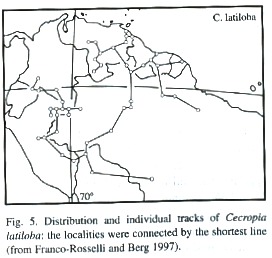
Distribution: Cecropia is a neotropical genus comprising 60 species mainly of humid areas between sea level and 2 600 m elevation. Species of Cecropia occur from southern Mexico to northern Argentina (Franco-Rosselli and Berg 1997). The Andean species are connected to the central Amazonian region by Cecropia species of wide distribution, such as C. sciadophylla, C. distachya and C. latiloba (Franco-Rosselli and Berg 1997).
The geographical distribution of C. latiloba is throughout Amazonian floodplains of whitewater rivers in Brazil, Peru, Colombia, Venezuela, and the Guayanas (Fig. 5; Kubitzki 1989, Ziburski 1991, Lamotte 1992, Prieto et al. 1995). It is almost absent in the floodplains of nutrient-poor blackwater floodplains, with some exceptions, for example in the Anavilhanas archipelago in the Rio Negro. In the inundation gradient in the floodplains, C. latiloba occurs from the high-lying, shortly flooded areas down to levels at 23 m asl subjected to long periods of flooding (Junk 1989).
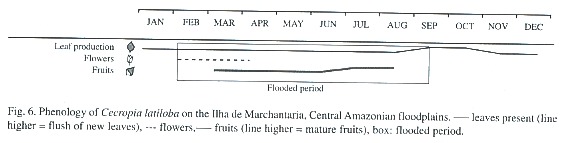
Leaf phenology: C. latiloba is an evergreen species which constantly produces new leaves. With flooding, new leaf production is reduced but not stopped: new leaves are produced also with prolongued flooding (Parolin 1997). Submerged leaves rot fast and are shed after few days.
Flowering, fruiting, and seed production: C. latiloba flowers at the beginning of the flooded period, from February to April (Fig. 6). It is pollinated by wind (Epperson and Alvarez-Buylla 1997), and fruits (Fig. 6) are present during most of the flooded period, from March to July, but they are mature only at the end of the high water phase, in July and August. When mature, the fruits fall apart and the fruit parts with seeds are dispersed by water and fish. Hairs on the fruits which retain air enable the fruits to float (Waldhoff et al. 1996). The fish species Brycon sp. (matrinchã), Cichlasoma sp. (cará), Colossoma macropomum (tambaqui), Lithodoras dorsalis (bacúpedra), Mylossoma sp. (pacú), and Triportheus sp. (sardinha) (Goulding 1980, Ziburski 1991, Roubach and Saint-Paul 1994, Waldhoff and Maia 2000). In general, the diaspores of the genus Cecropia are dispersed by bats and birds (Kubitzki 1985, Galindo-Gonzalez et al. 2000), and other small mammals (Guillaumet 1984). In C. latiloba, birds, bats and also monkeys feed on the fruits and disperse the seeds (Huber 1910, Roosmalen 1985).
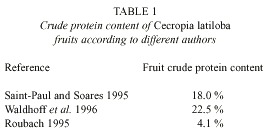
There are contradicting measurements of the protein content of C. latiloba fruits (Table 1): Saint-Paul and Soares 1995 measured 18%, in a range of 11% (Vitex cymosa) to 36% (Pseudbombax munguba). Waldhoff et al. (1996) measured a crude protein content of 22.5%, and Roubach (1995) says that the fruit of Cecropia sp. was the one that presented the lowest crude protein (4.1%) and lipid (0.9%) content in its composition, as well as the highest water content, among several tested species. Total caloric value of the fruits and seeds was 314 kcal(M.E.)/100 g, which was lower than in other plants, and fish of the species Colossoma macropomum that were fed with fruits of Cecropia did not seem to present any weight gain (Roubach 1995).
Water content of the fruit was 78%, and of the seed 58%, compared to an average of 63% water content of the fruits and 47% of the seeds in 27 tree species from the floodplains (Parolin unpubl.).
Fruit production of C. latiloba was 1l280lkg ha-1 (Adis, pers. comm.). Every fruit produces an average of 5 000 seeds (Parolin 1997) which survive prolongued submersion. In contrast to other species from Amazonian floodplains, the Cecropia species of the várzea (C. latiloba, C. membranacea) have a soil seed bank but its importance for the reproductive biology of the species is not known (Ziburski 1991).
Germination: In contrast to Cecropia species from terra firme, the seeds of C. latiloba do not have a period of dormancy, and do not necessarily require strong, direct light for seed germination (Ziburski 1991).
The seeds germinate within 5-13 days, but only if not submerged: germination rate of non-flooded seeds is 96% within three weeks (Ziburski 1991), compared to 0% in submerged seeds (Parolin 2001a). It does not play a role for germination whether the seeds are directly deposited on soil, or whether they are subjected to submersion or intestinal passages (Ziburski 1991).
C. latiloba has the epigean germination mode with foliaceous cotyledons (Hladik and Miquel 1990). Cotyledons opened two days after shoot emergence, which was shorter than in most other analysed species (Parolin 2001a). Cotyledon longevity, i.e. the duration until their fall or deterioration, 25 days at an average and the first leaf was expanded after 9 days, which both is shorter than in other floodplain species (Parolin 2001a).
Growth and productivity: C. latiloba is a light-demanding tree that grows rapidly in the first months after establishment, reaching 1lm of height in the 7 months before the first flooded period. Young individuals do not form branches and spread laterally very much (Worbes 1996). They compensate the reduced crown development by the production of many large leaves which form a large photosynthetically active surface (Worbes 1994). Height growth increases continuously until a height of 15-20 m is achieved. The growth form of adult trees corresponds to Rauhs model (Hallé and Oldeman 1970), which is typical for species with a high capacity of reiteration after damage (Oldeman 1990). The architecture of trees growing according to Rauhs model is characterized by a monopodial trunk which grows rhythmically and so develops tiers of branches, the branches themselves morphogenetically identical with the trunk. Flowers are always lateral and without effect on the growth of the shoot system. This model is one of the most frequent among seed plants, especially among weedtrees. The shoot system is rather unspecialized and is inherently very adaptable because all meristems are equivalent and rhythmic (Hallé et al. 1978). Trees with this architectural model have advantages in early succession because of the inherent simplicity of expression and because of the ability to regenerate readily: if the trunk meristem is destroyed, it is readily replaced, usually by the uppermost lateral meristem (Hallé et al. 1978). C. latiloba belongs to the life-history type in tropical trees that germinate, establish and grow to maturity only in gaps, in contrast to persistent species (Sposito and Santos 2001).
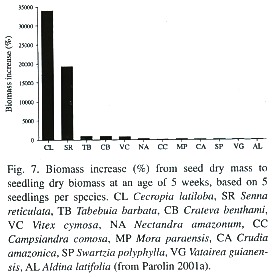
Biomass increase from seed mass (=l100%) to seedling biomass at an age of five weeks was 33 892 %, compared to 19 300% in Senna reticulata, another pioneer of the várzea, and to 900 – 50% in non-pioneer species like Tabebuia barbata or Aldina latifolia (Fig. 7; Parolin 2001a). Seedlings subjected to 12 weeks of waterlogging had 45% lower total biomass than well-watered seedlings of the control (Parolin 1997).
C. latiloba has an annual rhythm of shoot extension (Worbes 1997), and wood increment as determined by tree ring analysis is 9.1 mm y-1 (Worbes 1994), but can be as much as 15 mm y-1 (Parolin et al. 1998). Nitrogen concentrations of the wood are 0.09%, which is among the lowest measured in Amazonian floodplain trees – average nitrogen concentrations are 0.20% (Martius 1992). The C/N ratio of C. latiloba was 730, an individual reached as much as 1 700, whereas in the other 13 analysed species C/N ratios varied between 95 and 700 (Martius 1992).
Productivity of a 12-year-old C. latiloba stand is 8.1 t ha-1 yr-1 of wood (Worbes 1997). In the early successional stands where C. latiloba dominates, it can contribute one third to basal area and above ground biomass, and 50% to total bole volume of the stand (Klinge et al. 1995).
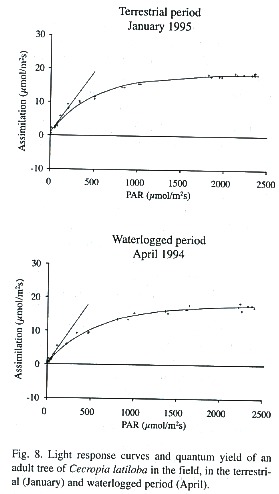
Metabolism: Photosynthetic assimilation, light response and transpiration. As is typical for pioneer species, C. latiloba performs high photosynthetic assimilation. Mean photosynthetic assimilation of C. latiloba is 17.1 ± 0.8 µmol CO2 m-2 s-1 but values of up to 21 µmol CO2 m-2 s-1 could be measured in adult trees in the field. The optimum performance can be measured in the terrestrial period, in well watered plants in the morning, before light incidence becomes too strong and photoinhibition reduces assimilation. Light saturation is reached at 1 000 µmol m-2 s-1 PAR (Fig. 8).
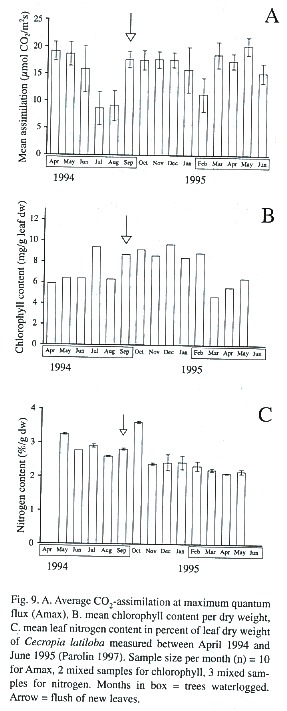
In the annual cycle, characteristic changes of CO2 -assimilation can be measured (Fig. 9A, Parolin 1997, Parolin 2000c). With the beginning of flooding, CO2 -uptake is reduced for some weeks. Then new leaves are sprouted, and photosynthetic assimilation rises again to values comparable to the terrestrial phase. After about six months of flooding leaf senescence increased and CO2 –assimilation decreased, but before the end of flooding new leaves were flushed and photosynthetic assimilation rose.
In waterlogged adult trees in the aquatic phase, average assimilation at maximum light incidence (Amax) was about 11% lower than in the terrestrial period (average non-flooded: 16.8 ± 1.5 µmol CO2 m-2 s-1 , flooded: 15.0 ± 4.6 µmol CO2 m-2 s-1 ), which is shown also by the lower light response curves (Fig. 8; Parolin 1997). Average quantum yield was 4.5% higher in the flooded than in the non-flooded period.
Transpiration was significantly lower in the aquatic period: in the flooded trees, an average of 5 700 µmol m-2 s -1 was measured, compared to an average of 8 200 µmol m-2 s-1 in the terrestrial period (Parolin 1997). Maximum measured transpiration was 9 600 µmol m-2 s-1 .
Chlorophyll fluorescence
Chlorophyll fluorescence is a parameter of potential photosynthesis, expressed by the photochemical yield Fv/Fm, where Fm is maximum fluorescence, and Fv is the difference between Fm and the minimal fluorescence level Fo (Bolhàr-Nordenkampf and Götzl 1992). In adult trees, the average photochemical yield was not significantly different in the terrestrial and aquatic periods, and ranged between 0.65 and 0.68 (Fv/Fm) (Parolin 1997).
Chlorophyll content of the leaves
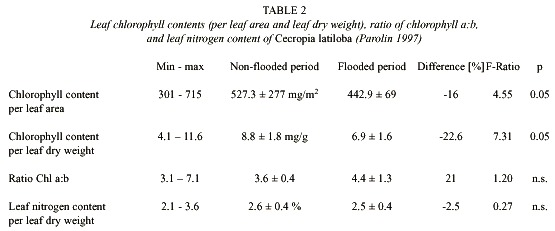
Leaf chlorophyll contents (per leaf dry weight) were significantly higher in the non-flooded trees than in the flooded trees (Table 2, Fig. 9B). The ratio of chlorophyll a:b was around 3.6 in the terrestrial period, and in the flooded period there was an increase of 21% compared to the non-flooded period (Table 2).
Nitrogen content of the leaves
Leaf nitrogen content was 2.5% at an average, but up to 3.6%, which is very high compared to other species (Parolin 1997). Differences between the flooded and the non-flooded period are neglectable (Table 2, Fig. 9C).
Flood tolerance: C. latiloba is a very flood tolerant species, as it occurs mainly at low levels in the flooding gradient. When the plants are submerged, the water destroys the leaves, but below the apex new leaves are produced from new lateral buds as soon as the water recedes. If the strong water currents damage the apex, the high capacity of reiteration compensates the losses (Lamotte 1992, Worbes 1994). Reiteration here is a clear adaptation to the water currents and high sedimentation rates of the Amazon river and its affluents, where sedimentation can be as much as 1lm per year (Junk 1989).
Tolerance to waterlogging is very high (Parolin 1997): seedlings were left in buckets filled with water for 12 months at the INPA / Manaus. Root volume was reduced because it was planted in a 21 plastic bag, and water was changed only at weekly intervals – thus it was subjected to even more extreme waterlogging conditions than juvenile plants are exposed to in the field. After a year the plants were less vital than non-flooded well watered individuals with the same root volume, and the leaves were smaller and had reduced photosynthetic activity. Nevertheless, they continuously produced new leaves and adventitiuos roots, and as soon as the waterlogging ended, the plant resprouted and grew vigorously.
The seedlings and saplings tolerate complete submergence for several months. They shed all the leaves, but new leaves expanded soon after flood end. In an experiment, leaf buds were sprouted before the end of flooding, below the water (Parolin 2001b).
Morphological adaptations to flooding: In waterlogged seedlings in a flooding experiment (Parolin 2001b), C. latiloba produced adventitious roots and a high amount of lenticels. Additionally, pneumatophore-like roots, stem dimorphism and hypertrophied lenticels were observed (Waldhoff et al. 1998). The additional root aeration provided by these formations diminishes the oxygen deficiency in the rhizosphere and enhances growth and photosynthetic activity (Crawford 1989, 1992). A special mechanism for root aeration, pressure ventilation, was shown to occur in seedlings and supposedly occurs also in adult trees of C. latiloba (Graffmann 2000). Therefore height growth, new leaf production, and photosynthetic activity were not severely affected in the waterlogged seedlings in the flooding experiment. C. latiloba was the only species among several tested that produced all, and not only one of these adaptations (Waldhoff et al. 1998). Another function of the adventitious roots is to increase nutrient uptake: Lamotte (1992) observed that C. latiloba develops adventitious roots in the direction of decomposing organic matter, for example fallen tree stems.
Myrmecophyly and secondary compounds: As most Cecropia species, C. latiloba is a myrmecophytic genus. Ants of the genera Azteca, Camponotus and Crematogaster are typically associated with the genus Cecropia (Bandeira and Harada 1991, Ayala et al. 1996) and occur on C. latiloba in the floodplains (Ana Harada pers. comm.). Azteca alfari is found on C. latiloba (Suzana Ketelhut, Ana Harada pers. comm.) as well as the black ants of the genus Lasius (Pio Correa 1978).
Chemical compounds (leaf tannins) and other forms of defenses may augment the biotic defenses (Garay-Arroyo and Alvarez-Buylla 1997). Cecropia may also be protected by physical defenses such as leaf consitutents contributing to toughness, and by phenological traits such as rapid leaf expansion (Folgarait and Davidson 1994). Nevertheless, in few cases high leaf predation could be observed on adult trees in the field (Fig. 10).
Dynamics and colonization strategy: C. latiloba colonizes a large spectrum of disturbed habitats over a wide range in the flood level gradient. It belongs to the mid-level tree community which reaches down to 23 m a.s.l on the shores of the Amazon river, where flooding lasts 230 days per year (Junk 1989). Although single individuals may be flooded for up to 8 months and it is considered as a characteristic tree of the chavascal, i.e. a physiognomic type of várzea with mostly low, arbustive vegetation (Ayres 1993), its main distribution occurs at levels in the flooding gradient with less than 7 months of flooding. C. latiloba preferrably establishes on long-term flooded sites which are less exposed to sedimentation (1-5 cm of sediment deposit per year, silt fraction of 60%; Wittmann 2001). It may therefore establish below an open canopy formed by Salix humboldtiana (Worbes 1994, Oliveira 1998) where sedimentation rates are lower because upright stems break the flow of water, effecting an active deposition of sediment at the water-vegetation interface (Terborgh and Petren 1991).
According to the classification of Worbes et al. (1992), it is a typical representant of the species-poor early secondary stage, after the dominance of the extremely sediment-tolerant Salix humboldtiana, and before the late secondary and climax communities, with Pseudobombax munguba and Piranhea trifoliata, respectively, as typical dominating species.
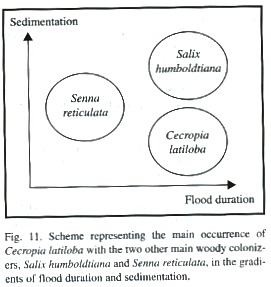
The limiting factors for the establishment of C. latiloba are high sediment deposition on new sand banks and competition with other fast-growing pioneer species, as for example Senna reticulata (Fig. 11). The highest competitivity is reached on sunny open spaces with high nutrient content, for example at sites which were subjected to human disturbances, or on landslides and and sites with erosion, which are frequent in the highly dynamic floodplain system. Senna reticulata is both a primary and secondary colonizer, but competes only as long as the newly colonized sites are not subjected to high floods because it does not tolerate complete sumergence (Parolin 1999, Parolin 2001b). C. latiloba and Senna reticulata are highly adapted to the colonization of open areas in nutrient-rich white-water floodplains and form large monospeficic stands, but they have different strategies concerning germination, seedling establishment and growth (Parolin 1999) and are hardly found on the same sites. Along a transect of 335 m on Ilha do Careiro (Fazenda Pec) in a disturbed area, an abandoned pasture, several seedlings of C. latiloba, and of the concurring species Senna reticulata, were found, but never did the two species occur in the same transect plot of 1 m 2 (Parolin unpubl.).
C. latiloba grows more slowly but constantly, and its high flood tolerance allows vertical evasion from concurring species (Williamson et al. 1998). Senna reticulata has fast initital growth and intense lateral growth, and outshades plants competing for space and light. Seedling growth of two fast-growing pioneer species, C. latiloba and Senna reticulata, was analyzed in the vicinity of Manaus (Parolin 1999). Young plants of C. latiloba grew 1 m in the first 6 months, while Senna reticulata grew up to four meters in the 8 months before the first flood. The strategy of C. latiloba is efficient in várzea environmentswith average to long periods of flooding. When other fast-growing species occur on favourable nutrient-rich sites, C. latiloba may be excluded from dominance because of its intrinsically slow growth, early and high commitment to biotic and other defenses (Folgarait and Davidson 1994). With shorter periods of flooding, and high nutrient availability (e.g. on abandoned pastures), the strategy of Senna reticulata is more efficient and leads to its complete dominance.
Economical importance: The high cellulose content gives this species economical importance for the production of paper (Hueck 1966, Lamotte 1992). It is also used for carbon production (Le Cointe 1922), and for the construction of boxes, rafts and floating houses (Lamotte 1992). An infusion of the leaves and the bark is used against fever (Soukoup 1987). The leaves and bark of Cecropia pachystachya from Northern Argentina are used in phytotherapy for their balsamic and expectorant action (Cativiela et al. 1998).
In conclusion C. latiloba can be considered to be one of the most efficient colonizers of open areas in Amazonian floodplains. Its main strategy to be successful is the high tolerance towards waterlogging and submergence, and the fast vertical growth and reiteration capacity. This, and the tolerance of high irradiation and sediment deposition allow C. latiloba to form large monospecific stands on open sites, and thus the first closed canopy which represents the initial phase of a successional sequence where less light-demanding species can penetrate and establish, resulting in stands with 130 species per hectare (Junk 1989) and high floristic diversity.
Acknowledgments
I wish to thank the technicians of INPAfor help received in the field, and Suzana Ketelhut for additional information. This work was made possible by financial support from the INPA/Max-Planck Project.
Resumen
Cecropia latiloba puede ser considerada una de las especies colonizadoras más eficientes de áreas abiertas en las llanuras inundadas de agua dulce, rica en nutrientes, del río Amazonas. Su principal estrategia para ser exitosa es la tolerancia al transporte por el agua y a permanecer sumergida, y su rápido crecimiento vertical y capacidad de reiteración. Esto, y la tolerancia a la alta irradiación y a la deposición de sedimentos permite a C. latiloba formar grandes parches monoespecíficos en sitios abiertos, y así el primer dosel cerrado el cual representa la fase inicial de la secuencia de sucesión que conduce a bosques altamente diversos. Este árbol, está extremadamente bien adaptado a las condiciones adversas de crecimiento en el terreno aluvial amazónico, con prolongados períodos de inundación y sumersión. La especie está presente en los niveles inferiores en la gradiente de inundación. Aunque pertenece a las especies más frecuentemente citadas en aspectos de taxonomía, distribución y descripción general del ecosistema, poco ha sido publicado respecto a su ecología. En el presente artículo se describen las características ecológicas, fisiológicas y fenológicas de C. latiloba. Es una especie siempre verde que constantemente produce hojas. Con las inundaciones, la producción de hojas se reduce pero nuevas hojas brotan también durante las inundaciones prolongadas. El pico de floración y fructificación es durante el período inundado. Cuando maduran, las frutas son dispersadas principalmente por el agua y los peces. La germinación de las semillas ocurre, sin latencia, entre 513 días después de la retirada de las aguas. En los 7 meses anteriores al primer período de inundación las plantas alcanzan 1 m de altura y el crecimiento se incrementa hasta que se alcanza una altura de 15-20 m. La asimilación fotosintética es alta, con valores de hasta 21 µmol CO2 m-2 s-1 . C. latiloba es una especie muy tolerante a las inundaciones, y el transporte de troncos por el agua continuamente produce nuevas hojas y raíces adventicias.
References
Ayala, F.J., J.K. Wetterer, J.T. Longino, D.L Hartl. 1996. Molecular phylogeny of Azteca ants (Hymenoptera: Formicidae) and the colonization of Cecropia trees. Mol. Phylogenet. Evol. 5: 423-428. [ Links ]
Ayres, J.M.C. 1993. As matas de várzea do Mamirauá. pp. 1-123. In: Estudos de Mamirauá. Sociedade Civil Mamirauá (ed.). CNPq, Brasília, DF, Brasil. Vol.I. Bandeira, A.G., Harada, A.Y. 1991. Cupins e formigas na Amazônia. In: Val A.L., R. Figliuolo and E. Feldberg (eds.). Bases cientificas para estratégias de preservaçao e desenvolvimento da Amazônia: Fatos e perspectivas. Manaus 1: 387-395. [ Links ]
Berg, C.C. 1978. Espécies de Cecropia da Amazônia Brasileira. Acta Amazonica 8(2): 149-182. [ Links ]
Bolhár-Nordenkampf, H.R., M. Götzl. 1992. Chlorophyllfluoreszenz als Indikator der mit Seehöhe zunehmenden Streßbelastung von Fichtennadeln. FBVA Berichte. Schriftenreihed. Forstl. Bundesveranst. 67: 119-131. [ Links ]
Cativiela M., M. Torres & M. Gattuso. 1998. Micrographic parameters for Cecropia pachystachya. Fitoterapia 69(4): 313-321. [ Links ]
Coombs, J., D.O. Hall, S.P. Long & L.H.O. Scurlock, 1985. Techniques in bioproductivity and photosynthesis. 2nd ed. Pergamon Press. 283 pp. [ Links ]
Crawford, R.M.M. 1989. The anaerobic retreat. In: Studies on plant survival. Ecological case histories of plant adaptation to adversity. R.M.M. Crawford (ed.). Blackwell Scientific Publications. Studies in Ecology Vol. 11: 105-129. [ Links ]
Crawford, R.M.M. 1992. Oxygen availability as an ecological limit to plant distribution. Adv. Ecol. Res. 23: 93-185. [ Links ]
Epperson BK. & E.R. Alvarez-Buylla 1997: Limited seed dispersal and genetic structure in life stages of Cecropia obtusifolia. Evolution 51: 275-282. [ Links ]
Ferreira, L.V. 1997. Effects of the duration of flooding on species richness and floristic composition in three hectares in the Jaú National Park in floodplain forests in central Amazonia. Biodiv. and Conserv. 6: 1353-1363. [ Links ]
Fleming, T.H., C.F. Williams. 1990. Phenology, seed dispersal, and recruitment in Cecropia peltata (Moraceae) in Costa Rican tropical dry forest. J. Trop. Ecol. 6: 163-178. [ Links ]
Folgarait, P.J., D.W. Davidson. 1994. Antiherbivore defenses of myrmecophytic Cecropia under different light regimes. Oikos 71: 305-320. [ Links ]
Franco-Rosselli, P., C.C. Berg. 1997. Distributional patterns of Cecropia (Cecropiaceae): A panbiogeographic analysis. Caldasia 19(1-2): 285-296. [ Links ]
Galindo-Gonzalez, J., S. Guevara & V.J. Sosa. 2000: Bat-and bird-generated seed rains at isolated trees in pastures in a tropical rainforest. Conservation Biol. 14(6): 1693-1703. [ Links ]
Garay-Arroyo, A., E.R. Alvarez-Buylla. 1997. Isozyme variation in a tropical pioneer tree species (Cecropia obtusifolia, Moraceae) with high contents of secondary compounds. Biotropica. 29: 280-290. [ Links ]
Goulding, M. 1980. The fruit- and seed-eating large characins. pp. 55-216. In: M. Goulding (ed.) The fishes and the forest. Explorations in Amazonian natural history. Univ. of California. [ Links ]
Graffmann, K.C. 2000. Die Bedeutung der Druckventilation für die Sauerstoffversorgung des Wurzelsystems bei Bäumen der amazonischen Über-schwemmungswälder. Dissertation Uni Köln, 91 pp. [ Links ]
Guillaumet, J.-L. 1984. Observações sobre a frutificação e disseminação das sementes de algumas espécies do gênero Cecropia (Moraceae). pp. 143-163. In: XXXV Congresso Nacional de Botânica, Anais, SBB, Manaus. [ Links ]
Hallé, F., Oldeman, R.A.A. 1970. Essai sur larchitecture et la dynamique de croissance des arbres tropicaux. Monographie 6, Mansson. Paris. 178 p. [ Links ]
Hallé, F., Oldeman, R.A.A., Tomlinson, P.B. 1978. Tropical trees and forests. An architectural analysis. Springer, Berlin Heidelberg New York. 441 p. [ Links ]
Hladik, A., & S. Miquel. 1990. Seedling types and plant establishment in an African rain forest. In: Bawa K.S. & M. Hadley (eds.). Reproductive Ecology of tropical forest plants. Man and the Biosphere Series 7:261-282. [ Links ]
Huber, J. 1910. Mattas e madeiras amazonicas. Bol. Museu Goeldi 6: 91-225. [ Links ]
Hueck, K. 1966. Die Wälder Südamerikas. Ökologie, Zusammensetzung und wirtschaftliche Bedeutung. Gustav Fischer, Stuttgart. 358 p. [ Links ]
Junk, W.J. 1989. Flood tolerance and tree distribution in Central Amazonian floodplains. pp. 47-64. In: L.B. Nielsen, I.C. Nielsen and H. Balslev (eds.). Tropical forests: Botanical dynamics, speciation and diversity. Academic Press London. [ Links ]
Junk, W.J., Bayley, P.B., Sparks, R.E. 1989. The flood pulse concept in river-floodplain systems. In: D.P. Dodge (ed.). Proceedings of the International Large River Symposium. Can. Publ. Fish. Aquat. Sci. 106: 110-127. [ Links ]
Klinge, H., J. Adis, M. Worbes. 1995. The vegetation of a seasonal várzea forest in the lower Solimões River, Brazilian Amazonia. Acta Amazonica 25(3/4): 201-220. [ Links ]
Kubitzki, K. 1985. The dispersal of forest plants. In: Prance G.T. & T.E. Lovejoy (eds.). Amazonia – Key environments. Pergamon. pp. 129-163. [ Links ]
Kubitzki, K. 1989 a. The ecogeographical differentiation of Amazonian inundation forests. Plant Syst. Evol. 162: 285-304. [ Links ]
Lamotte, S. 1992. Essai dinterprétation dynamique des végétations en milieu tropical inondable. La plaine alluviale de haute Amazonie. PhD Thesis Academie de Montpellier. 423 p. [ Links ]
Le Cointe, P. 1922. LAmazonie brésilienne. Augustin Challamel (ed.). Librairie Maritime et Coloniale, Paris. [ Links ]
Martius, C. 1992. Density, humidity, and nitrogen content of dominant wood species of floodplain forests (Várzea) in Amazonia. Holz als Roh- und Werkstoff. 50: 300-303. [ Links ]
Medina E. 1983. Adaptations of tropical trees to moisture stress. pp. 225-237. In: Golley F.B. (ed.). Ecosystems of the world: Tropical rain forest ecosystems. Elsevier Scientific Publ. Comp. [ Links ]
Milton, K. 1991. Leaf change and fruit production in six neotropical Moraceae species. J. Ecol. 79:1-26. [ Links ]
Oginuma, K. & H. Tobe. 1995. Karyomorphology of some Moraceae and Cecropiaceae (Urticales). J. Plant Research 108(1091): 313-326. [ Links ]
Oldeman, R.A.A. 1990. Forests: Elements of Silvology. Springer, Berlin. 623 p. [ Links ]
Oliveira, A.C. de. 1998. Aspectos da dinâmica populacional de Salix martiana Leyb. (Salicaceae), em áreas de várzea da Amazônia Central. Master Thesis INPA/FUA. 83 p. [ Links ]
Parolin, P. 1997. Auswirkungen periodischer Vernässung und Überflutung auf Phänologie, Photosynthese und Blattphysiologie von Baumarten unterschiedlicher Wachstumsstrategie in zentralamazonischen Überschwemmungsgebieten. Herbert Utz, Wissenschaft, München. 166 p. [ Links ]
Parolin, P., L.V. Ferreira, W.J. Junk. 1998. Central Amazonian floodplains: effect of two water types on the wood density of trees. Verh. Internat. Verein. Limnol. 26: 1106-1112. [ Links ]
Parolin, P. 1999. Growth strategies of Senna reticulata and Cecropia latiloba, two pioneer tree species of Central Amazonian floodplains. Bielefelder Ökologische Beiträge 14 :272-277. [ Links ]
Parolin, P. 2000a. Physiology of Trees in the Flood Plains of the Amazon. The Botanica 50: 10-24. [ Links ]
Parolin, P. 2000b. Seed mass in Amazonian floodplain forests with contrasting nutrient supplies. J. Trop. Ecol. 16: 417-428. [ Links ]
Parolin, P. 2000c. Phenology and CO 2 -assimilation of trees in Central Amazonian floodplains. J. Trop. Ecol. 16: 465-473. [ Links ]
Parolin, P. 2001a. Seed germination and early establishment in 12 tree species from nutrient-rich and nutrient- poor Central Amazonian floodplains. Aquat. Bot. 70: 89-103. [ Links ]
Parolin, P. 2001b. Effects of waterlogging, flooding and drought on tree seedlings from Amazonian flood-plains. Oecologia 128: 326-335. [ Links ]
Pio Correa, M. 1978. Dicionário de plantas úteis do Brasil e das exóticas cultivadas. Min. Agric., Inst. Brasil. Desenv. Florest., 6 volumes. [ Links ]
Prance, G.T. 1979. Notes on the vegetation of Amazonia. III. Terminology of Amazonian forest types subjected to inundation. Brittonia, New York Botanical Garden, 31: 26-38. [ Links ]
Prieto, A., J.O. Rangel, A. Rudas, P. Palacios. 1995. Structural aspects and vegetation types from Mocagua Island, Amazon River. Caldasia 17(82-85): 463-479. [ Links ]
Roosmalen, M.G.M. 1985. Habitat preferences, diet, feeding strategy and social organization of the black spider monkey (Ateles paniscus paniscus Linnaeus 1758) in Surinam. Acta Amazonica 15: 1-290. [ Links ]
Roubach, R., Saint-Paul, U. 1994. Use of fruits and seeds from Amazonian inundated forests in feeding trials with Colossoma macropomum (Cuvier, 1818) (Pisces, Characidae). J. Appl. Ichthyol. 10: 134-140. [ Links ]
Roubach, R. 1995. Growth performance of Colossoma macropomum (Serrasalminae) fed with fruits from inundated forests. pp. 52-53. In: 2nd SHIFT Workshop, Cuiabá, 1995. Genus Ed. [ Links ]
Saint-Paul, U., M.G.M. Soares. 1995. The Amazonian inundated forest: relation between fish and environment. pp. 41-43. In: 2nd SHIFT Workshop, Cuiabá, 1995. Genus Ed. [ Links ]
Soukup, J. 1987. Vocabulario de los nombres vulgares de la flora peruana y catálogo de los géneros. Salesiana Ed., Lima, 436 p. [ Links ]
Speck, T., Atger, C., Bogenrieder, A., Galtier, J., Rowe, N.P. & Spatz, H.C. 1998. Mechanical properties of two hollow stemmed tropical trees (Cecropia obtusa, Cecropia sciadophylla). J. Exp. Bot. 49 (Suppl.): 79. [ Links ]
Sposito, T.C. & F.A.M. Santos. 2001. Architectural patterns of eight Cecropia (Cecropiaceae) species of Brazil. Flora 196: 215-226. [ Links ]
Terborgh, J. & Petren, K. 1991. Development of habitat structure through succession in an Amazonian flood-plain forest. pp. 28-46. In: S.S. Bell et al., (eds.): Habitat Structure. Chapman and Hall, London. [ Links ]
Waldhoff, D., L. Maia Alencar 2000. Production and chemical composition of fruits from trees in flood-plain forests of Central Amazonia and their importance for fish production. In: W.J. Junk, J. Ohly, M.T.F. Piedade & Soares, M.G. (eds.): The Central Amazon floodplain: Actual Use and Options for a Sustainable Management. Backhuys, Leiden. [ Links ]
Waldhoff, D., U. Saint-Paul, B. Furch. 1996. Value of fruits and seeds from the floodplain forests of Central Amazonia as food resource for fish. Ecotropica 2: 143-156. [ Links ]
Waldhoff, D., W.J. Junk, B. Furch. 1998. Responses of three Central Amazonian tree species to drought and flooding under controlled conditions. Intern. J. Ecol. Environm. 24: 237-252. [ Links ]
Whitmore, J.L. 1973. Wood density variation in Costa Rican balsa. Wood Sci. 5(3): 223-229. [ Links ]
Williamson, G.B., R.C.G. Mesquita, K. Ickes, G. Ganade. 1998. Estratégias de colonizaçao de árvores pioneiras nos Neotrópicos. pp. 131-144. In: Gascon C., P. Mouthino (eds.). Floresta Amazônica: Dinâmica, Regeneração e Manejo. Instituto Nacional de Pesquisas da Amazônia (INPA). [ Links ]
Wittmann, F. 2001. Artenverbreitung und Bestandesstruktur amazonischer Várzea-Waelder und die Erfassung von Waldtypen mittels fernerkundlichen Methoden. PhD thesis Univers. Mannheim. [ Links ]
Worbes, M. 1994. Grundlagen und Anwendungen der Jahresringforschung in den Tropen. Habilitationsschrift Uni Hamburg. 211 p. [ Links ]
Worbes, M. 1996. Rhythmisches Wachstum und anatomisch-morphologische Anpassungen an Lebensstrategien von Bäumen in zentralamazonischen Überschwemmungswäldern. Mitt. Dtsch. Dendrol. Ges. 82: 155-172. [ Links ]
Worbes, M. 1997. The forest ecosystem of the floodplains. pp. 223-266. In: W.J. Junk (ed.). The Central Amazon floodplain: Ecology of a pulsing system. Ecological Studies 126, Springer, Heidelberg. [ Links ]
Worbes, M., H. Klinge, J.D. Revilla, C. Martius. 1992. On the dynamics, floristic subdivision and geographical distribution of Várzea forests in Central Amazonia. J. Vegetation Sci. 3: 553-564. [ Links ]
Ziburski, A. 1991. Dissemination, Keimung und Etablierung einiger Baumarten der Überschwem-mungswälder Amazoniens. In: W. Rauh (ed.). Tropische und subtropische Pflanzenwelt. Akademie der Wissenschaften und der Literatur. 77: 96. [ Links ]