Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO  uBio
uBio
Share
Revista de Biología Tropical
On-line version ISSN 0034-7744Print version ISSN 0034-7744
Rev. biol. trop vol.50 n.2 San José Jun. 2002
in LLC-MK2 cells revealed by thin sections, negative staining,
and scanning electron microscopy
Francisco Hernández-Chavarría 1, 2* , Libertad Alzamora-González 1, 3 , Libia Herrero-Uribe2
1 Centro de Investigación en Estructuras Microscópicas, Universidad de Costa Rica, San José, Costa Rica.
2 Facultad de Microbiología, Universidad de Costa Rica, San José, Costa Rica.
3 Facultad de Biología, Universidad Cayetano Heredia, Lima, Perú.
* Author for correspondence: Centro de Investigación en Estructuras Microscópicas, Universidad de Costa Rica, San José, Costa Rica. e-mail: hchavarr@cariari.ucr.ac.cr
Recibido 22-VII-2001. Corregido 21-I-2002. Aceptado 19-III-2002.
Abstract
Previous electron microscope studies of ultrastructural events during hepatitis A virus replication in experimentally infected cells have used only ultrathin section techniques. Nevertheless, no important differences were observed between infected and uninfected cells. This study was carried out using scanning electron microscopy and negative staining of whole LLC-MK2 cells grown directly on grids covered with support membranes, and then infected with an hepatitis A virus strain. Thin sections of infected and unifected controls were also analyzed. An intricate web of projections forming a net between cell interfaces was observed only in infected cells. Some of these projections were more than 700 nm long and had ballooning tips. Nevertheless, HAV particles were not visualized in the infected cells.
Key words: Hepatitis A virus, negative staining, scanning electron microscopy, thin sections, ultrastructure.
Ultrathin sections represent the most practical and common methodology to study the ultrastructure of cells, including those infected by viruses. The thickness of the sections analyzed using transmission electron microscope (TEM) depend on the acceleration voltage used; for example, in a standard TEM using acceleration voltages of 75 to 125 kV, it is possible to observe sections less than 100 nm thick. For this reason, a thin section represents only a very small part of the cell volume. Other methods have been used to study distribution of virions inside the cell, such as observation of thick sections or whole cells using ultra high voltage electron microscopy (Mazzone et al. 1985, Yoshida et al. 1986, Albrecht et al. 1989, Hiramatsu et al. 1999). Nevertheless, this instrumentation is not widely available.
Negative staining methods are used almost exclusively for the analysis of isolated virions or bacteria. This method can also be used to analyze cells grown on grids with support membrane. When this preparation is analyzed using a standard electron microscope, it is possible to visualize clearly the thin periphery of flat cells, such as epitheliod cells. The polarity of the cells is maintained since the monolayer is not disturbed during the procedure. This technique can be used in combination with scanning electron microscopy of cell monolayers grown on cover glasses.
The method proposed above was used to analyze LLC-MK2 cells infected with a strain of hepatitis A virus (HAV) adapted to grow in cell monolayers (Lemon et al. 1992). Previous studies of the ultrastructural events during replication of HAV were carried out using thin sections of infected cells, and showed that infection is always noncytocidal and persistent, with a restricted yield (Hollinger & Ticehurst 1996). Virions were demonstrated in small cytoplasmic vesicles of marmoset hepatocytes. Those vesicles may be bound by multilayer membranes (Provost et al. 1975).
Few ultrastructural alterations have been detected in experimentally infected cell-monolayers (Kiernan et al. 1987, Tinari et al. 1989, Hollinger and Ticehurst 1996). The most conspicuous finding is the appearance of multilayer arrangements of membranes, but those were also observed in uninfected control cells (Kiernan et al. 1987). Thus, both infected and control cells were indistinguishable with the exception of annulate lamellae that were more frequently in the infected cells (Marshall et al. 1996); sometimes this membrane arrangement appeared to be continuous with the smooth endoplasmic reticulum (Goser et al. 2000). Kiernan et al. (1987) were unable to observe HAV like particles as previously described in vivo by Provost et al. (1975). The visualization of viral particles was done only when the specimens were labeled with specific antibodies (Asher et al. 1987, 1988, Tinari 1989).
In contrast, we have detected marked differences between HAV infected cells and their uninfected controls, using a combination of scanning electron microscopy and negative staining of whole cells.
Materials and methods
Confluent monolayers of LLC-MK2 (ATCC- CCL 7) cells, cultured on cover-glasses, were infected with the culture adapted HAX-70 strain of Hepatitis A virus (kindly provided by Lousiana State University – International Center for Medical Research and Training (LSU-ICMRT)) and incubated at 37°C under a 5% CO2 atmosphere. Both infected and uninfected monolayers were processed for electron microscopy 14, 21, and 31 days after infection. Cell monolayers were fixed for 2 hours at room temperature (2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 mM, pH 7.2 in phosphate buffer). Post-fixation was carried out in 1% OsO4 for one hour in the same buffer. The specimens were dehydrated through an ascending ethanol series and processed for scanning electron microscope (SEM) or for transmission electron microscope (TEM). Samples for SEM were dried using the t-butyl alcohol method, mounted on aluminum studs, ion-sputtered with platinum and analyzed using SEM (Hitachi S-570). Samples for TEM were embedded in Spurr resin, thin-sectioned and contrasted with uranyl acetate and lead citrate, and analyzed under TEM (Hitachi 7100). Grids covered with formvar membrane were placed on microtiter plates and then inoculated with cells until the monolayer became confluent and covered the grids, after which monolayers were infected with HAV. Other cell monolayers were kept as uninfected controls. Three weeks later the cells on the grids were fixed with 2.5% glutaraldehyde, negatively stained with phosphotunstic acid and analyzed under TEM. HAV infection of the infected cell monolayers was confirmed by radio immunoassay (Villarejos et al. 1982).
Results
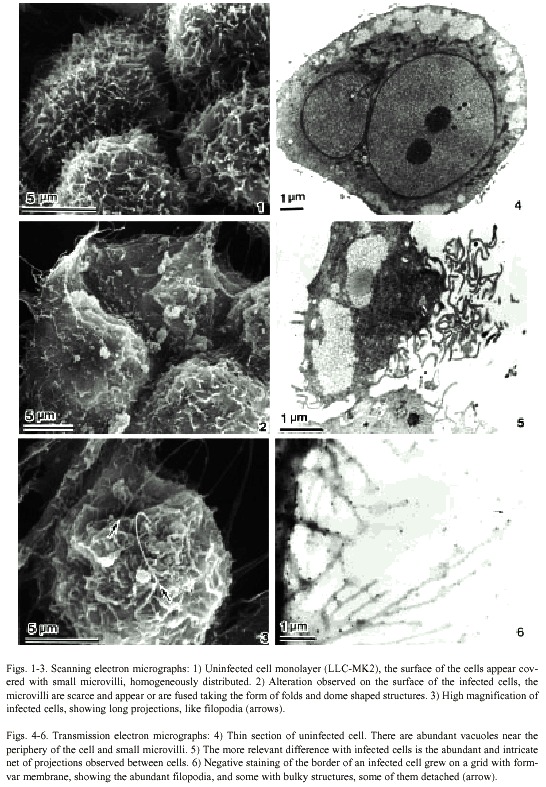
SEM analysis of both infected and uninfected cells showed abundant microvilli homogeneously distributed on the apical surfaces (Fig.1). However, their density was apparently reduced on infected cells, on which folds and dome shaped structures (Fig. 2), as well as long projections along cell interfaces, were observed on infected cells (Fig. 3).
In thin sections, both infected and uninfected cells appeared similar. The nucleus was rounded or bilobulated and the chromatin was homogeneously distributed with one or two well developed nucleoli; electron lucid vacuoles with an extensive light fibrillar component were localized mainly at the periphery of the cell (Fig 4). These were also observed in infected cells (Fig. 5). The main difference between both cells was the abundant and intricate projections forming a kind of net surrounding the infected cells (Fig. 5).
Infected cells grown on grids with formvar membrane showed abundant projections, some of them more than 700 nm long. Several of these showed ballooning tips of approximately 500 nm in diameter (Fig. 6), and some of these tips appeared detached from the projections.
Discussion
The infection of cell lines with HAV, even with well-adapted viral strains, is noncytocidal and persistent, with a restricted yield (Lemon et al. 1992, Teterina et al. 1997, Goset et al. 2000). Virions are usually recovered only by mechanical lysis of cell monolayers, because the progeny of most HAV strains remain cell-associated. The mechanism of virus spread from cell to cell is still unknown (Hollinger and Ticehurst 1996, Cuthbert 2001).
Previous electron microscopic studies using thin sections of HAV infected cell cultures have shown no marked ultrastructural changes in infected cells, with the exception of some cytoplasmic accumulations of multilayered membranes (Asher et al. 1987, 1988, Kiernan et al. 1987) or annulate lamellae (Marshall et al. 1996). However, our analysis of thin sections and negative staining of whole cells using a standard TEM (acceleration voltage of 100 kV), in conjunction with scanning electron microscopy, demonstrated that LLC-MK2 cells, infected with HAV, showed an intricate web of projections forming a net between adjacent cells, not previously described, which was not observed in uninfected controls. Nevertheless, HAV viral particles were not visualized in these structures.
The mechanism of viral release from infected cells is unknown, and does not depend on cell destruction, because high viral titers are found in stools previously any evidence of cell necrosis in natural infections (Cuthbert 2001). We can speculate, if this intricate net of cytoplasmic projections appears only in infected cells, a possible mechanism of viral transmission cell to cell, that could involve those projections without cell destruction. Is possible that those cytoplasmic projections were not reported before, because the previously ultra-structural studies used thin sections, a methodology that makes difficult the analysis of the whole border of each cell. In our work, we surmount this obstacle using other methods to observe easily the complete cells, such as negative stain or scanning electron microscopy.
Acknowledgments
We thank Jorge D. García for their suggestions and critical review of the manuscript, and the International Center for Medical Research and Training (ICMRT) for provided the Hepatitis A strain (HAX-70) used in this study. The project was supported in part by the Vicerrectoría de Investigación of the University of Costa Rica and the Japan International Cooperation Agency.
Resumen
Los estudios previos sobre los eventos ultraestructurales en la replicación del virus de la hepatitis Aen cultivos de células infectadas experimentalmente, se han realizado exclusivamente mediante cortes ultrafinos. No obstante, no se han observado diferencias importantes entre células infectadas y no infectadas. Este estudio fue realizado empleando microscopia electrónica de rastreo, tinción negativa de células (LLC-MK2) cultivadas directamente sobre rejillas para microscopia electrónica de transmisión e infectadas con el virus de la hepatitis A. Adicionalmente se analizaron cortes ultrafinos de células infectadas y no infectadas. Se observó una intrincada red de proyecciones entre las superficies de las células, que apareció solo en las células infectadas. Algunas de esas proyecciones medían más de 700 nm de largo y terminaban en extremos abultados. Sin embargo, no se observaron viriones en las células infectadas.
References
Albrecht, R.M., S.L. Goodman & S.R. Simmon. 1989. Distribution and movement of membrane-associated platelet glycoproteins: Uses of colloidal gold with correlative video-enhanced light microscopy, low-voltage high-resolution scanning electron microscopy, and high-voltage transmission electron microscopy. Am. J. Anat. 185: 149-164. [ Links ]
Asher, L.V.S., L.N. Binn & R.H. Marchwicki. 1987. Demonstration of hepatitis A virus in cell culture by electron microscopy with immunoperoxidase staining. J. Virol. Meth. 15: 323-328. [ Links ]
Asher, L.V.S., L.N. Binn, R.H. Marchwicki, T.L. Mensing & R.J. Feighny. 1988. Electron Microscopy and immunoelectron microscopy of hepatitis A virus in cell culture. pp. 19-23. In A.J. Zuckerman (ed.). Viral hepatitis and liver disease. New York: Alan R. Liss. [ Links ]
Binn, L.N., S.M. Lemon, R.H. Marchwicki, R.R. Redfield, N.L. Gates & W.H. Bancroft. 1984. Primary isolation and serial passage of hepatitis A virus strains in primate cell cultures. J. Clin. Microbiol. 20: 28-33. [ Links ]
Cuthbert, J.A. 2001. Hepatitis A: Old and new. Clin. Microbiol. Rev. 14: 38-58. [ Links ]
Gosert, R., D. Egger & K. Bienz. 2000. A cytopathic and a cell culture adapted hepatitis A virus strain differ in cell killing but not in intracellular membrane rearrangements. Virology. 266: 157-169. [ Links ]
Hiramatsu, Y., F. Uno, M. Yoshida, Y. Hatano & S. Nii 1999. Poxvirus virions: Their surface ultrastructure and interaction with the suerface membrane of host cells. J. Electron. Microsc. 48: 937-946. [ Links ]
Hollinger, F.B. & J.R. Ticehurst. 1996. Hepatitis A Virus. pp. 735-782. In B.N. Fields et al. (eds.). Virology. Lippincott – Raven, Philadelphia. [ Links ]
Kiernan, R.E., J.A. Marshall, A.G. Coulepis, D.A. Anderson & I.D. Gust. 1987. Cellular changes associated with persistent hepatitis A infection in vitro. Arch. Virol. 94: 81-95. [ Links ]
Lemon, S.M., R.W. Jansen & E.A. Brown. 1992. Genetic, antigenic and biological differences between strains of hepatitis A virus. Vaccine, Vienna. Suppl. pp. 1-5. [ Links ]
Marshall, J.A., J. Borg, A.G. Coulepis & D.A. Anderson. 1996. Annulate lamellae and lytic HAV infection in vitro. Tissue Cell. 28: 205-214. [ Links ]
Mazzone, H.M., G. Wray & W.F. Engler. 1985. The high voltage electron microscope in virology. Adv. Virus. Res. 35: 47-59. [ Links ]
Ticehurst, J.R., J.I. Cohen, S.M. Feinstone, R.H. Purcell, R.W. Jansen & S.M. Lemon. 1989. Replication of hepatitis A virus: new ideas from studies with cloned cDNA. pp. 27-50. In E. Ehrenfeld & B.L. Semler. (eds.). Molecular Aspects of Picornavirus Infection and Detection. A.S.M., Washington DC. [ Links ]
Provost, P.J., B.S. Wolanski, W.J. Miller, O.L. Ittensohn, M.J. McAleer & M.R. Hilleman. 1975. Physical, chemical and morphological dimensions of human hepatitis A virus strain CR326(38578). Proc. Soc. Exp. Biol. Med. 148: 532-539. [ Links ]
Siegl, G., J. De Chastonay & G. Kronaver. 1984. Propagation and assay of hepatitis A virus in vitro. J. Virol. Meth. 9, 53-67. [ Links ]
Teterina, N.L., K. Bienz, D. Egger, A.E. Gorbalenya & E. Ehrenfeld. 1997. Induction of intracellular membrane rearrangements by HAV proteins 2C and 2BC. Virology 237: 66-77. [ Links ]
Tinari, A., F.M. Ruggeri, A.M. Divizia, A. Pana & G. Donelli. 1989. Morphological changes in HAV-infected Frp/3 cells and immunolocalization of HAAg. Arch. Virol. 104: 209-224. [ Links ]
Villarejos, V.M., J. Serra, K. Anderson-Visoná & J. Mosley. 1982. Hepatitis A in households. Am. J. Epidemiol. 115: 577-586. [ Links ]
Yoshida, M., F. Uno & S. Un. 1986. High voltage electron microscopy of whole cells infected with herpes simples virus type 1. J. Electron. Microsc. 35: 47-59. [ Links ]